Summary
Background
Transient isolated brainstem symptoms (eg, isolated vertigo, dysarthria, diplopia) are not consistently classified as transient ischaemic attacks (TIAs) and data for prognosis are limited. If some of these transient neurological attacks (TNAs) are due to vertebrobasilar ischaemia, then they should be common during the days and weeks preceding posterior circulation strokes. We aimed to assess the frequency of TNAs before vertebrobasilar ischaemic stroke.
Methods
We studied all potential ischaemic events during the 90 days preceding an ischaemic stroke in patients ascertained within a prospective, population-based incidence study in Oxfordshire, UK (Oxford Vascular Study; 2002–2010) and compared rates of TNA preceding vertebrobasilar stroke versus carotid stroke. We classified the brainstem symptoms isolated vertigo, vertigo with non-focal symptoms, isolated double vision, transient generalised weakness, and binocular visual disturbance as TNAs in the vertebrobasilar territory; atypical amaurosis fugax and limb-shaking as TNAs in the carotid territory; and isolated slurred speech, migraine variants, transient confusion, and hemisensory tingling symptoms as TNAs in uncertain territory.
Findings
Of the 1141 patients with ischaemic stroke, vascular territory was categorisable in 1034 (91%) cases, with 275 vertebrobasilar strokes and 759 carotid strokes. Isolated brainstem TNAs were more frequent before a vertebrobasilar stroke (45 of 275 events) than before a carotid stroke (10 of 759; OR 14·7, 95% CI 7·3–29·5, p<0·0001), particularly during the preceding 2 days (22 of 252 before a vertebrobasilar stroke vs two of 751 before a carotid stroke, OR 35·8, 8·4–153·5, p<0·0001). Of all 59 TNAs preceding (median 4 days, IQR 1–30) vertebrobasilar stroke, only five (8%) fulfilled the National Institute of Neurological Disorders and Stroke (NINDS) criteria for TIA. The other 54 cases were isolated vertigo (n=23), non-NINDS binocular visual disturbance (n=9), vertigo with other non-focal symptoms (n=10), isolated slurred speech, hemisensory tingling, or diplopia (n=8), and non-focal events (n=4). Only 10 (22%) of the 45 patients with isolated brainstem TNAs sought medical attention before the stroke and a vascular cause was suspected by their physician in only one of these cases.
Interpretation
In patients with definite vertebrobasilar stroke, preceding transient isolated brainstem symptoms are common, but most symptoms do not satisfy traditional definitions of TIA. More studies of the prognosis of transient isolated brainstem symptoms are required.
Funding
Wellcome Trust, UK Medical Research Council, Dunhill Medical Trust, Stroke Association, National Institute for Health Research (NIHR), Thames Valley Primary Care Research Partnership, and the NIHR Biomedical Research Centre, Oxford.
Introduction
The diagnosis of transient ischaemic attack (TIA) is often based purely on the description of symptoms, particularly in patients with vertebrobasilar events in whom positive diffusion weighted imaging is less common1, 2 and inter-observer agreement in diagnosis by experienced neurologists is only moderate.3, 4 Rapid access to secondary prevention after TIA reduces the risk of early recurrent stroke5, 6 and is recommended in clinical guidelines.7, 8, 9, 10, 11, 12, 13 However, a false-positive diagnosis of TIA can adversely affect patient confidence, employment, lifestyle, and insurance costs and can result in unnecessary preventative treatments. Nevertheless, missing the diagnosis means that the opportunity to prevent a disabling stroke is lost. Indeed, evidence exists that apparently non-TIA transient neurological attacks (TNAs) are associated with an increased risk of stroke, coronary events, and dementia.14, 15, 16
The inconsistency in clinical diagnosis of vertebrobasilar TIA is due mainly to uncertainty about the nature of several transient isolated brainstem symptoms. Most clinical guidelines do not specify which of these symptoms should be deemed as TIA,7, 8, 9, 10, 11, 12, 13 although some cite the National Institute of Neurological Disorders and Stroke (NINDS) criteria.17, 18 The NINDS criteria state that most transient isolated brainstem symptoms, such as vertigo, dysarthria, dizziness or wooziness, focal symptoms suggestive of migraine, confusion, and amnesia, should not be defined as TIAs,19 although many clinicians take a more flexible view in routine practice. Previous studies of the generality of TIAs or TNAs have not looked specifically at isolated transient brainstem symptoms and there have been no prospective unselected population-based studies of incidence and prognosis on which to base clinical decisions. Indeed, such studies would be difficult because many (probably most) patients with transient neurological symptoms do not report them to medical attention. However, one useful epidemiological approach, which was helpful in confirming the high risk of stroke after clinically definite TIA,20 is to determine the frequency of events during the days and weeks immediately preceding a stroke. This temporal relation can be estimated fairly reliably because most patients do present to medical attention after a stroke,20 and it also focuses on the most clinically important subset of TNAs—ie, those that are associated with impending stroke, irrespective of whether the individual sought medical attention after the warning symptoms. We therefore aimed to assess the frequency of isolated brainstem TNAs preceding vertebrobasilar stroke and to compare the frequency with the background rate of such symptoms in patients with stroke in the carotid territory.
