Abstract
Aims
We examined the relationship between forced expiratory volume in 1 s (FEV1), airflow obstruction, and incident heart failure (HF) in black and white, middle-aged men and women in four US communities.
Methods and results
Lung volumes by standardized spirometry and information on covariates were collected on 15 792 Atherosclerosis Risk in Communities (ARIC) cohort participants in 1987–89. Incident HF was ascertained from hospital records and death certificates up to 2005 in 13 660 eligible participants. Over an average follow-up of 14.9 years, 1369 (10%) participants developed new-onset HF. The age- and height-adjusted hazard ratios (HRs) for HF increased monotonically over descending quartiles of FEV1 for both genders, race groups, and smoking status. After multivariable adjustment for traditional cardiovascular risk factors and height, the HRs [95% confidence intervals (CIs)] of HF comparing the lowest with the highest quartile of FEV1 were 3.91 (2.40–6.35) for white women, 3.03 (2.12–4.33) for white men, 2.11 (1.33–3.34) for black women, and 2.23 (1.37–3.59) for black men. The association weakened but remained statistically significant after additional adjustment for systemic markers of inflammation. The multivariable adjusted incidence of HF was higher in those with FEV1/forced vital capacity <70% vs. ≥70%: HR 1.44 (95% CI 1.20–1.74) among men and 1.40 (1.13–1.72) among women. A consistent and positive association with HF was seen for self-reported diagnosis of emphysema and chronic obstructive pulmonary disease, but not for asthma.
Conclusions
In this large population-based cohort with long-term follow-up, low FEV1 and an obstructive respiratory disease were strongly and independently associated with the risk of incident HF.
Keywords: Lung function, COPD, Heart failure, Risk factors, Cohort study
See page 348 for the editorial comment on this article (doi:10.1093/eurjhf/hfs022)
Introduction
Heart failure (HF) is a major cause of disability and premature mortality.1 It has a poor prognosis, and the costs associated with its treatment exceed those for coronary artery disease and cancer combined.1 Chronic obstructive pulmonary disease (COPD) remains one of the leading causes of morbidity and mortality in the developed world2.
Chronic obstructive pulmonary disease is a common co-morbidity among patients with HF, and vice versa.3 A large body of literature suggests that higher cardiovascular mortality occurs in individuals with COPD and low lung volumes.2,4 A connection between HF and COPD has been alluded to in the literature. In patients with COPD, the predicted percentage of forced expiratory volume in 1 s (FEV1) was inversely associated with left ventricular ejection fraction,5 and cor pulmonale in particular is a well-recognized complication of severe COPD; further, severe COPD is associated with impaired left ventricular filling.5,6 Recently published studies suggest that left ventricular diastolic and systolic dysfunction are frequently seen in mild and even subclinical COPD7 and emphysema.8 However, only a few recently published studies have reported an association between subclinical or spirometry-based airflow obstruction and incident HF in middle-aged men,9 in healthy older adults,10 and in those with either systolic or diastolic dysfunction at baseline.11
To our knowledge, there are limited population-based reports that systematically examine the relationship between COPD (spirometry-based airflow obstruction, clinical definition, and self-reported diagnosis) and the risk of incident HF. We hypothesized that low FEV1 as well as airflow obstruction in individuals without HF is associated with increased risk of subsequent HF, and that this relationship would be consistent across groups defined by race, gender, and smoking status. We addressed these questions in a large biracial cohort of middle aged men and women sampled from four US communities.
Methods
Study population
The Atherosclerosis Risk in Communities (ARIC) study enrolled 15 792 men and women aged 45–64 years sampled from four US communities: Forsyth County, NC; Jackson, MS; seven north-western suburbs of Minneapolis, MN; and Washington County, MD. Enrolment at the Jackson, MS site was restricted to black residents, while black residents were oversampled in Forsyth County. The ARIC study protocol was approved by the institutional review board of each participating university, and written informed consent was obtained from study participants at the baseline visit. Baseline examinations of the cohort were conducted from 1987 to 1989 to collect standardized information on socioeconomic indicators, medical history, family history, cardiovascular risk factors, serum chemistries, electrocardiograms (ECGs), medication use, and lung volumes. Three re-examinations followed the baseline visit, as well as annual telephone interviews and active surveillance of hospitalizations and death.
