In addition to non-pharmacological and pharmacological interventions, patient counselling and modification of lifestyle play a key role diabetes management.[1] Patient counselling is necessary to ensure adherence to pharmacological therapy.[2] Although insulin remains the most powerful tool to achieve glycaemic control, it is sometimes initiated too late in the course of diabetes, due to various physician- and patient-originated barriers.[3] Recently, newer injectable drugs have become available for management of diabetes. These include glucagon-like peptide-1 (GLP-1) analogues such as liraglutide and exenatide, and amylin analogues like pramlintide.[4] No recommendation is available to guide diabetes care professionals regarding the science and art of counselling for these drugs. In this article we focus on counselling patients for liraglutide (Victoza®; Novo Nordisk A/S, Denmark) and exenatide (Byetta and Bydureon; Amylin/Eli Lilly, USA). GLP-1 agonists have shown significant and clinically relevant benefits over other anti-diabetic medications.[5–12] Since its introduction in India in June 2010, we have initiated liraglutide in over 100 type-2 diabetic subjects. This article focuses on the specific aspects of GLP-1 analogue counselling and highlights the subtle differences between counselling for GLP-1 analogue and insulin, based on our clinical work with a large, heterogeneous patient cohort.
The unique features of GLP-1-based therapy, and differences between insulin and GLP use and adverse effects profiles, make counselling for GLP-1 analogues a challenge. The GLP-1 agonists liraglutide and exenatide differ from insulin in several aspects: Mechanism of action, benefits, side-effect profile, injection timing, need for blood glucose monitoring, and complexity of the regimen.[4] In addition, onset of action is usually faster with rapid-acting insulin than GLP agonists. Acutely, insulin may be a life-saving drug with immediate dramatic effects. The benefits of GLP-1 analogues, on the other hand, may not be visible so quickly. The side-effect profile is dissimilar: Insulin is associated with hypoglycaemia and weight gain, whereas GLPs are associated with gastrointestinal symptoms, which are for the most part mild and transient. Rules for self-adjustment of dose and sick day management still need to be formulated for GLPs. In a pay-from-pocket scenario, counselling has to be done about the relatively higher cost of GLP-1 analogues, as compared with insulin.
Some patients may find it difficult to accept injectable therapy at first suggestion. One should introduce the concept of the drug, inform the patient about its potential benefits and risks, and if necessary, offer time to think it over. The discussion can be continued in the next consultation, after a reasonable ‘contemplation period’. In our experience, longer contemplation periods may lead to better compliance and lower discontinuation rates.
For a patient to accept insulin or GLP-1 analogues, and to continue taking it regularly, it may help to explain the mechanism of action. One should use simple, easy to understand analogies to explain the concept of GLP-1- and GLP-1-based therapy. For example, we have used explanations such as:
GLP-1 is a hormone which increases the good hormone secreted by the pancreas (insulin) while reducing the bad hormone (glucagon).
GLP-1 helps your pancreas to work better by putting out more insulin, but only when needed.
Clinical trials of GLP-1 analogues have shown numerous favourable outcomes: Better glycaemic control, reduction in body weight, improvement in lipid profile, reduction in systolic blood pressure, improvement in β-cell function, any of which may be of interest to or pertinent to many diabetic patients.[5–12]
The counsellor should build on existing knowledge and needs of the patient. For example, an obese patient would benefit from explanation about the weight-reducing property of liraglutide. Another patient who has a strong family history of cardiovascular disease may appreciate learning about the blood pressure- and lipid-lowering action of GLPs. Someone who is aware of the concept of insulin resistance might prefer information on insulin sensitivity. GLP-1 use is linked with weight loss. This is an attractive property for most patients, and should be reinforced. A simple way of describing this is:
“Using the drug will help you will likely help you achieve two important goals: A more healthful weight and better glucose control.”
Because GLP-1 agonists only stimulate β-cells to produce insulin when glucose levels are elevated, when used as monotherapy there is minimum risk of hypoglycaemia. While counselling a potential patient, the diabetes care professional should focus on the safety and efficacy of these drugs, highlighting the lesser chance of hypoglycaemia. This can be done by using a simple analogy such as:
“GLPs can help you achieve safe and effective glucose control. Because other choices might be more likely to cause hypoglycaemia, we think this is a safer method.
Patients are sometimes apprehensive of injectable therapy. They feel it will be difficult to administer, complex to adjust, involve frequent monitoring, be linked to rigid meal patterns, and impact their freedom of lifestyle.