Methods
Study design and patients
The Oxford Vascular Study (OXVASC) is a prospective, population-based study of all stroke and TIA in 91 105 individuals of all ages registered with 63 primary care physicians in Oxfordshire, UK. We restricted analysis to patients with a first stroke presenting to medical attention from April 1, 2002, to March 31, 2010.
The study methods have been described elsewhere.21, 22 Briefly, various overlapping methods of hot and cold pursuit were used to achieve near complete ascertainment of all individuals presenting to medical attention with TIA or stroke.21, 22 These included:
(1) An open-access TIA service available 7 days per week to which participating general practitioners and the local accident and emergency department send all individuals with suspected TIA or minor stroke whom they would not usually admit to hospital, with emergency provision at weekends supplementing a weekday clinic; (2) daily searches of admissions to the stroke unit, general medical, neurology, and other relevant wards; (3) daily searches of the local accident and emergency department and eye hospital attendance register; (4) monthly computerised searches of general practitioner diagnostic coding and hospital discharge codes; (5) monthly searches of all cranial and carotid imaging studies done in local hospitals; and (6) monthly reviews of all death certificates and coroners' reports.
OXVASC was approved by our local ethics committee (Oxfordshire Ethics Committee A: 05/Q1604/70). All patients provided written informed consent and were seen by study physicians as soon as possible after their initial presentation. Relatives provided written assent and clinical details for those unable to provide written consent.
Procedures
The study senior neurologist (PMR) reviewed all cases and classified them as stroke or other disorder using standard definitions.21 Severity of event was assessed with the National Institutes of Health stroke scale (NIHSS)23 and clinical features. We classified events as minor stroke if there was a focal neurological deficit lasting more than 24 h and an NIHSS score of 5 or lower5 at time of assessment by a study physician. We classified vascular territory (carotid or vertebrobasilar) of stroke on the basis of brain imaging and by use of the NINDS criteria applied to clinical features of the event.19 We excluded strokes of uncertain vascular territory. Vascular assessment was done with CT, MRI, or catheter angiography as clinically appropriate.
A detailed history about preceding events was obtained from every patient with a standardised questionnaire.21, 22 This questionnaire included date of symptom onset, duration and type of symptoms, baseline characteristics, and time of first seeking medical attention. All patients were asked about TIA or TNA symptoms during the previous 90 days. Additionally, we searched hospital notes and family doctors' records for TIA or TNA symptoms in the previous 90 days for all patients. We prospectively classified TNAs using a predefined, diagnostic classification system. We classified isolated vertigo, vertigo with non-focal symptoms, isolated double vision, transient generalised weakness, and binocular visual disturbance as TNAs in the vertebrobasilar territory. Binocular visual disturbance includes patients with partial or complete visual field loss that is not sufficiently clear cut to be a definite TIA; this category would not include a homonymous hemianopia or quadrantinopia but does include patients with lone bilateral blindness, lone bilateral visual blurring, visual scrolling, or other unusual visual perceptions that are not clearly related to migraine or other non-vascular cause. These cases differ from migraine in that so-called positive symptoms (such as fortification spectra or photopsia) will not be prominent. Atypical amaurosis fugax (atypical monocular visual disturbance, such as isolated visual blurring, a sensation of looking through water) and limb-shaking episodes (presumed to be due to low flow in the ipsilateral carotid artery) were classified as TNAs in the carotid territory. Isolated slurred speech, migraine variants, transient confusion, and hemisensory tingling symptoms were classified as TNAs in uncertain territory. All TNAs were further classified according to whether they fulfilled NINDS TIA criteria.19
Statistical analysis
We compared baseline characteristics of patients with stroke in the vertebrobasilar territory (vertebrobasilar stroke) versus those with stroke in the carotid territory (carotid stroke) using the χ2 test for categorical variables and t test for age. Where significant differences were noted, appropriately stratified analyses were done. We used the χ2 test to compare the frequency and type of TNAs reported during the 90 days preceding a vertebrobasilar stroke versus a carotid stroke and to compare the frequency of events preceding stroke with or without symptomatic arterial stenosis. Given that some patients experience multiple TNAs before stroke, to avoid double counting, analysis was based on the first TNA or TIA during the 90 days preceding stroke, rather than the most recent TNA or TIA. We assumed an arbitrary level of 5% significance.
