Abstract
Background
Egg white proteins are usually subjected to heating, making them edible for the majority of egg-allergic children.
Objective
We sought to investigate the underlying mechanisms responsible for the reduced allergenicity displayed by heat-treated egg white allergens.
Methods
C3H/HeJ mice were orally sensitized with ovalbumin (OVA) or ovomucoid (OM) and challenged with native or heated proteins to evaluate their allergenicity. Immunoreactivity was assessed by immunoblotting using sera from egg-allergic children. In vitro gastrointestinal digestion of native and heated OVA and OM was studied by SDS-PAGE and liquid chromatography. Intestinal uptake of intact native and heated OVA and OM by human intestinal epithelial (Caco-2) cells was investigated. Rat basophil leukemia (RBL) cells passively sensitized with mouse serum and human basophils passively sensitized with egg-allergic children’s serum were used to assess the effector cell activation by heated, digested and transported OVA and OM.
Results
Heated OVA and OM did not induce symptoms of anaphylaxis in sensitized mice when administered orally. Heating did not completely destroy IgE-binding capacity of OVA or OM but enhanced in vitro digestibility of OVA. Digestion of both OVA and OM diminished mediator release in RBL assay and basophil activation. Heating of allergens prevented transport across human intestinal epithelial cells in a form capable of triggering basophil activation or T cell activation.
Conclusions
Heat treatment reduces allergenicity of OVA and OM. This is partially due to the enhanced gastrointestinal digestibility of heated OVA and the inability of heated OVA or OM to be absorbed in a form capable of triggering basophils.
Clinical implications
Reduced allergenicity of heated egg white proteins partially resulting from altered digestion and absorption in the gastrointestinal tract may explain the clinical tolerance of extensively heated egg in the majority of egg-allergic children.
Capsule summary
The majority of egg-allergic children tolerate extensively heated egg. This study demonstrates that the decreased allergenicity of heated ovalbumin and ovomucoid in large part results from altered digestion and processing in the gastrointestinal tract.
Keywords: egg allergy, ovalbumin, ovomucoid, heat treatment, heating, gastrointestinal digestion, antigen absorption, mice oral sensitization, anaphylaxis, basophil activation, passive sensitization
INTRODUCTION
Food processing and gastrointestinal degradation are fundamentally important for food protein allergenicity. Numerous reports1–6 addressed the effect of thermal and non-thermal processing on the final food allergenicity, which can be either enhanced or reduced depending on the particular allergen. Moreover, structural stability under the extreme degradative environment found within the gastrointestinal tract is often a requisite for a protein to elicit an allergic response.7, 8
Food processing is of particular relevance for egg white allergens, since egg proteins are usually subjected to heat treatment such as boiling or baking and are likely to undergo important structural changes affecting their secondary and tertiary structure. It has been reported that approximately 70% of egg-allergic children tolerated baked egg ingestion. 9–12
It is usually argued that heating induces protein denaturation leading to the loss of conformational epitopes, suggesting that heated egg-tolerant children would present IgE antibodies mostly against conformational epitopes.11,13 Heat resistant proteins like ovomucoid (OM), the dominant egg white allergen, can retain both linear and conformational epitopes upon heating. However, OM-specific IgE levels were found to be poorly predictive of heated egg reactivity in a study enrolling 117 egg allergic subjects.12 Heat-induced aggregation of milk allergens was shown to prevent their absorption through enterocytes and subsequent onset of allergic symptoms in mice14, pointing at an additional explanation for tolerance to heated allergens.
These collective data suggest that extensive heating diminishes the allergenicity of egg white proteins, although the underlying mechanisms remain elusive. We sought to investigate the factors behind the reduced allergenicity displayed by the two major egg white allergens, ovalbumin (OVA) and ovomucoid, when they are subjected to heat-treatment. We utilized in vivo and in vitro methods to compare digestion resistance, intestinal transport and effector cell-triggering capacity of native and heated egg white proteins.
METHODS
Heating of ovalbumin and ovomucoid
OVA (Grade VI, 99% of purity, Sigma, St. Louis, MO) and OM (Trypsin inhibitor from chicken egg white, Type III-O, free of ovoinhibitor, Sigma) were dissolved as required for the different assays and heated in a boiling water bath for 30 minutes.
In vitro digestion of ovalbumin and ovomucoid
Gastric digestion
OVA and OM were dissolved in simulated gastric fluid (SGF, 35 mM NaCl) at pH 2, preheated for 15 min at 37 °C, and subjected to an in vitro gastric digestion with porcine pepsin (EC 3.4.23.1, 3440 units/mg, Sigma) at an enzyme:substrate ratio of 1:20, w/w (172 units/mg). The reaction was stopped after 60 minutes with 1 M NaHCO3, giving a final protein concentration of 5 mg/mL and pH 7.
Duodenal digestion
The starting material were gastric digests adjusted to pH 7, by adding 1 M CaCl2, 0.25 M Bis-Tris pH 6.5 and a 0.125 M bile salt mixture containing equimolar quantities of sodium taurocholate (Sigma) and sodium glicodeoxycholate (Sigma). After preheating at 37 °C for 15 min, porcine pancreatic lipase (EC 232-619-9, Sigma), colipase (EC 259-490-1, Sigma) and a commercial pancreatic mix, Corolase® PP (AB Enzymes GmbH, Darmstadt, Germany) prepared in 35 mM NaCl adjusted to pH 7 were added to the duodenal mix. The final composition of the mixture was: 4.15 mg/mL OVA/OM, 6.15 mM of each bile salt, 20.3 mM Bis-Tris, 7.6 mM CaCl2; and the enzymes referred to the quantity of protein were: 28.9 units/mg lipase, Corolase® PP (enzyme:substrate ratio of 1:25, w/w) and colipase (enzyme:substrate ratio 1:895 w/w).
Digoxigenin labeling of egg white proteins
Proteins were incubated with digoxigenin-3-0-succinyl-ε-aminocaproic acid-N-hydroxy-succinimide ester (DIG, Roche Diagnostics, Indianapolis, IN) for 2h at room temperature under constant shaking. Free DIG was eluted with PBS through a Sephadex PD-10 Column (Amersham Biosciences).