The unmatched advantages of GLP-1 analogues in this regard need to be emphasised in simple words. For example:
“Using GLP-1 agonists is generally quite simple; short acting agents are given once or twice daily, and long-acting exenatide is given once weekly. Time of day does not appear to make a big difference, and most patients find the injection easier than they expected.”
“As the risk of hypoglycemia is quite low, one need not follow a strict six meal pattern, or monitor glucose levels multiple times a daily.”
Some GLPs are available in pen devices, making injections more acceptable. The technique, site, and rotation for GLP-1 analogues are similar to that for insulin. Long-acting exenatide is also available, but the technique for mixing it prior to injection is different than liraglutide or short-acting exenatide.
The most common adverse effects of GLP-1 analogues are related to the gastrointestinal tract. The minority of patients who experience nausea and/or vomiting should be reassured that such symptoms are typically short-lived and mild, and are only rarely a cause of discontinuation of therapy
One should specify the advantages of these effects in advance:
“The loss of appetite may help you reduce weight and achieve glycemic control.”
No counselling session on GLP-1 analogues is complete without a discussion of its cost, especially in a pay-from-pocket scenario. The cost of GLP-1 analogues should be put in perspective. Compared with the cost of oral hypoglycaemic drugs (especially dipeptidyl peptidase-4 (DPP-4) inhibitors), anti-obesity drugs, and the complications of diabetes, these molecules are not that all expensive.
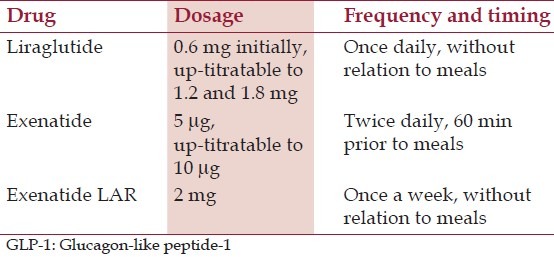
The doses of various GLP-1 analogues are mentioned in Table 1.
Table 1.
Dosage of GLP-1 analogues
DPP-4 inhibitors are often compared with GLP agonists. Although as a class, incretins have the potential to improve satiety, slow gastric emptying, blunt glucagon secretion, and stimulate glucose-dependent insulin secretion, because the plasma levels of GLP-1 are significantly lower with DPP-4 inhibitors than with GLP-1 agonists, DPP-4 inhibitors produce GLP levels that are only sufficient to blunt glucagon and stimulate glucose-dependent insulin secretion. This difference in GLP-1 plasma levels also explains the difference in weight loss seen between GLP-1 agonists and DPP-4 inhibitors.
In conclusion, counselling is an essential part of GLP-1 analogue prescription. We hope that this communication assists clinicians to counsel their patients in the use of GLP-1 agonists.
References
- 1.Morrison F, Shubina M, Turchin A. Lifestyle counseling in routine care and long-term glucose, blood pressure, and cholesterol control in patients with diabetes. Diabetes Care. 2012;35:334–41. doi: 10.2337/dc11-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratanawongsa N, Crosson JC, Schillinger D, Karter AJ, Saha CK, Marrero DG. Getting under the skin of clinical inertia in insulin initiation: The translating research into action for diabetes (TRIAD) insulin starts project. Diabetes Educ. 2012;38:94–100. doi: 10.1177/0145721711432649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational global attitudes of patients and physicians in insulin therapy study. Diabet Med. 2012;29:682–9. doi: 10.1111/j.1464-5491.2012.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeFronzo RA. Current issues in the treatment of type 2 diabetes.Overview of newer agents: Where treatment is going. Am J Med. 2010;123:38–48. doi: 10.1016/j.amjmed.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Marre M, Shaw J, Brändle M, Bebakar WM, Kamaruddin NA, Strand J, et al. LEAD-1 SU study group. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26:268–78. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: The LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: A 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–56. doi: 10.1016/S0140-6736(10)60307-8. [DOI] [PubMed] [Google Scholar]
- 8.Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): A randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–81. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 9.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: A 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 10.Buse JB, Sesti G, Schmidt WE, Montanya E, Chang CT, Xu Y, et al. Liraglutide effect action in diabetes-6 study group.Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care. 2010;33:1300–3. doi: 10.2337/dc09-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): A randomized controlled trial. Diabetologia. 2009;52:2046–55. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. LEAD-4 Study Investigators.Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD) Diabetes Care. 2009;32:1224–30. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]