Role of the funding source
The funding sources had no role in study design, collection, analysis, and interpretation of data, writing of the report, or the decision to submit for publication. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Results
Of 1141 consecutive patients with definite stroke, vascular territory could be identified in 1034 (91%). Of these, 742 (72%) patients had a minor stroke and 292 (28%) had a major stroke. 759 (73%) patients had a stroke in the carotid territory and 275 (27%) in the vertebrobasilar territory (table 1). Patients with carotid stroke were older than those with vertebrobasilar stroke, but other baseline characteristics were similar (table 1).
Table 1.
Baseline characteristics, vascular risk factors, and premorbid medication in patients with definite ischaemic stroke, by territory
| Carotid stroke (n=759) | Vertebrobasilar stroke (n=275) | p value | |
|---|---|---|---|
| Mean age (years) | 75·9 (11·8) | 73·3 (13·1) | 0·002 |
| Male sex | 372 (49%) | 133 (48%) | 0·88 |
| Hypertension | 450 (59 %) | 173 (63%) | 0·31 |
| Diabetes | 85 (11 %) | 35 (13%) | 0·51 |
| Angina or myocardial infarction | 145 (19%) | 64 (23%) | 0·16 |
| Peripheral vascular disease | 59 (8%) | 28 (10%) | 0·25 |
| Previously diagnosed atrial fibrillation | 156 (21%) | 50 (18%) | 0·43 |
| Current smoker | 107 (14%) | 34 (12%) | 0·54 |
| Previous TIA | 65 (9%) | 20 (7%) | 0·61 |
| Previous stroke | 126 (17%) | 33 (12%) | 0·08 |
| Previous antiplatelet treatment | 336 (44%) | 109 (40%) | 0·20 |
| Previous statin treatment | 168 (22%) | 61 (22%) | 1·0 |
| Previous antihypertensive treatment | 443 (58%) | 171 (62%) | 0·28 |
Data are number of patients (%) or mean (SD). TIA=transient ischaemic attack.
Events (TIAs and other TNAs) were more common in the 90 days preceding a vertebrobasilar stroke than before carotid stroke (in 59 of 275 patients before a vertebrobasilar stroke vs 65 of 759 patients before a carotid stroke; OR 2·92, 95% CI 1·99–4·28, p<0·0001). Of 275 patients with vertebrobasilar stroke, 59 (22%, 95% CI 16·6–26·4) had at least one event within the preceding 90 days (median 4 days, IQR 1–30) of which only five (8%) had events that fulfilled the NINDS criteria for TIA (figure). In the remaining 54 patients, 45 had isolated brainstem TNAs (23 patients with isolated vertigo, nine with non-NINDS binocular visual disturbance, ten with vertigo with other non-NINDS symptoms, two with isolated diplopia, and one with transient generalised weakness), and nine had TNAs of uncertain territory (four patients with isolated slurred speech, two with isolated hemisensory symptoms, two with transient confusion, and one with a migraine variant). In seven (13%) of these 54 patients, multiple TNAs occurred, with isolated vertigo being the most common (four of seven). In all four cases, the vertigo occurred suddenly and without provocation by movement.
Figure.
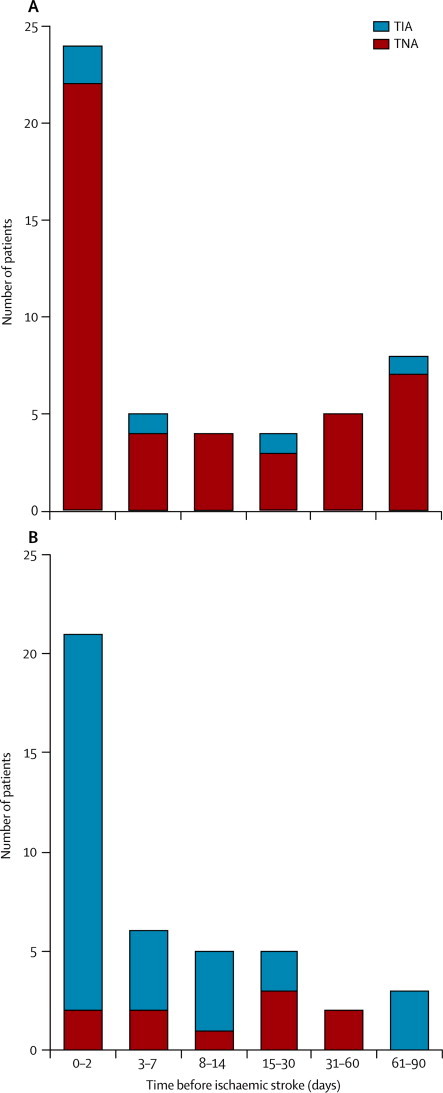
Occurrence of vertebrobasilar TNA or TIA in the 90 days before vertebrobasilar stroke (A) versus occurrence of carotid TNA or TIA in the 90 days before carotid stroke (B)
TIA=transient ischaemic attack. TNA=transient neurological attack.
