Abstract
Carbamazepine and other anticonvulsants are commoner cause of severe adverse cutaneous drug reactions such as erythema multiforme, toxic epidermal necrolysis (TEN), and Stevens–Johnson syndrome (SJS). We report a case of SJS rapidly progressing to TEN with a combination of haloperidol and carbamazepine in a patient with bipolar affective disorder. The pathophysiological mechanism underlying this reaction is discussed.
Keywords: Carbamazepine, haloperidol, Stevens–Johnson syndrome, toxic epidermal necrolysis
Antiepileptic drugs are associated with several dermatological adverse effects including rashes, urticaria, and photosensitivity reactions, whereas, severe and life threatening acute adverse cutaneous drug reactions such as erythema multiforme, toxic epidermal necrolysis (TEN) and Stevens–Johnson syndrome (SJS) are reported rarely. Among them, TEN is clinically characterized by erythematous macules and targetoid lesions throughout the body along with more than 30% of body surface having full thickness epidermal necrosis; whereas SJS may have the <10% body surface area having full-thickness epidermal necrosis with detachment of the cutaneous surface along with mucous membrane involvement in two or more areas.[1] Most of the reported cases of SJS and TEN (>90%) occur during first 2 months of antiepileptic drugs use. The estimated risk ranges between one and ten cases per 10 000 new users for carbamazepine, lamotrigine, phenytoin, phenobarbital, whereas lower rates have been reported for valproate.[2] Highest rates of SJS-TEN have been reported to occur with carbamazepine (14/10,000 users).[2,3] We report a case of SJS rapidly progressing to TEN with a combination of haloperidol and carbamazepine in a patient with bipolar affective disorder.
CASE REPORT
A 27-year-old unmarried Asian male diagnosed with bipolar affective disorder, presented with 5 years of cannabis (ganja) intake in dependence pattern and increased talk, disturbed sleep, hyperactivity for past 3 years. He had one past episode of mania 10 years back and one episode of depression 4 years back that remitted following treatment with carbamazepine without any adverse reaction. There was no family history of any psychiatric or medical illness or drug reactions. Mental status examination showed domineering attitude, voluble speech, euphoric affect, and delusion of grandiosity. He was administered intramuscular haloperidol injection 10 mg and promethazine injection 50 mg twice a day for 2 days to control agitation that was changed to oral haloperidol 5 mg twice a day. Considering his previous response he was started on carbamazepine tablet 400 mg per day; dose was increased by 100 mg every 3 days to 700 mg per day. On 13th day, patient developed fever (temperature 101.3 °F), sore throat along with flushing of face, and subsequently developed maculopapular rashes, starting from the face spreading to the neck and then to the trunk. Within 6 to 8 h vesicular eruptions were noted in the lips along with conjunctival congestion. There were no rashes below the trunk or presence of target lesions. Within 24 h of development of rashes, vesico-bullous eruptions developed in the face and neck with involvement of mucosa of lips, palate and throat [Figure 1]. There was rapid spread of lesion and within next 24 h more than 30% of body surface area had vesico-bullous eruptions with positive Nikolsky sign. A diagnosis of drug-induced Stevens–Johnson syndrome progressing to toxic epidermal necrolysis was made after dermatologist consultation. Laboratory investigations revealed high erythrocyte sedimentation rate (ESR) titers (50 mm in the first hour), white blood cell (WBC) count 7000 per mm3, and neutrophil count 74.5%; rest were within the normal range. All his medications were stopped and referred to dermatology department for further management. He was treated with dexamethasone injection 4 mg b.i.d., ceftriaxone injection 1 gm b.i.d., topical betamethasone, and ciprofloxacin eye drops along with supportive care and after 7 days his condition improved. He was subsequently managed with sodium valproate 1000 mg and olanzapine 10 mg per day with complete resolution of manic symptoms and without any emergent adverse effect.
Figure 1.
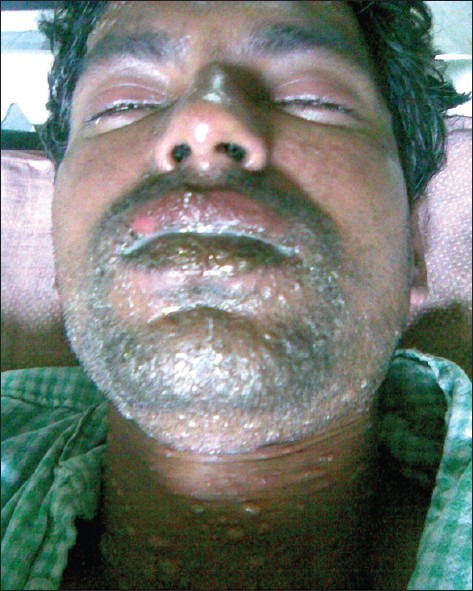
Vesico-bullous lesions over face and neck with swelling of eyelids and ulceration over lips
DISCUSSION
In our case, the patient had two prior exposures to carbamazepine without developing any cutaneous adverse reactions. On third exposure to a combination of carbamazepine and haloperidol, he developed SJS on day 13, which progressed very rapidly to TEN. The summary of product characteristics for haloperidol mentions TEN and SJS to have been reported to occur, though true incidence of these associations is not known.[4] But, there have been case studies describing SJS associated with carbamazepine-neuroleptic combination, specifically with haloperidol.[5] Also, similar increased risk of SJS has been reported to occur with lamotrigine-aripiprazole combination, where two out of three cases developed SJS.[6] Therefore, it is probable that SJS/TEN was associated with haloperidol-carbamazepine combination.