Those with prevalent HF (n = 775) or missing (n = 325) data on HF at baseline, missing (n = 127) or implausible (n = 12) lung volume data, missing information on important covariates (n = 780), and race other than black or white (n = 48) were excluded from these analyses. The final study sample was 13 660.
Exposure
Pulmonary function was assessed at the baseline visit (1987–89) using a water-sealed Collins Survey II volume displacement spirometer (Collins Medical, Inc.) and Pulmo-Screen II software (PDS Healthcare Products, 496 Inc.). Spirometry was conducted using American Thoracic Society (ATS) guidelines.12 From at least three acceptable readings, of which at least two were reproducible (volumes within ±5%), a best reading was selected by computer software and confirmed by a technician using ATS criteria for acceptability. The protocol was standardized across the four field centres, with training and certification of pulmonary technicians and extensive quality assurance throughout the study.
History of respiratory disease was obtained via a standardized questionnaire by trained personnel.13 Participants were also asked whether they were ever diagnosed with bronchitis/emphysema/asthma, and whether these were confirmed by a doctor. Chronic bronchitis was defined as productive cough for at least 3 months in two contiguous years. COPD was defined by a positive response to a physician diagnosis of either emphysema or chronic bronchitis. Post-bronchodilator measurements are required to define COPD optimally per GOLD classification,2 but only pre-bronchodilator measurements were available in this study. An FEV1/forced vital capacity (FVC) ratio of <0.7 was also used to define airflow obstruction. Those with airflow obstruction were further divided into mild (FEV1 ≥80% of predicted value) and moderate/severe (FEV1 <80% of predicted).2
Covariates
At baseline, prevalent coronary heart disease (CHD) was ascertained by self-report and a 12-lead ECG. Hypertension was defined by diastolic pressure ≥90 mmHg or systolic pressure ≥140 mmHg on the average of the last two of three measurements, or the use of antihypertensive medications. Diabetes was defined by the presence of a serum glucose level ≥200 mg/dL, an 8 h fasting glucose level ≥126 mg/dL, a self-reported history of physician-diagnosed diabetes, or the current use of hypo-/antihyperglycaemic medications. Heart rate was derived from the 12-lead ECG. Medication intake including beta-blockers was self-reported or coded from medicines brought by participants during the exam. Cigarette smoking status was defined as currently smoking, or a history of ever smoking ≥400 cigarettes. The average number of cigarettes/day and number of years of smoking reported were multiplied to derive cigarette-years of smoking. Body mass index was defined as the ratio of measured weight (kg) and measured height2 (in m2). Participants identified themselves as American/Alaskan Indian, Asian, black, or white race.
Heart failure
Incident HF was defined as the first HF hospitalization with an HF discharge code or HF as the underlying cause of death on a death certificate (n = 84). Hospital discharge diagnoses were coded using the International Classification of Diseases Code, Ninth Revision (ICD-9, code 428), and deaths were coded using ICD-9 or ICD-10 (codes 428 and I 50, respectively). Cohort follow-up for the current analysis ended on 31 December 2004.
Statistical methods
Means (standard deviations) and proportions of characteristics at baseline and follow-up HF event rates were calculated for quartiles of FEV1. A higher proportion of blacks and women were in the lower quartiles of FEV1 and FVC; thus all analyses (except for Table 1) used race- and gender-specific quartiles of FEV1. Incidence density (rate) of HF in various strata was estimated assuming a Poisson distribution. Cox regression models were used to assess the relationship between race- and gender-specific quartiles of FEV1 (and FVC), and airflow obstruction with incident HF for each race and gender group.