The temporal relation between isolated vertebrobasilar TNA and vertebrobasilar stroke was very similar to that of definite carotid TIA and carotid stroke (figure), both being concentrated during the 2 days preceding the stroke. The rate of definite carotid TIA in the 2 days preceding carotid stroke was about 60 times greater than that during the preceding 3–90 days (9·5 events per day, 19 TIAs in 0–2 days, vs 0·15 events per day, 13 TIAs in 3–90 days; relative rate 64·3, 95% CI 31·76–130·21). This relative rate was similar for isolated brainstem TNAs preceding vertebrobasilar stroke (11·0 events per day, 22 TNAs in 0–2 days vs 0·26 events per day, 23 TNAs in 3–90 days).
Isolated TNAs in the vertebrobasilar territory were about 15 times more likely before a vertebrobasilar stroke than before a carotid stroke (table 2), slightly more so when cardioembolic strokes were excluded (41 of 219 events before vertebrobasilar stroke vs eight of 558 events before carotid stroke, OR 15·8, 95% CI 17·0–37·3, p<0·0001), but strikingly more so for events within the 2 days immediately preceding the stroke (OR 35·8, 8·4–153·5, p<0·0001; table 2). The difference in proportion remained high for events occurring 8–90 days preceding the stroke (OR 9·3, 3·7–23·6, p<0·0001; table 2).
Table 2.
TNA or TIA in the 90 days preceding stroke by territory
|
Territory of stroke |
OR (95%CI) | p value | |||
|---|---|---|---|---|---|
| Vertebrobasilar | Carotid | ||||
| TNA in vertebrobasilar territory* | 45/275 (16%) | 10/759 (1%) | 14·7 (7·3–29·5)† | <0·0001 | |
| 0–2 days | 22/252 (9%) | 2/751 (<1%) | 35·8 (8·4–153·5) | <0·0001 | |
| 3–7 days | 4/256 (2%) | 2/753 (<1%) | 6·0 (1·1–32·7) | 0·04 | |
| 8–90 days | 19/275 (7%) | 6/759 (1%) | 9·3 (3·7–23·6) | <0·0001 | |
| TNA in uncertain territory‡ | 9/225 (4%) | 17/717 (2%) | 1·7 (0·8–3·9) | 0·20 | |
| Definite TIA | 5/221 (3%) | 32/732 (4%) | 0·5 (0·2–1·3) | 0·16 | |
TNA=transient neurological attack. TIA=transient ischaemic attack. Data are n/N (%) unless otherwise stated.
Isolated vertigo, vertigo plus other symptoms of any type, isolated double vision, transient generalised weakness, and binocular visual disturbance. Days indicate the time of the transient event before the stroke event.
This association was independent of age: OR 12·8 (95% CI 3·6–45·9) for ages <65 years; OR 14·7 (7·3–29·5) for ages 65–74 years; and OR 11·0 (4·6–26·4) for ages ≥75 years.
Isolated slurred speech, migraine variant, transient confusion, and isolated hemisensory tingling. Denominators are based on first TNA or TIA during the 90 days preceding stroke; therefore patients with TNAs or TIAs during the initial period were censored.
TNAs thought to be of carotid territory origin, such as atypical amaurosis fugax and limb shaking episodes, occurred only before definite carotid stroke (six of 759 patients, three with atypical amaurosis fugax and three with limb-shaking). Of the 759 patients with definite carotid territory stroke, 65 (9%) had at least one TNA or TIA within the preceding 90 days (median 7·0 days, IQR 1–19·5) of which 32 (49%) fulfilled the NINDS criteria for TIA (figure, table 2).
Only ten (22%) of the 45 patients with isolated brainstem TNAs sought medical attention before the stroke and a vascular cause was suspected by their physician in one of these cases. In the remainder of cases, the initial diagnosis was documented as postural hypotension (three cases), peripheral vestibular disturbance (two cases), sepsis (two cases), migraine (one case), and cranial nerve palsy (one case). Vascular risk factors were more common in those with isolated vertigo, compared with other vertebrobasilar TNAs (table 3).
Table 3.
Vascular risk factors and duration of isolated vertigo versus other vertebrobasilar TNAs preceding vertebrobasilar stroke
| Isolated vertigo (n=23) | All other vertebrobasilar TNA (n=22) | ||
|---|---|---|---|
| Age ≥60 years | 17 (74%) | 15 (68%) | |
| Any vascular risk factors (diabetes mellitus, hypertension, smoking, or atrial fibrillation) | 19 (83%) | 11 (50%) | |
| Previous vascular events (myocardial infarction, peripheral vascular disease, TIA, or stroke) | 4 (17%) | 9 (41%) | |
| Any vascular medication* | 12 (52%) | 17 (77%) | |
| Duration of symptoms | |||
| <1 h | 11 (48%) | 15 (68%) | |
| ≥1 h | 12 (52%) | 7 (32%) | |
Data are number of patients (%) unless otherwise stated. TNA=transient neurological attack. TIA=transient ischaemic attack.