Although the mechanism underlying SJS/TEN is not clear, an idiosyncratic, delayed hypersensitivity reaction (type-IV) has been implicated in the pathophysiology.[7] It has been suggested that TEN is a rapidly developing equivalent of SJS, in which predominantly a drug-induced cytotoxic immune reaction specifically causes epidermal layer skin destruction along with other systemic manifestations.[3] The only difference between SJS and TEN is the body surface area involvement which if exceeds 10% of body surface is considered in the SJS-TEN spectrum. In carbamazepine-induced drug reactions, bioactivation of various metabolites by the cytochrome P-450 system are implicated. Most of the metabolites are inactivated either through hydrolysis by liver microsomal epoxide hydrolase-1 or by glutathione transferases. Nevertheless, some of the metabolites that are left unhydrolyzed may activate CD8+ T-cell mediated cytotoxic reactions leading to SJS-TEN complex through epidermal cell apoptosis.[7] It has been suggested that, slow acetylators with concomitant antiepileptics are among those at most risk as they do not achieve complete detoxification of reactive drug metabolites which can act as haptens that interact with host tissues making them antigenic. Once apoptosis starts, the dying cells produce more chemokines that perpetuates the inflammatory process leading to extensive epidermal necrolysis.[7] It is interesting to note that SJS appears within 2 weeks with such combination, as was observed in our case study. This might be because of the combination of carbamazepine and haloperidol causing faster immunological reaction.
Furthermore, the association between SJS/TEN with HLA-BF*1502 allele has been reported to be ethnicity specific; it is common in Asian origin Chinese population.[8] This allele has some role in controlling the metabolism of carbamazepine and as it is commonly seen in Asian origin Chinese population; further studies of these reactions at the genetic level will help to predict when such drug reactions might occur. Moreover, Indian studies have also highlighted risk factors predicting increase possibility of carbamazepine-induced SJS, such as female gender, younger age, HIV positive, past history of hypersensitivity, drug naïve, and positive family history of SJS or serious drug reaction, polytherapy with nonsteroidal anti-inflammatory drugs (NSAIDs), and lamotrigine or sulfonamides.[9] The maximum number of cases of SJS-TEN is reported to occur in their twenties as was seen in our case. Anticonvulsants were observed more frequently in the Indian and Asian reports, whereas antimicrobials and NSAIDs were more commonly implicated in the western literature.[9]
The investigations at admission and after development of SJS-TEN, when compared showed a very high rise in titers of ESR (50 mm in the first hour), doubling of WBC count to 7000/mm3, doubling of neutrophil count to 74.5%, and reduction of lymphocyte count to almost half from baseline. The median duration of developing carbamazepine-induced SJS-TEN has been reported to occur between 25 to 90 days. [10] Patients who have been taking carbamazepine for more than 3 months without developing skin reactions are at low risk of carbamazepine-induced acute cutaneous drug reaction. According to Roujeau and Stern,[3] SJS-TEN complex is invariably accompanied with elevated blood ESR, which was also noted in our case. Although nonspecific, weekly monitoring of ESR may be an indicator of possible severe adverse cutaneous drug reactions during initial 3 months after starting on carbamazepine.
In summary, it may be suggested that caution is required when initiating haloperidol along with carbamazepine, which may trigger severe form of SJS as was seen in our case. Though, a lack of histopathological evaluation to confirm the diagnosis was a limitation in our case study. Also, the reaction may appear earlier, within 2 weeks, when carbamazepine-haloperidol combination is used. Further prospective studies might be required to substantiate these findings.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92–6. [PubMed] [Google Scholar]
- 2.Devi K, George S, Criton S, Suja V, Sridevi PK. Carbamazepine-the commonest cause of toxic epidermal necrolysis and Stevens-Johnson syndrome: A study of 7 years. Indian J Dermatol Venereol Leprol. 2005;71:325–8. doi: 10.4103/0378-6323.16782. [DOI] [PubMed] [Google Scholar]
- 3.Roujeau JC, Stern RS. Severe Adverse Cutaneous Reactions to drugs. N Engl J Med. 1994;331:1272–85. doi: 10.1056/NEJM199411103311906. [DOI] [PubMed] [Google Scholar]
- 4.Summary of product characteristics. Haloperidol. [Last accessed on 2010 Mar 20]. Available from: http://emc.medicines.org.uk/medicine/17426/SPC/Haldol 10mg Tablets/
- 5.Wong KE. Stevens-Johnson syndrome in neuroleptic-carbamazepine combination. Singapore Med J. 1990;31:432–3. [PubMed] [Google Scholar]
- 6.Shen YC, Chen SJ, Lin CC, Chen CH. Concomitant use of lamotrigine and aripiprazole increases risk of Stevens-Johnson syndrome? Int Clin Psychopharmacol. 2007;22:247–8. doi: 10.1097/01.yic.0000224789.21406.81. [DOI] [PubMed] [Google Scholar]
- 7.Leeder JS. Mechanisms of idiosyncratic hypersensitivity reactions to antiepileptic drugs. Epilepsia. 1998;39(Suppl 7):S8–16. doi: 10.1111/j.1528-1157.1998.tb01679.x. [DOI] [PubMed] [Google Scholar]
- 8.Lim KS, Kwan P, Tan CT. Association of HLA-BF*1502 allele and carbamazepine induced severe adverse cutaneous drug reaction among Asians, a review. Neurol Asia. 2008;13:15–21. [Google Scholar]
- 9.Sharma VK, Sethuraman G, Minz A. Stevens Johnson syndrome, toxic epidermal necrolysis and SJS-TEN overlap: A retrospective study of causative drugs and clinical outcome. Indian J Dermatol Venerol Leprol. 2008;74:238–40. doi: 10.4103/0378-6323.41369. [DOI] [PubMed] [Google Scholar]
- 10.Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, et al. Medication use and the risk of Stevens–Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333:1600–7. doi: 10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]