Table 1.
Characteristics of study participants at baseline and incident heart failure across quartiles of forced expiratory volume in 1 s (n = 13 360)
| FEV1 quartile at baselinea |
||||
|---|---|---|---|---|
| Characteristicb | Q1 (0.5–2.3 L), n = 3415 | Q2 (2.3–2.8 L), n = 3415 | Q3 (2.8–3.3 L), n = 3415 | Q4 (3.3–5.9 L), n = 3415 |
| Demographic/lifestyle/clinical | ||||
| Age (in years) | 56.06 (5.6) | 54.15 (5.7) | 53.45 (5.7) | 52.62 (5.4) |
| Male | 14.6 | 21.5 | 51.2 | 93.3 |
| African American | 39.1 | 27.2 | 20.6 | 11.6 |
| Hypertension | 41.8 | 31.7 | 29.7 | 23.5 |
| Diabetes | 15.1 | 9.6 | 9.4 | 7.4 |
| Current smoker | 36.5 | 25.3 | 21.6 | 17.7 |
| Former smoker | 21.3 | 26.6 | 34.7 | 44.8 |
| Cigarette-years of smoking | 361.6 (467.9) | 269 (397.5) | 305.6 (434.5) | 307.9 (386.3) |
| Usual ethanol intake (g/week) | 27.9 (82.3) | 31.2 (75.6) | 49.1 (107.1) | 62.6 (106.3) |
| Height (cm) | 161.9(7.5) | 164.8 (7.1) | 169.9 (7.3) | 177.6 (6.5) |
| Systolic blood pressure (mmHg) | 124.9 (20.4) | 120.1 (18.4) | 119.4 (18.1) | 118.4 (15.6) |
| Diastolic blood pressure (mmHg) | 73.2 (11.8) | 72.6 (10.9) | 73.5 (11.0) | 74.4 (10.3) |
| LDL-cholesterol (mg/dL) | 139.4 (42.0) | 136.2 (38.4) | 136.3 (38.8) | 138.2 (36.6) |
| HDL-cholesterol (mg/dL) | 55.4 (17.7) | 55.4 (16.9) | 52.2 (17.4) | 45.5 (13.8) |
| Body mass index (kg/m2) | 28.2 (6.4) | 27.4 (5.5) | 27.1 (4.9) | 27.2 (3.7) |
| Inflammatory markers | ||||
| White blood count (×103/mm3) | 6.4 (2.1) | 6 (1.9) | 6 (1.8) | 5.9 (2.1) |
| Fibrinogen (mg/dL) | 322.4 (71.0) | 302 (61.3) | 297 (61.6) | 284.5 (55.9) |
| Albumin (g/dL) | 3.2 (0.7) | 3.2 (0.6) | 3.1 (0.6) | 3.1 (0.6) |
| von Willebrand's factor (%) | 126.1 (53.2) | 117.1 (46.6) | 114.1 (44.2) | 110 (42.6) |
| Study exposure (S) | ||||
| FEV1 (L) | 1.9 (0.3) | 2.5 (0.1) | 3 (0.2) | 3.9 (0.4) |
| FVC (L) | 2.8 (0.5) | 3.4 (0.4) | 4 (0.4) | 5.1 (0.6) |
| FEV1/FVC | 70.8 (11.0) | 75.1 (6.8) | 75.6 (6.4) | 76.3 (5.4) |
| FEV1/FVC <70% | 41.1 | 22.5 | 20.6 | 15.8 |
| Chronic bronchitisc | 7.6 | 4.1 | 4.3 | 3.8 |
| Self-reported diagnosis of bronchitis | 13.3 | 6.9 | 5.3 | 3.7 |
| Self-reported diagnosis of emphysema | 3.7 | 0.9 | 0.8 | 0.7 |
| Self-reported diagnosis of COPDd | 15.4 | 7.5 | 5.9 | 4.3 |
| Self-reported diagnosis of asthma | 8.5 | 4.3 | 4.2 | 3.5 |
| Follow-up | ||||
| Heart failure events | 16.2 | 9.3 | 8.3 | 6.3 |
| Follow-up duration (in years) | 14 (4.3) | 15.1 (3.4) | 15.1 (3.3) | 15.4 (3.0) |
| Heart failure rate/1000 person years | 11.5 | 6.1 | 5.5 | 4.1 |
Data are from the Atherosclerosis Risk in Communities (ARIC) study baseline examination (1987–89) in a subsample without prevalent HF and not missing information on important covariates, followed up to 31 December 2004.