RP-HPLC
Proteins and the corresponding hydrolysates at 4.15 mg/mL, were separated in a Hi-Pore RP-318 (250 × 4.6 mm internal diameter) column (Bio-Rad, Richmond, CA), in a Waters 600 HPLC (Waters Corporation, Milford, MA). The samples were eluted with 0.37% (v/v) trifluoroacetic acid in double-distilled water as solvent A and 0.27% (v/v) trifluoroacetic acid in acetonitrile as solvent B, at 1mL/min, and 220 nm. Data were processed with Empower 2 Software (Waters Corporation).
SDS-PAGE
Proteins were separated by SDS-PAGE (NuPAGE 4%–12%, 15 wells; Invitrogen, Carlsbad, CA) as per manufacturer's instructions; 6 µg protein were loaded per well. Proteins were transferred onto Immobilon-P PVDF membranes (Millipore, Bedford, MA) and probed with egg-allergic children’s sera.
Serum samples
A serum pool was made of equal parts of serum from 8 egg-allergic, heated egg-reactive children as documented with an oral challenge. Levels of specific-IgE antibodies were measured with UniCAP (Phadia US, Portage, MI); lower limit of detection 0.35, upper limit of detection 100 kUA/L. Pool’s specific-IgE levels were: egg white 12.8, OVA 14.0, and OM 13.9 kAU/L.
Immunoblotting
Immunoblots for detection of IgE binding were performed with native and heated OVA and OM. Membranes were incubated with an egg-allergic serum pool 1:10 dilution in PBS containing 0.05% Tween 20, 1% BSA, 10% normal goat serum for 60 minutes. PBS-rinsed membranes were incubated with 125I-goat anti-human IgE (DiaMed, Windham, ME) for 1 hour, washed, and exposed to Kodak BioMax MS Film (Carestream Health Inc, Rochester, NY) for 1 to 12 days. As a negative control, serum from a non-atopic adult was used.
Sensitization and oral challenge of mice
Five-week-old female C3H/HeJ mice (NCI, Fredrick, MD) were sensitized orally with 1mg of native OVA (N=15) or OM (N=24) in 0.2 M bicarbonate buffer plus 10 µg cholera toxin (CT; List Biologicals, Campbell, CA) per week for 6 weeks. On week 7, all sensitized mice were orally challenged with either native or heated OVA and OM. Five OVA-sensitized mice were challenged one week apart with both heated and unheated OVA. Total doses of 30 and 42 mg of OVA and OM respectively were administered in two increments, 15 minutes apart. If no symptoms were observed, the mice were then challenged with 100 µg of allergen intraperitoneally (i.p.). Animal studies were approved by the Institutional Animal Care and Use Committee of Mount Sinai School of Medicine.
Anaphylaxis assessment
Symptoms were scored as previously published.14 Rectal temperature (World Precision Instruments, Sarasota, FL) was measured as a further assessment of anaphylaxis severity.
Measurement of antigen-specific IgE
Mouse OVA- and OM-specific IgE was quantified by ELISA. A 96 well plate was coated overnight at 4°C with rat anti-mouse IgE antibody (BD Biosciences, San Jose, CA), then blocked with 10% normal mouse serum, 1% BSA in PBS 0.05 %Tween. After incubation with serum from sensitized mice, dioxigenin (DIG) conjugated OVA or OM were added. Finally, HRP-labeled Anti-DIG antibody (Fab fragments, Roche Diagnostics) was incubated with tetramethylbenzidine (BD Biosciences, San Jose, CA) as a substrate. Reaction was stopped with 1.2 M sulfuric acid and absorbance measured at 450nm.
In vitro cytokine responses
Splenocytes were plated at a density of 5 × 106 cells/mL in 24-well cell tissue culture plates (Nalge Nunc, Naperville, IL) with 50 µg/mL of OVA and OM proteins, respectively, or medium alone (RPMI 1640) in 10% FCS for 72 hours at 37°C in 5% CO2. Cytokines in culture supernatants were measured by ELISA (eBiosciences, San Diego, CA).
Mediator release assay
Rat Basophil Leukemia cells RBL-2H3 (kind gift from Dr. Stefan Vieths) were cultured in Eagle minimal essential medium with 10% FCS and the assay was performed as published.15 Briefly, RBLs (at 3 × 106 cells/mL) were incubated with serum at a final dilution of 1:60 at 37°C in 5% CO2 overnight in 96-well tissue-culture plates (BD Falcon; BD, Bedford, MA). Both OVA- and OM-sensitized mouse pool sera were used. Sensitized cells were stimulated with 100 µL per well of the dilutions of allergens. Rat anti-mouse IgE (Pharmingen) was used as a positive control for IgE-mediated degranulation; RBLs were lysed with 1% Triton X-100 (Sigma) for total release. β-N-acetylhexosaminidase release (NHR) upon stimulation with allergen was determined. Spontaneous release (stimulation with buffer only) was subtracted from the allergen-induced NHR at every dilution point. Values were expressed as percentage of the total release and the protein concentration that gave 50% of the maximum NHR (IC50) was determined.16, 17
Transcytosis studies
Caco-2 cells (Clone C2Bbe1, American Type Culture Collection, Rockville, MD) were seeded on 0.4 µm transwell filters (Cole-Parmer, Vernon Hills, IL) inserts at a concentration of 5×105 cells/mL. After one week transepithelial resistance was checked by ohmmeter (World Precision Instruments) and monolayers used if resistance was greater than 300 Ohms. Then FITC-labeled native and heated OVA and OM were added in triplicates to the apical side (0.5mL at 0.5 mg/mL) and incubated at 37°C / 5%CO2 for 20 hours. Samples from the basolateral side were collected after the incubation period.
OVA-specific T cell activation
CD4+ T cells were isolated from spleens and lymph nodes of DO11.10 mice by negative selection (StemCell, Vancouver, Canada) and labeled with CFSE (Invitrogen). 3 – 5 × 106 cells were injected into naïve Balb/c recipients intravenously. The next day, mice were fed with 25 mg of native or heated OVA in a total volume of 1 mL, given as two doses 1 hour apart. After 72 h, mice were euthanized and mesenteric lymph node (MLN) and Peyer’s patch (PP) cells were isolated. Cells were stained with antibodies against CD4 and the DO11 TCR (KJ1–26), and dead cells identified and excluded with a violet live/dead staining kit (Invitrogen). Cells were acquired on a LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software (Treestar, Ashland, OR).