Any antiplatelet, statin, or antihypertensive.
In patients with stroke in whom 50% or higher symptomatic stenosis was documented, TNAs were also more frequent preceding vertebrobasilar stroke than preceding carotid stroke (table 4). This excess remained when patients with definite TIA and 50% or higher symptomatic stenosis were added to the analysis (table 4). Among the 16 patients with vertebrobasilar TIA and 50% or higher symptomatic vertebrobasilar stenosis, preceding TNAs were more commonly binocular visual disturbance (n=7) than isolated vertigo (n=2).
Table 4.
Events in the 90 days preceding ischaemic stroke or TIA with ≥50% symptomatic stenosis
|
Strokes with ≥50% symptomatic stenosis |
Strokes and TIAs with ≥50% symptomatic stenosis |
|||||||
|---|---|---|---|---|---|---|---|---|
| Vertebrobasilar stenosis (n=39) | Carotid stenosis (n=45) | OR (95% CI) | p value | Vertebrobasilar stenosis (n=72) | Carotid stenosis (n=95) | OR (95% CI) | p value | |
| NINDS TIA | 1 (3%) | 4 (9%) | 0·27 (0·01–2·8) | 0·37 | 5 (7%) | 17 (18%) | 0·3 (0·1–1·1) | 0·04 |
| TNA | 11 (28%) | 3 (7%) | 5·50 (1·3–27·6) | 0·02 | 27 (38%) | 12 (13%) | 4·2 (1·8–9·7) | 0·002 |
| No TIA or TNA | 27 (69%) | 38 (84%) | 0·41 (0·1–1·3) | 0·12 | 40 (56%) | 66 (69%) | 0·5 (0·3–1·1) | 0·08 |
Data are n (%) unless otherwise stated. TNA=transient neurological attack. TIA=transient ischaemic attack. OR=odds ratio. NINDS=National Institute of Neurological and Disorders and Stroke. Vascular evaluation was done using CT, MRI, or catheter angiography as clinically appropriate. Stenosis was defined as 50% or greater symptomatic stenosis. Derived from a total of 873 strokes with vascular imaging and 83 TIAs with 50% or greater symptomatic stenosis.
Discussion
Our study shows that there is a temporal relation between transient isolated brainstem symptoms and vertebrobasilar stroke (panel). Such events occurred in the days and weeks immediately preceding 16% of vertebrobasilar strokes. Of all events occurring during the 90 days preceding a vertebrobasilar stroke, fewer than 10% satisfied the NINDS definition of TIA. We also noted that most patients with such symptoms failed to present to medical attention at the time but presented only after a subsequent stroke, potentially leading to major bias in determining risk prospectively even in a population-based study of TNAs.
Panel. Research in context.
Systematic review
We searched Medline (1950–2011) and Embase (1988–2011) and also hand-searched relevant journals and the reference lists of included papers using the search terms “transient ischaemic attack”, “transient neurological attack (TNA)”, “stroke”, “vertebrobasilar”, and “stroke symptoms”. Searches were restricted to human studies. All types of study with at least three patients were included. Several studies14, 15, 16, 24, 25, 26, 27, 31, 32, 33, 34, 35, 36, 37, 38 have shown an association between TNAs and stroke, particularly isolated vertigo, but such TNAs do not satisfy traditional definitions of TIA.
Interpretation
We studied all potential ischaemic events during the 90 days preceding an ischaemic stroke in patients ascertained within a prospective, population-based incidence study in Oxfordshire, UK (the Oxford Vascular Study). Isolated brainstem TNAs were more frequent before a vertebrobasilar stroke than before a carotid stroke, particularly during the preceding 2 days. Our study shows a close temporal relation between vertebrobasilar stroke and preceding isolated brainstem transient neurological attacks that do not satisfy the NINDS definition of TIA. More studies of the prognosis of the different transient isolated brainstem symptoms are needed.