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
aForced expiratory volume in 1 s measured using standardized spirometry at study baseline.
bExpressed as mean and standard deviation (SD) or percentages.
cChronic bronchitis was defined as productive cough for at least 3 months in two contiguous years.
dSelf-reported diagnosis of COPD is the presence of self-reported diagnosis of either bronchitis or emphysema.
All Cox models were adjusted for age, height, and squared height (height2), since use of percentage predicted lung function(s) can violate the assumptions of homogeneity of variance required to interpret the estimates correctly and has been discouraged, in favour of adjustment using height2 and height.14 The potentially strong confounding due to cigarette smoking was addressed by stratified analysis by smoking for each race and gender group. Further, models were adjusted for prevalent CHD and its traditional risk factors. As chronic inflammation may act as both a confounder and an intermediary for this association, a change in the magnitude of the estimates after additional adjustment for pro-inflammatory, pro-coagulability markers and obesity was evaluated with an understanding that either of the estimates may be biased and it requires multiple values of both exposure and confounder/intermediary to be able to adjust for only the confounding effect and not more. Statistical adjustment for an intervening variable or effect may result in bias towards the null; conversely, residual confounding may result in bias away from the null.15 Lastly, adjustment for maximum inspiratory pressure was done to adjust for overall decreased muscle strength.
Multivariable adjusted cumulative failure estimates were plotted by quartiles of FEV1, as the inverse of the survival function [1 – S (t + 0)], to yield the estimated cumulative proportion of those who developed heart failure at any time point t. To evaluate the potential influence of a misdiagnosis of incident HF on studied association, additional sensitivity analyses were carried out (i) by excluding those with baseline COPD, CHD, or both; and (ii) by censoring HF events with a concomitant discharge code for acute exacerbation of COPD, respiratory failure, or both. Results of the sensitivity analysis are reported in Supplementary material online, Table S1. Compliance with the proportional hazards assumption was examined for the main exposure using log–log curves, and no obvious violation was seen for FEV1 or FVC quartiles. SAS, version-9.1 and STATA-IC, version-10 software were used for analysis. A P < 0.05 for a two-sided test was considered statistically significant.
Results
The 25th, 50th, and 75th percentiles of FEV1 in litres were 1.9, 2.2, and 2.5 in black females, 2.6, 3.0, and 3.4 in black males, 2.2, 2.5, and 2.8 in white females, and 3.0, 3.5, and 3.9 in white males. Characteristics of all study participants by decreasing gender-specific quartiles of FEV1 are shown in Table 1. At study baseline, those in the lower quartiles of FEV1 had a higher mean age, cigarette-years of smoking, systolic blood pressure, and markers of inflammation, a lower height, and a higher proportion were current smokers, black, women, or had some respiratory disease.