Stripping and passive sensitization of basophils from non-allergic donors
PBMCs were isolated using Ficoll from egg-tolerant adult donors. For stripping of bound IgE18, the PBMC pellet was re-suspended in 3mL lactic acid solution (13.4 mM lactate, 140 mM NaCl, 5 mM KCl, pH 3.9) and incubated on ice for 5 minutes. 0.5% human serum albumin 7 mL (HSA, Sigma) in RPMI medium with glutamine (Fisher Scientific, Pittsburg, PA) was added and solution neutralized with 15 µL 12%Tris. After centrifuging and washing the pellet with HSA / RPMI, it was resuspended in 1/100 of the original blood volume. Stripped PBMCs were placed in a 96 U-bottom well plate and incubated for one hour at 37°C with 1:2 diluted serum (20 µL PBMCs + 20 µL diluted serum) from a pool of 3 egg-allergic children (OVA-specific and OM-specific IgE levels were 63.4 and 29.8 kUA/L, respectively).
Basophil activation assay
PBMCs sensitized as above were incubated with basophil stimulation buffer RPMI with IL-3 (25 µg/mL, R&D Systems, Minneapolis, MN)) as a negative control or with anti-IgE antibody (25 µg/mL, Bethyl Laboratories, Montgomery, TX) as a positive control. Samples (4 µg/mL) were incubated with native, heated and / or digested OVA and OM or with basolateral supernatants from transcytosis experiments at a 1:5 dilution. The reaction was stopped after 30 min with EDTA in cold PBS. Cells were stained for CD63, CD123, HLA-DR (BD Biosciences), CD203c (Beckman Coulter), and fixed with FACS Lysing Solution (BD Biosciences). Cells were acquired as described above.
Statistical analysis
Differences between mice groups were analyzed by independent t-test. Donors’ basophil stimulation percentages for heated and digested samples were compared by ratio paired t-test. P-values below 0.05 were considered significant. Statistical analysis was performed using GraphPad Prism software (La Jolla, CA).
RESULTS
Heated ovalbumin and ovomucoid do not induce anaphylaxis in an animal model
Mice were sensitized to native OVA (n=15) or OM (n=24) with cholera toxin. Upon oral challenge with the native proteins, OM-sensitized mice developed higher anaphylaxis scores and lower body temperature than OVA-sensitized mice, (P<.001) confirming that OM is a stronger allergen. Following oral challenge with native OM and native OVA, all mice presented symptoms of anaphylaxis. In contrast, mice challenged with the heated allergens did not develop anaphylaxis, as assessed by body temperature measurement (FIG. 1) and symptom score (median score of 4 and 2 for native OM and OVA respectively, compared to median score of 0 for both heated OM and OVA), p<0.01. However, when mice were subsequently systemically (i.p.) challenged with the heated allergens, the majority of them (3/5 for heated OVA and 6/7 for heated OM) showed mild symptoms and a drop in body temperature. Serum specific-IgE levels were similar in all mice groups (FIG. 1B), as were OVA and OM-induced IL-13 and IFN-γ production from spleen cells (Supplemental FIG 1).
Figure 1.
A) Mice body temperature upon oral challenge with native (N) or heated (H) OVA or OM. B) Serum OVA-specific and OM-specific IgE levels of the different mice groups before challenge. OD values correspond to 1/100 serum dilution. * denotes statistically significant differences between native and heated challenged groups, P<.001.
Heat treatment does not completely destroy ovalbumin and ovomucoid IgE-binding epitopes
Native and heated OVA and OM were immunolabeled with a serum pool from extensively heated egg-reactive children to assess the binding of serum IgE. There was no appreciable decrease in binding to heated OM and some decrease in binding to heated OVA. Both OVA and OM showed strong binding regardless of heat treatment (FIG. 2), suggesting the persistence of linear epitopes recognized by IgE.
Figure 2.
Immunoblotting of unheated or heated (H-) OM and OVA using pooled sera from heated egg-reactive children.
Heat treatment makes ovalbumin more susceptible to digestion whereas it does not affect ovomucoid
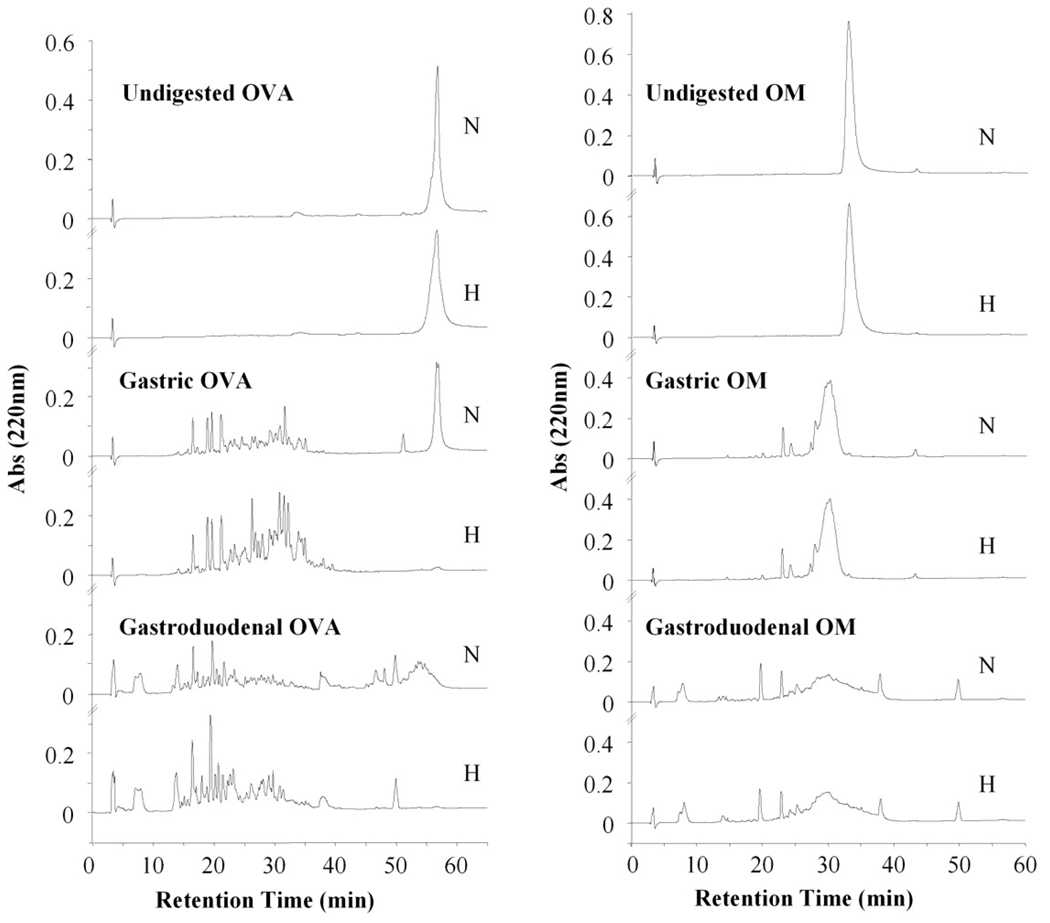
We used an in vitro digestion model to evaluate the effect of heating on the final outcome of OVA and OM. Samples were collected after 60 minutes (gastric phase) and 120 min (duodenal phase) and analyzed by RP-HPLC (FIG. 3). Native OVA was poorly hydrolyzed by pepsin. However, heated OVA became completely hydrolyzed after 60 minutes. In contrast, heat treatment did not alter OM susceptibility to either pepsin or duodenal enzymes, producing similar digestion profiles where OM was quickly digested giving raise to fragments of lower molecular weight.