Results from large cohort studies have shown that 12–18% of people without a previous diagnosis of stroke reported TIA-like symptoms.24, 25, 26, 27 However, none of these studies determined the risk of stroke during the subsequent days and weeks and there are very few prospectively collected data of the prognosis after TNA. Prospective studies can underestimate stroke risk by missing those who do not seek medical attention at the time of the TNA. Questionnaire-based surveys of patients looking at the frequency of previous TNAs have various recall biases and exclude those who had a major or fatal stroke after the TNA and are therefore unable to respond.24, 25, 26, 27 Given these difficulties, large prospective population-based studies with complete ascertainment are probably impractical. However, it is possible to identify and study the most important subset of TNAs—ie, those that are associated with a subsequent stroke and which should therefore not be missed or misdiagnosed. Since half of all recurrent strokes that occur in the 5 years after a definite TIA occur in the first 90 days,28 with a similar front-loaded risk after vertebrobasilar TIA,29, 30 if we assume a similar time course for atypical TIAs (or TNAs) then useful information on the nature of high-risk TNAs can be gleaned from studying those events that occur in the 90 days preceding a definite stroke.
Isolated vertigo was the most common transient symptom preceding vertebrobasilar stroke in our study. Results from the Atherosclerosis Risk in Communities Study (ARIC) study24 showed that dizziness was the most frequently reported atypical symptom (36%) in the underlying population, although only 1·2% were classified as having a TIA or stroke.24 In elderly people, the prevalence of vertigo in the year before stroke has been reported to be 15%.31 Results from a prospective study of patients admitted to hospital with isolated vertigo showed a three-fold increase in stroke risk compared with controls up to 4 years after hospital discharge.32 Results from a retrospective study of patients with vertigo identified from MRI requests made after emergency department attendance showed 9·2% had acute stroke. Gait instability and subtle neurological examination findings were associated with increased stroke risk.33 Studies using Doppler sonography, magnetic resonance angiography, or catheter angiography have shown that isolated vertigo can be the only manifestation of vertebrobasilar ischaemia.33, 34, 35, 36 In a study of 29 patients admitted to hospital with possible vertebrobasilar TIA, isolated vertigo was present in 21%.34 In our study, the close temporal relation between vertigo and vertebrobasilar stroke suggests that these TNAs are often secondary to vertebrobasilar ischaemia.
Our findings have implications for public education to improve recognition and awareness of the significance of symptoms of TIA. In our study, of those patients who did present to medical attention after the initial event, most were not managed as having TIAs, suggesting that broader diagnostic criteria are needed. Although we do not know the rate of TNAs in the population, in patients with isolated vertigo plus vascular risk factors, referral for specialist opinion would appear to be reasonable.
The main strengths of our study were the population-based design, the rigorous case-ascertainment, and the prospective definition and classification of the various types of TNA. However, our study has limitations. First, the prevalence of TNAs before stroke events might be inaccurate if patients failed to report them during direct questioning or if recall bias increased recollection and reporting of TNAs. However, the very rapid fall in rate of preceding TNAs over the few days before the stroke is not what would be expected on the basis of recall bias—people would recall events for the preceding few weeks and not just the preceding few hours. Most importantly, in previous studies of definite TIA before stroke, this same time course of preceding TIA was noted20 and was subsequently confirmed in prospective studies.21 We might also have missed TNAs in patients who were unable to give a clear history after stroke because of aphasia or reduced consciousness. Second, although our attribution of the probable vascular territory of TNAs was predefined, it might not be accurate in all cases. Finally, we do not have data of the total number of patients with TNAs in our population who sought medical attention but were not considered to have TNAs and were not referred to secondary care during the period of the study. Without these data on the denominator we cannot identify the absolute risk of vertebrobasilar stroke after an isolated brainstem TNA reported to medical attention. Moreover, we do not, of course, know how many patients had such symptoms and did not seek medical attention. Furthermore, a previous study of patients with TNAs who were referred to hospital suggested that family doctors tend to refer those with vascular risk factors, potentially leading to a referral bias.37 Nevertheless, in those studies that have attempted to determine the incidence of TNAs that are reported to medical attention, the numbers of events have been similar to the number of definite TIAs ascertained over the same period,38 suggesting that the number of denominator events (ie, the total number of TNAs in the population) might not be so large as to substantially dilute the risk of stroke.
In conclusion, our study shows a close temporal relation between vertebrobasilar stroke and preceding isolated brainstem TNAs which do not satisfy the NINDS definition of TIA. A high index of suspicion is particularly important in view of the short-time window available for prevention of stroke. However, because of the adverse effects of a false-positive diagnosis of TIA, more studies of the prognosis of the different transient isolated brainstem syndromes are needed.
Acknowledgments
Acknowledgments
PMR is in receipt of an NIHR Senior Investigator Award and a Wellcome Trust Senior Investigator Award.