Figure 3.
RP-HPLC chromatograms corresponding to native (N) and heated (H) OVA and OM undigested and subjected to gastric and gastroduodenal digestion.
Heat treatment and digestion significantly reduce the capacity of ovalbumin and ovomucoid to trigger basophil activation and degranulation in the RBL-based mediator release assay
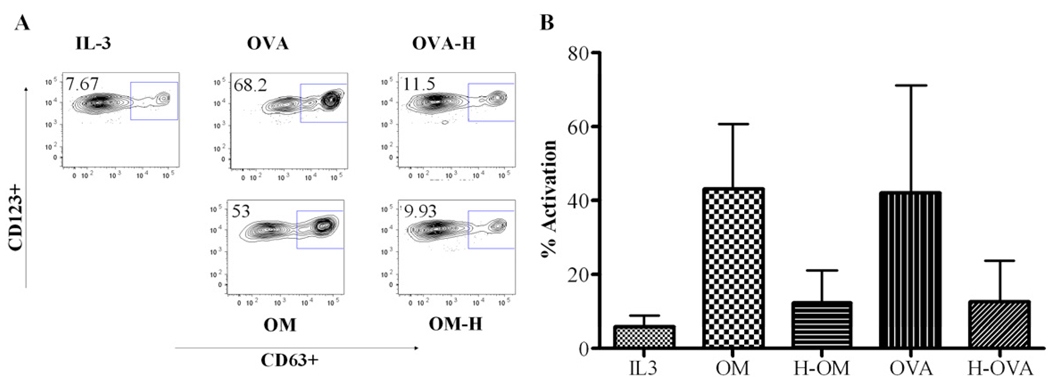
We aimed to determine whether heated proteins or the peptides generated following digestion were able to activate basophils comparably to native proteins. We isolated basophils from 3 egg-tolerant adult donors and passively sensitized them with pooled allergic children’s sera. Passively sensitized basophils were stimulated with either native or heated OVA and OM, as well as the corresponding gastroduodenally digested proteins. The population of activated basophils analyzed by flow cytometry is shown in FIG 4. Challenge of basophils with native OVA or OM induced basophil activation as measured by upregulation of CD63. OVA exhibited a significant reduction in basophil activation only when it was both heated and digested. OM stimulation was reduced after digestion regardless of heating, while heating alone did not reduce basophil activation.
Figure 4.
Percentage of activated basophils (CD63+, CD123+, CD203c+, HLA-DRlow) upon stimulation with native, heated (H) and/or digested (Dig) OVA or OM. A) Detailed activated basophil population from one donor. B) Activation percentages from 3 donors.
We verified these results using RBL cells passively sensitized with serum from OM-sensitized mice (FIG. 5). Digested OM gave reduced NHR compared to undigested OM, regardless of heating status. The IC50 of OM was approximately 100-fold higher upon digestion; 10 µg/mL for digested OM versus 0.1 µg/mL for un-digested OM. These data suggest that heating does not affect OM basophil activation capacity and mediator release from RBLs but proteolysis by gastroduodenal enzymes greatly decreases it.
Figure 5.
NHR of RBL cells passively sensitized with ovomucoid (OM)-reactive mice’s serum and stimulated with different concentrations of undigested/digested native and heated OM. NHR release by negative controls (digestive enzymes, buffer) was not significantly different from spontaneous release, which was subtracted in the final graph. Positive control, anti-IgE stimulation, was 60%.
Heating prevents transcytosis of intact allergen across intestinal epithelial cells and Peyer’s patches
Food allergens must cross the intestinal epithelial barrier before activating allergic effector cells. To model this, native and heated OVA and OM were added to the apical side of a polarized monolayer of Caco-2 human intestinal epithelial cells grown in transwells. Supernatants were collected from the basolateral compartment after an overnight culture, and we then evaluated the basophil activation by the transcytosed proteins (FIG. 6). Native OVA and OM were readily transported across Caco-2 monolayers and triggered significant basophil activation. In contrast, heated OVA and heated OM triggered markedly decreased basophil activation.
Figure 6.
Percentage of activated basophils (CD63+, CD123+, CD203c+, HLA-DRlow) upon stimulation with native or heated (H) OVA or OM transcytosed by Caco-2 cells. A) Detailed activated basophil population from one experiment. B) Mean activation percentages from 3 replicates. Error bars represent SD.
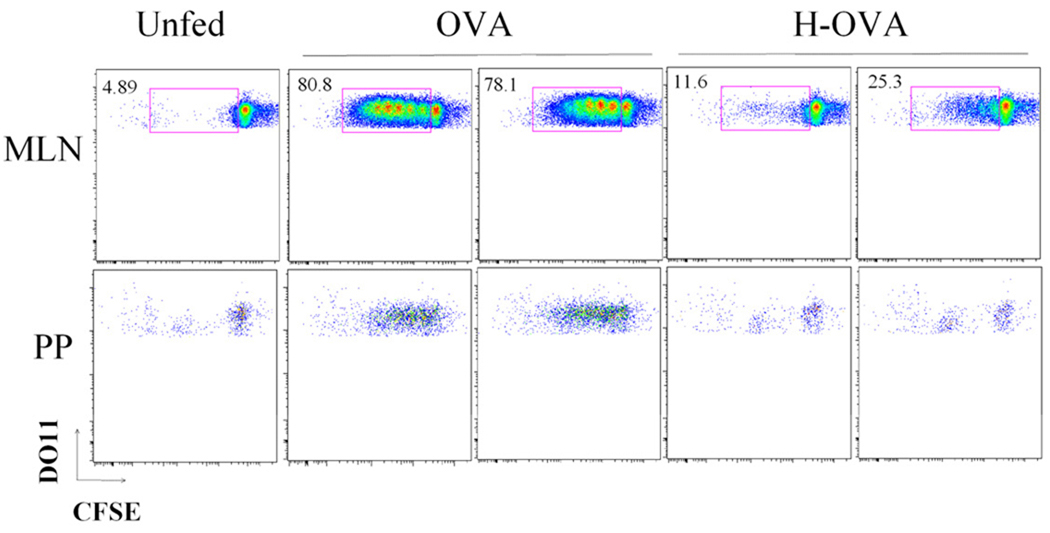
To assess the impact of heating on gastrointestinal uptake of antigen in vivo, we assessed antigen-specific T cell activation in the gastrointestinal-associated lymphoid tissue using an adoptive transfer model. CFSE-labeled OVA-specific DO11.10 T cells were injected into naive Balb/c mice prior to oral feeding with native or heated OVA. Proliferation of DO11.10 T cells was assessed in the MLN and (PP) (FIG. 7). Mice fed with native OVA presented extensive proliferation in both the MLN and the PP compared to unfed mice. In contrast, mice fed with heated OVA had minimal T cell proliferation in either the MLN or the PP. Thus, in addition to facilitating digestion of OVA, heating of ovalbumin and ovomucoid abrogates their intestinal absorption in an intact form capable of triggering effector cells and T cells.
Figure 7.
OVA-specific T cell activation in the gastrointestinal lymphoid tissue. CFSE-labeled DO11.10 T cells were transferred to naïve Balb/c mice prior to gavage feeding with 25 mg of OVA or heated OVA (H-OVA). After 72 h, proliferation of DO11.10 T cells was assessed by flow cytometry using cells isolated from mesenteric lymph node (MLN) or Peyer’s patch (PP). Data are shown from 2 individual OVA-fed or H-OVA-fed mice.
DISCUSSION
The in vivo murine model of egg-induced anaphylaxis used here reproduces the observation that the majority (over 70%) of egg-allergic children can tolerate extensively-heated egg.9, 10, 12, 19 Mice were completely tolerant to heated egg delivered by the oral, but not the systemic, route. This suggests that heating may influence the handling of egg allergens in the gastrointestinal tract in addition to altering the conformation of IgE-binding epitopes. We showed that heating did not completely destroy epitopes recognized by IgE antibodies from egg-allergic children. This was true for IgE-binding, and for activation of basophils passively sensitized with serum from egg-allergic children or mice. Therefore, our goal was to determine why ingestion of heated OVA and OM did not cause symptoms despite the persistence of IgE- binding epitopes.
We confirmed that OVA changes its structure upon heating and forms high molecular weight aggregates (observed by inability to enter SDS-PAGE gels, supplemental FIG 2). In contrast, OM remains largely unaffected in the gel after heating, although there is strong evidence of an irreversibly heat-denatured form of OM with newly appearing specific IgE epitopes.20
In order for food allergens to trigger anaphylaxis, they must escape digestion and be absorbed in a sufficiently intact or immunologically active form across the epithelial barrier.21–23 We examined how heating of egg white proteins altered each of these factors. In globular proteins like OVA, heating is known to induce a partially folded molten globule structure responsible for their gelling and emulsifying properties.24 This structure is also favored at the low pH of the gastric phase25 and it is differentiated from the native state by the absence of close packing throughout the molecule.26 It has been reported that OVA high resistance to pepsin27 is lost when heated28. Heat treatment would therefore facilitate the access of gastrointestinal proteases to potential cleavage sites resulting in a complete degradation of OVA. In contrast, OM structure comprising 9 disulfide bonds and 25% of carbohydrate content is likely responsible for its high thermal stability and limited denaturation.29 This could explain the unchanged enzymatic susceptibility of OM observed under gastrointestinal conditions.
Resistance to digestion is a common characteristic shared by many allergens. 2S albumins from mustard30, Brazil nuts31, or sesame seeds32; lipid transfer proteins from grape33 or cherry34 are only some examples of highly stable allergens. In addition, there are allergens that despite being rapidly digested, give raise to stable fragments which retain allergenicity, as in the case of Ara h 135–38, the major peanut allergen.. Impaired gastrointestinal function entails an increased risk of systemic absorption of food antigens. Hindered degradation of codfish allergens occurring at increased gastric pH conditions results in the maintenance of biological activity even after two hours of digestion, as evidenced by their histamine releasing capacity.7 Incomplete digestion of kiwifruit at increased gastric pH has also been reported39 and the use of antacid medication has been related to sensitization to dietary proteins due to incomplete enzymatic degradation.40 Hence, food processing such as heat treatment, which facilitates OVA degradation within the gastrointestinal tract, would have a beneficial effect on preventing adverse reactions in allergic individuals.