Contributors
NLMP contributed to acquisition of data, draft and revision of the report, statistical analysis, and interpretation of data. MS contributed to the revision of the report content and acquisition of data. PMR contributed to study concept and design, draft and revision of the report, analysis and interpretation of data, and study supervision.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Schulz UGR, Briley D, Meagher T, Molyneux A, Rothwell PM. Diffusion-weighted MRI in 300 patients presenting late with subacute transient ischaemic attack or minor stroke. Stroke. 2004;35:2459–2465. doi: 10.1161/01.STR.0000143455.55877.b9. [DOI] [PubMed] [Google Scholar]
- 2.Schulz UGR, Briley D, Meagher T, Molyneux A, Rothwell PM. Abnormalities on diffusion weighted magnetic resonance imaging performed several weeks after a minor stroke or transient ischaemic attack. J Neurol Neurosurg Psychiatry. 2003;74:734–738. doi: 10.1136/jnnp.74.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraaijeveld CL, van Gijn J, Schouten HJ, Staal A. Interobserver agreement for the diagnosis of transient ischemic attacks. Stroke. 1984;15:723–725. doi: 10.1161/01.str.15.4.723. [DOI] [PubMed] [Google Scholar]
- 4.Flossmann E, Redgrave JN, Briley D, Rothwell PM. Reliability of clinical diagnosis of the symptomatic vascular territory in patients with recent transient ischemic attack or minor stroke. Stroke. 2008;39:2457–2460. doi: 10.1161/STROKEAHA.107.511428. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, Giles MF, Chandratheva A. Effect of urgent treatment of transient ischemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432–1442. doi: 10.1016/S0140-6736(07)61448-2. [DOI] [PubMed] [Google Scholar]
- 6.Lavallee PC, Meseguer E, Abboud H. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6:953–960. doi: 10.1016/S1474-4422(07)70248-X. [DOI] [PubMed] [Google Scholar]
- 7.Intercollegiate Stroke Working Party . National clinical guideline for stroke. 3rd edn. Royal College of Physicians; London: 2008. [Google Scholar]
- 8.National collaborating centre for chronic conditions Diagnosis and initial management of acute stroke and transient ischaemic attack (TIA). NICE clinical guideline 68. http://guidance.nice.org.uk/CG68/NICEGuidance/pdf/English (accessed May 3, 2011).
- 9.Scottish Intercollegiate Guidelines Network Management of patients with stroke or TIA: assessment, investigation, immediate management and secondary prevention: a national clinical guideline. 2008. http://www.sign.ac.uk (accessed May 3, 2011).
- 10.American Heart Association. American Stroke Association Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 11.National Stroke Foundation (Australia): Melbourne Clinical guidelines for acute stroke management. 2007. http://www.strokefoundation.com.au/acute-clinical-guidelines-for-Acute-stroke-management (accessed May 3, 2011).
- 12.The European Stroke Organisation (ESO) Executive Committee. the ESO Writing Committee Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 13.Johnston SC, Nguyen-Huynh MN, Schwarz ME. National stroke association guidelines for the management of TIA. Ann Neurol. 2006;60:301–313. doi: 10.1002/ana.20942. [DOI] [PubMed] [Google Scholar]
- 14.Grimley Evans J. Transient neurological dysfunction and risk of stroke in an elderly English population: the different significance of vertigo and non-rotatory dizziness. Age Ageing. 1990;19:43–49. doi: 10.1093/ageing/19.1.43. [DOI] [PubMed] [Google Scholar]
- 15.Koudstaal PJ, Algra A, Pop GAM, Kappelle LJ, van Latum JC, van Gijn J. Risk of cardiac events in atypical transient ischaemic attack or minor stroke. Lancet. 1992;340:630–633. doi: 10.1016/0140-6736(92)92170-k. [DOI] [PubMed] [Google Scholar]
- 16.Bos MJ, van Rijn MJE, Witteman JCM, Hofman A, Koudstaal PJ, Breteler MMB. Incidence and prognosis of transient neurological attacks. JAMA. 2007;298:2877–2885. doi: 10.1001/jama.298.24.2877. [DOI] [PubMed] [Google Scholar]
- 17.Stroke foundation of New Zealand New Zealand guideline for the assessment and management of people with recent transient ischaemic attack (TIA) 2008. www.stroke.org.nz (accessed May 3, 2011).