We next assessed the effect of heating on transcytosis of intact egg white allergens across human intestinal epithelium. Heating of OVA and OM completely abrogated the delivery of immunologically intact forms of allergen across the epithelial monolayer. Heat treatment of milk proteins has been shown to cause aggregation of the whey proteins, redirecting antigen uptake away from absorptive enterocytes to Peyer’s patches (PP). The immediate consequence of this pathway switch was the abrogation of anaphylactic response in mice, probably because aggregated antigens transported into PP bypass lamina propria mast cells or fail to reach the systemic circulation.14 Here, however, the lack of proliferating OVA-specific CD4 T cells in the MLN or PP argues that OVA is not getting absorbed into the gut mucosa, and unlike the heated milk proteins does not seem to be getting taken up into the PP either. The aggregation of OVA was likely responsible for this blockade of absorption, whereas OM does not form aggregates when heated.11 Moreover, gastrointestinal digestibility of OM was unaffected by heat treatment. However, the finding that heated OM was not transported across epithelial cells in a form capable of triggering basophils could indicate enhanced intracellular degradation as it crosses the monolayer of cells. In ex vivo studies of animal and human intestinal mucosa, only small amounts of intact food antigens were transcytosed (~0.1% of luminal concentration) by intestinal epithelial cells.41 Large proteins taken up by intestinal epithelial cells were released in their basal pole either as immunogenic peptides (~40%) or fully degraded into aminoacids (~50%) with only a minor fraction crossing the epithelium in their intact form.42 We have shown that OM degradation by enzymes diminished its basophil activation capacity to a great extent, yet we did not address the additional impact of intracellular enzymes in enterocytes. In the case of OM, we hypothesize that heating could render it more susceptible to enterocytic intracellular enzymes generating non-allergenic peptides.
Another factor that might affect both the integrity of IgE-binding epitopes and gastrointestinal digestibility and absorption is interaction between heated egg white proteins and complex food matrix.6, 43 A study by Kato et al demonstrated a marked decrease in the solubility of ovomucoid when egg white was mixed with wheat flour and wheat gluten and then heated at 180°C for 10 minutes, mimicking the process of bread-making.44 Immunoblotting suggested that ovomucoid polymerized and formed high-molecular weight complexes with gluten leading to aggregation and insolubilization of ovomucoid. This phenomenon might further decrease the accessibility to digestion and slow the absorption of intact allergenic particles, relevant to human studies. 12
The use of heated proteins arises as an attractive strategy for oral immunotherapy. Our data confirm the relative safety of heated egg white proteins. However, it is important to remark that despite their diminished capacity to trigger effector cells, alteration of the allergen structure may either enhance sensitizing potential or abrogate tolerogenic capacity. Heat-treatment of milk proteins was shown to promote sensitization in C3H/HeJ mice14. Boiling of egg white proteins abrogated the suppression of Th2 responses in BALB/c mice receiving the antigens prior to sensitization. 45 Furthermore, glycated OVA as a result of the Maillard reaction during thermal processing was shown to induce enhanced activation of OVA-specific CD4+ T cells on co-culture with myeloid dendritic cells compared with native OVA and OVA processed without glucose. 46
Our findings emphasize that food processing can fundamentally alter the ability of food protein allergens to trigger reactions not solely by interfering with their IgE binding, but by altering their degradation and absorption within the gastrointestinal tract.
Supplementary Material
ACKNOWLEDGMENTS
We thank Wayne Shreffler, MD, PhD, from Mass General Hospital, Boston (MA), for sharing the methodology of basophil stripping. We thank Wei Wang for technical assistance with the DO11.10 transfer studies. GM is grateful to the Department of Pediatrics at Mount Sinai School of Medicine for their assistance and contribution to this work.
Funding
G. Martos was supported by the Spanish Research Council (CSIC) through the JAE program and from the Ministry of Science and Innovation through the project AGL2008-01740.
R. Bencharitiwong was supported in part by Nutricia Foundation.
M.C. Berin was supported by NIH NIAID AI044236
A. Nowak-Węgrzyn was supported in part by NIH NIAID AI 059318
The project was supported in part by Grant Number CTSA ULI RR 029887 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Abbreviations
- Bis-Tris
Bis(2-hydroxyethyl)-amino-tris(hydroxymethyl)-methane
- MLN
Mesenteric lymph nodes
- NHR
β-N-acetylhexosaminidase release
- OVA
Ovalbumin
- OM
Ovomucoid
- PBMC
Peripheral blood mononuclear cells
- PP
Peyer’s patches
- RP-HPLC
Reverse phase - High performance liquid chromatography
- SDS-PAGE
Sodium dodecyl sulfate – polyacrylamide gel electrophoresis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
All authors report no conflict of interest.
REFERENCES
- 1.Peyron S, Mouecoucou J, Fremont S, Sanchez C, Gontard N. Effects of heat treatment and pectin addition on beta-lactoglobulin allergenicity. J Agric Food Chem. 2006;54:5643–5650. doi: 10.1021/jf053178j. [DOI] [PubMed] [Google Scholar]
- 2.Poulsen OM, Hau J, Kollerup J. Effect of homogenization and pasteurization on the allergenicity of bovine milk analysed by a murine anaphylactic shock model. Clin Allergy. 1987;17:449–458. doi: 10.1111/j.1365-2222.1987.tb02039.x. [DOI] [PubMed] [Google Scholar]
- 3.Bernhisel-Broadbent J, Strause D, Sampson HA. Fish hypersensitivity. II: Clinical relevance of altered fish allergenicity caused by various preparation methods. J Allergy Clin Immunol. 1992;90:622–629. doi: 10.1016/0091-6749(92)90135-o. [DOI] [PubMed] [Google Scholar]
- 4.Fiocchi A, Restani P, Riva E, Restelli AR, Biasucci G, Galli CL, et al. Meat allergy: II--Effects of food processing and enzymatic digestion on the allergenicity of bovine and ovine meats. J Am Coll Nutr. 1995;14:245–250. doi: 10.1080/07315724.1995.10718503. [DOI] [PubMed] [Google Scholar]
- 5.Restani P, Fiocchi A, Restelli AR, Velona T, Beretta B, Giovannini M, et al. Effect of technological treatments on digestibility and allergenicity of meat-based baby foods. J Am Coll Nutr. 1997;16:376–382. doi: 10.1080/07315724.1997.10718700. [DOI] [PubMed] [Google Scholar]
- 6.Thomas K, Herouet-Guicheney C, Ladics G, Bannon G, Cockburn A, Crevel R, et al. Evaluating the effect of food processing on the potential human allergenicity of novel proteins: international workshop report. Food Chem Toxicol. 2007;45:1116–1122. doi: 10.1016/j.fct.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Untersmayr E, Vestergaard H, Malling HJ, Jensen LB, Platzer MH, Boltz-Nitulescu G, et al. Incomplete digestion of codfish represents a risk factor for anaphylaxis in patients with allergy. J Allergy Clin Immunol. 2007;119:711–717. doi: 10.1016/j.jaci.2006.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breiteneder H, Mills EN. Molecular properties of food allergens. J Allergy Clin Immunol. 2005;115:14–23. doi: 10.1016/j.jaci.2004.10.022. quiz 24. [DOI] [PubMed] [Google Scholar]
- 9.Des Roches A, Nguyen M, Paradis L, Primeau MN, Singer S. Tolerance to cooked egg in an egg allergic population. Allergy. 2006;61:900–901. doi: 10.1111/j.1398-9995.2006.01134.x. [DOI] [PubMed] [Google Scholar]
- 10.Konstantinou GN, Giavi S, Kalobatsou A, Vassilopoulou E, Douladiris N, Saxoni-Papageorgiou P, et al. Consumption of heat-treated egg by children allergic or sensitized to egg can affect the natural course of egg allergy: hypothesis-generating observations. J Allergy Clin Immunol. 2008;122:414–415. doi: 10.1016/j.jaci.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 11.Urisu A, Ando H, Morita Y, Wada E, Yasaki T, Yamada K, et al. Allergenic activity of heated and ovomucoid-depleted egg white. J Allergy Clin Immunol. 1997;100:171–176. doi: 10.1016/s0091-6749(97)70220-3. [DOI] [PubMed] [Google Scholar]
- 12.Lemon-Mule H, Sampson HA, Sicherer SH, Shreffler WG, Noone S, Nowak-Wegrzyn A. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol. 2008;122:977–983. doi: 10.1016/j.jaci.2008.09.007. e1. [DOI] [PubMed] [Google Scholar]
- 13.Cooke SK, Sampson HA. Allergenic properties of ovomucoid in man. J Immunol. 1997;159:2026–2032. [PubMed] [Google Scholar]
- 14.Roth-Walter F, Berin MC, Arnaboldi P, Escalante CR, Dahan S, Rauch J, et al. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer's patches. Allergy. 2008;63:882–890. doi: 10.1111/j.1398-9995.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 15.Nowak-Wegrzyn AH, Bencharitiwong R, Schwarz J, David G, Eggleston P, Gergen PJ, et al. Mediator release assay for assessment of biological potency of German cockroach allergen extracts. J Allergy Clin Immunol. 2009;123:949–955. doi: 10.1016/j.jaci.2009.01.070. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann A, Kaul S, Fotisch K, Danz N, Luttkopf D, Hatahet L, et al. Mediator release assays based on human and murine IgE: potential and limitations in allergen standardization. Arb Paul Ehrlich Inst Bundesamt Sera Impfstoffe Frankf A M. 2003:76–85. discussion 85-6. [PubMed] [Google Scholar]
- 17.Vieths S, Jankiewicz A, Aulepp H, Haustein D. Allergy to heated and processed foods. Arb Paul Ehrlich Inst Bundesamt Sera Impfstoffe Frankf A M. 1997:250–262. [PubMed] [Google Scholar]
- 18.Pruzansky JJ, Grammer LC, Patterson R, Roberts M. Dissociation of IgE from receptors on human basophils. I. Enhanced passive sensitization for histamine release. J Immunol. 1983;131:1949–1953. [PubMed] [Google Scholar]
- 19.Ando H, Moverare R, Kondo Y, Tsuge I, Tanaka A, Borres MP, et al. Utility of ovomucoid-specific IgE concentrations in predicting symptomatic egg allergy. J Allergy Clin Immunol. 2008;122:583–588. doi: 10.1016/j.jaci.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Hirose J, Kitabatake N, Kimura A, Narita H. Recognition of native and/or thermally induced denatured forms of the major food allergen, ovomucoid, by human IgE and mouse monoclonal IgG antibodies. Biosci Biotechnol Biochem. 2004;68:2490–2497. doi: 10.1271/bbb.68.2490. [DOI] [PubMed] [Google Scholar]
- 21.Taylor SL, Hefle SL. Will genetically modified foods be allergenic? J Allergy Clin Immunol. 2001;107:765–771. doi: 10.1067/mai.2001.114241. [DOI] [PubMed] [Google Scholar]
- 22.Metcalfe DD, Astwood JD, Townsend R, Sampson HA, Taylor SL, Fuchs RL. Assessment of the allergenic potential of foods derived from genetically engineered crop plants. Crit Rev Food Sci Nutr. 1996;36(Suppl):S165–S186. doi: 10.1080/10408399609527763. [DOI] [PubMed] [Google Scholar]
- 23.Taylor SL. Protein allergenicity assessment of foods produced through agricultural biotechnology. Annu Rev Pharmacol Toxicol. 2002;42:99–112. doi: 10.1146/annurev.pharmtox.42.082401.130208. [DOI] [PubMed] [Google Scholar]