- 18.The Stroke Prevention and Educational Awareness Diffusion (SPREAD) Collaboration The Italian guidelines for stroke prevention. Neurol Sci. 2006;21:5–12. doi: 10.1007/s100720070112. [DOI] [PubMed] [Google Scholar]
- 19.The Ad Hoc Committee on the classification and outline of cerebrovascular disease II. Stroke. 1975;6:566–616. doi: 10.1161/01.str.6.5.564. [DOI] [PubMed] [Google Scholar]
- 20.Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Lancet. 2005;366:29–36. doi: 10.1212/01.WNL.0000152985.32732.EE. [DOI] [PubMed] [Google Scholar]
- 21.Rothwell PM, Coull AJ, Giles MF. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study) Lancet. 2004;363:1925–1933. doi: 10.1016/S0140-6736(04)16405-2. [DOI] [PubMed] [Google Scholar]
- 22.Rothwell PM, Coull AJ, Silver LE. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 23.Brott T, Adams HP, Jr, Olinger CP. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 24.Toole JF, Lefkowitz DS, Chambless LE, Wijnberg L, Paton CC, Heiss G. Self-reported transient ischaemic attack and stroke symptoms: Methods and baseline prevalence, the ARIC study 1987–1989. Am J Epidemiol. 1996;144:849–856. doi: 10.1093/oxfordjournals.aje.a009019. [DOI] [PubMed] [Google Scholar]
- 25.Hart CL, Hole DJ, Davey Smith G. The relation between questions indicating transient ischaemic attack and stroke in 20 years of follow up in men and women in the Renfrew/Paisley study. J Epidemiol Community Health. 2001;55:653–656. doi: 10.1136/jech.55.9.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howard VJ, McClure LA, Meschia JF, Pulley L, Orr SC, Friday GH. High prevalence of stroke symptoms among persons without a diagnosis of stroke or transient ischaemic attack in a general population. Arch Intern Med. 2006;166:1952–1958. doi: 10.1001/archinte.166.18.1952. [DOI] [PubMed] [Google Scholar]
- 27.Howard G, Stafford MM, Meschia JF. Stroke symptoms in individuals reporting no prior stroke or transient ischaemic attack are associated with a decrease in indices of mental and physical functioning. Stroke. 2007;38:2446–2452. doi: 10.1161/STROKEAHA.106.478032. [DOI] [PubMed] [Google Scholar]
- 28.Paul NLM, Chandratheva A, Rothwell PM. Major unmet need for more effective secondary prevention in the subacute phase after minor ischaemic stroke: a population-based study. J Neurol Neurosurg Psychiatry. 2010;81:e27–e28. [Google Scholar]
- 29.Flossmann E, Rothwell PM. Prognosis of vertebrobasilar transient ischaemic attack and minor stroke. Brain. 2003;126:1940–1954. doi: 10.1093/brain/awg197. [DOI] [PubMed] [Google Scholar]
- 30.Marquardt L, Kuker W, Chandratheva A, Geraghty O, Rothwell PM. Incidence and prognosis of ≥50% symptomatic vertebral or basilar artery stenosis: prospective population-based study. Brain. 2009;132:982–988. doi: 10.1093/brain/awp026. [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson WE, Heyman A, Burch JG, Ostfeld A, Labarthe DR, Leviton A. Use of a self-administered questionnaire for detection of transient cerebral ischaemic attacks: I. Survery of elderly persons living in retirement facilities. Ann Neurol. 1979;6:40–46. doi: 10.1002/ana.410060110. [DOI] [PubMed] [Google Scholar]
- 32.Lee C-C, Su Y-C, Ho H-C, Hung S-K, Lee M-S, Chou P, Huang Y-S. Risk of stroke in patients hospitalized for isolated vertigo-a four year follow-up study. Stroke. 2011;42:48–52. doi: 10.1161/STROKEAHA.110.597070. [DOI] [PubMed] [Google Scholar]
- 33.Chase M, Joyce NR, Carney E, Salciccioli JD, Vinton D, Donnino MW, Edlow JA. ED patients with vertigo: can we identify clinical factors associated with acute stroke? Am J Emerg Med. 2012;30:587–591. doi: 10.1016/j.ajem.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Gomez CR, Cruz-Flores S, Malkoff MD, Sauer CM, Burch CM. Isolated vertigo as a manifestation of vertebrobasilar ischaemia. Neurology. 1996;47:94–97. doi: 10.1212/wnl.47.1.94. [DOI] [PubMed] [Google Scholar]
- 35.Moubayed SP, Saliba I. Vertebrobasilar insufficiency presenting as isolated positional vertigo or dizziness: a double-blind retrospective cohort study. Laryngoscope. 2009;119:2071–2076. doi: 10.1002/lary.20597. [DOI] [PubMed] [Google Scholar]
- 36.Norrving B, Magnusson M, Holtas S. Isolated acute vertigo in the elderly; vestibular or vascular disease? Acta Neurol Scand. 1995;9:43–48. doi: 10.1111/j.1600-0404.1995.tb05841.x. [DOI] [PubMed] [Google Scholar]
- 37.Dennis M, Warlow C. Migraine aura without headache: transient ischaemic attack or not? J Neurol Neurosurg Psychiatry. 1992;55:437–440. doi: 10.1136/jnnp.55.6.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bots ML, van der Wilk EC, Koudstaal PJ, Hofman A, Grobbee DE. Transient neurological attacks in the general population: prevalence, risk factors and clinical relevance. Stroke. 1997;28:768–773. doi: 10.1161/01.str.28.4.768. [DOI] [PubMed] [Google Scholar]