- 24.Hirose M. Molten globule state of food proteins. Trends Food Sci. Technol. 1993;4:48–51. [Google Scholar]
- 25.Koseki T, Kitabatake N, Doi E. Conformational changes in ovalbumin at acid pH. J Biochem. 1988;103:425–430. doi: 10.1093/oxfordjournals.jbchem.a122286. [DOI] [PubMed] [Google Scholar]
- 26.Kuwajima K. The molten globule state as a clue for understanding the folding and cooperativity of globular-protein structure. Proteins: Struc. Funct. Genet. 1989;6:87–103. doi: 10.1002/prot.340060202. [DOI] [PubMed] [Google Scholar]
- 27.Martos G, Contreras P, Molina E, Lopez-Fandino R. Egg white ovalbumin digestion mimicking physiological conditions. J Agric Food Chem. 2010;58:5640–5648. doi: 10.1021/jf904538w. [DOI] [PubMed] [Google Scholar]
- 28.Takagi K, Teshima R, Okunuki H, Sawada J. Comparative study of in vitro digestibility of food proteins and effect of preheating on the digestion. Biol Pharm Bull. 2003;26:969–973. doi: 10.1248/bpb.26.969. [DOI] [PubMed] [Google Scholar]
- 29.Djurtoft R, Pedersen HS, Aabin B, Barkholt V. Studies of food allergens: soybean and egg proteins. Adv Exp Med Biol. 1991;289:281–293. doi: 10.1007/978-1-4899-2626-5_21. [DOI] [PubMed] [Google Scholar]
- 30.Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nat Biotechnol. 1996;14:1269–1273. doi: 10.1038/nbt1096-1269. [DOI] [PubMed] [Google Scholar]
- 31.Moreno FJ, Mellon FA, Wickham MS, Bottrill AR, Mills EN. Stability of the major allergen Brazil nut 2S albumin (Ber e 1) to physiologically relevant in vitro gastrointestinal digestion. FEBS J. 2005;272:341–352. doi: 10.1111/j.1742-4658.2004.04472.x. [DOI] [PubMed] [Google Scholar]
- 32.Moreno FJ, Maldonado BM, Wellner N, Mills EN. Thermostability and in vitro digestibility of a purified major allergen 2S albumin (Ses i 1) from white sesame seeds (Sesamum indicum L.) Biochim Biophys Acta. 2005;1752:142–153. doi: 10.1016/j.bbapap.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Vassilopoulou E, Rigby N, Moreno FJ, Zuidmeer L, Akkerdaas J, Tassios I, et al. Effect of in vitro gastric and duodenal digestion on the allergenicity of grape lipid transfer protein. J Allergy Clin Immunol. 2006;118:473–480. doi: 10.1016/j.jaci.2006.04.057. [DOI] [PubMed] [Google Scholar]
- 34.Scheurer S, Lauer I, Foetisch K, San Miguel Moncin M, Retzek M, Hartz C, et al. Strong allergenicity of Pru av 3, the lipid transfer protein from cherry, is related to high stability against thermal processing and digestion. J Allergy Clin Immunol. 2004;114:900–907. doi: 10.1016/j.jaci.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Maleki SJ, Kopper RA, Shin DS, Park CW, Compadre CM, Sampson H, et al. Structure of the major peanut allergen Ara h 1 may protect IgE-binding epitopes from degradation. J Immunol. 2000;164:5844–5849. doi: 10.4049/jimmunol.164.11.5844. [DOI] [PubMed] [Google Scholar]
- 36.Eiwegger T, Rigby N, Mondoulet L, Bernard H, Krauth MT, Boehm A, et al. Gastro-duodenal digestion products of the major peanut allergen Ara h 1 retain an allergenic potential. Clin Exp Allergy. 2006;36:1281–1288. doi: 10.1111/j.1365-2222.2006.02565.x. [DOI] [PubMed] [Google Scholar]
- 37.van Boxtel EL, Koppelman SJ, van den Broek LA, Gruppen H. Determination of pepsin-susceptible and pepsin-resistant epitopes in native and heat-treated peanut allergen Ara h 1. J Agric Food Chem. 2008;56:2223–2230. doi: 10.1021/jf072907n. [DOI] [PubMed] [Google Scholar]
- 38.Koppelman SJ, Hefle SL, Taylor SL, de Jong GA. Digestion of peanut allergens Ara h 1, Ara h 2, Ara h 3, and Ara h 6: a comparative in vitro study and partial characterization of digestion-resistant peptides. Mol Nutr Food Res. 2010;54:1711–1721. doi: 10.1002/mnfr.201000011. [DOI] [PubMed] [Google Scholar]
- 39.Lucas JS, Cochrane SA, Warner JO, Hourihane JO. The effect of digestion and pH on the allergenicity of kiwifruit proteins. Pediatr Allergy Immunol. 2008;19:392–398. doi: 10.1111/j.1399-3038.2007.00678.x. [DOI] [PubMed] [Google Scholar]
- 40.Untersmayr E, Scholl I, Swoboda I, Beil WJ, Forster-Waldl E, Walter F, et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol. 2003;112:616–623. doi: 10.1016/s0091-6749(03)01719-6. [DOI] [PubMed] [Google Scholar]
- 41.Heyman MDJ, Kaiserlian D. In: Antigen handling by intestinal epithelial cells in antigen presentation by intestinal epithelial cells. Company RGL, editor. 1996. pp. 1–16. [Google Scholar]
- 42.Heyman M, Ducroc R, Desjeux JF, Morgat JL. Horseradish peroxidase transport across adult rabbit jejunum in vitro. Am J Physiol. 1982;242:G558–G564. doi: 10.1152/ajpgi.1982.242.6.G558. [DOI] [PubMed] [Google Scholar]
- 43.Teuber SS. Hypothesis: the protein body effect and other aspects of food matrix effects. Ann N Y Acad Sci. 2002;964:111–116. doi: 10.1111/j.1749-6632.2002.tb04136.x. [DOI] [PubMed] [Google Scholar]
- 44.Kato Y, Watanabe H, Matsuda T. Ovomucoid rendered insoluble by heating with wheat gluten but not with milk casein. Biosci Biotechnol Biochem. 2000;64:198–201. doi: 10.1271/bbb.64.198. [DOI] [PubMed] [Google Scholar]
- 45.Peng HJ, Chang ZN, Tsai LC, Su SN, Shen HD, Chang CH. Heat denaturation of egg-white proteins abrogates the induction of oral tolerance of specific Th2 immune responses in mice. Scand J Immunol. 1998;48:491–496. doi: 10.1046/j.1365-3083.1998.00432.x. [DOI] [PubMed] [Google Scholar]
- 46.Glycation of a food allergen by the Maillard reaction enhances its T-cell immunogenicity: role of macrophage scavenger receptor class A type I and II Ilchmann A, Burgdorf S, Scheuer S, Waibler Z, Nahai R, EWellner A, et al. J Allergy Clin Immunol. 2010;125:175–183. doi: 10.1016/j.jaci.2009.08.013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.