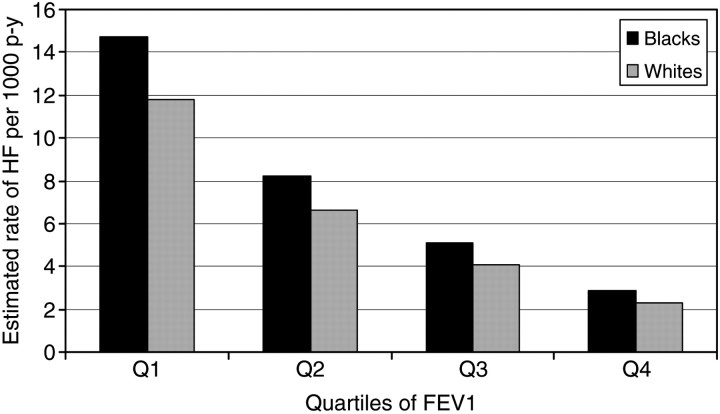
During an average follow-up of 14.9 years, incident HF was seen in 1369 (10%) of the study participants. A monotonic increase in the rate of incident HF with decreasing quartiles of FEV1 was seen in both blacks and whites, after adjusting for age and height (Figure 1). The age- and height-adjusted average incidence rates of HF per 1000 person years by quartiles of FEV1 (from the lowest to the highest quartile) were 14.7, 8.2, 5.1, and 2.9 for blacks, and 11.8, 6.6, 4.1, and 2.3 for whites.
Figure 1.
Estimated rate of new-onset heart failure (HF) per 1000 person years among black and white cohort members during an average study follow-up of 14.9 years by gender- and race-specific quartiles of forced expiratory volume in 1 s (FEV1). The estimated rates are adjusted for age, height, and height × height. The estimated rates shown are for an individual 55 years of age, and 5.5 feet in height. Data are from the Atherosclerosis Risk in Communities (ARIC) study baseline examination (1987–89) in a subsample without prevalent HF and not missing information on important covariates, followed up to 31 December 2004. p-y, person years.
Forced expiratory volume in 1 s and incident heart failure
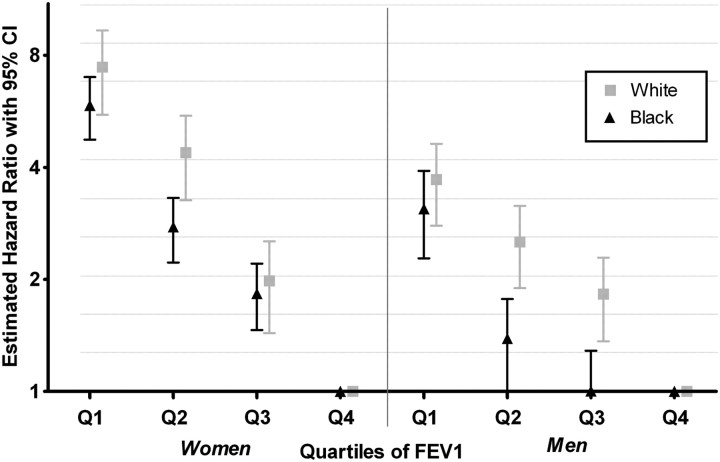
In Cox regression models adjusted for age, smoking, and height, the hazard ratios (HRs) of HF increased monotonically and inversely by quartiles of FEV1, for both genders in each race group (Figure 2). The age- and height-adjusted HRs and their 95% confidence interval (95% CIs) comparing those in the lowest quartile of FEV1 with those in the highest for HF were 3.48 (2.24–5.40) among black female, 2.88 (1.80–4.61) among black males, 6.95 (4.42–10.94) among white female, and 5.66 (4.04–7.91) among white males. The HRs comparing the highest with the lowest quartile were of greater magnitude in whites than in blacks, and in women than in men, and were statistically significant for all comparisons with the highest quartile except for the third quartile among black males. In similar models, HR estimates when comparing quartiles of FVC were slightly higher than for FEV1 (data not shown).
Figure 2.
Estimated hazard ratio [95% confidence intervals (CI)] of incident heart failure for the quartiles of forced expiratory volume in 1 s (FEV1) for each gender and race, adjusted for age, smoking, height, and height × height. The y-axis is plotted on a log scale with base 2. Data are from the Atherosclerosis Risk in Communities (ARIC) study baseline examination (1987–89) in a subsample without prevalent HF and not missing information on important covariates, followed up to 31 December 2004.
The above associations were observed across all smoking status categories. In analyses restricted to the never smokers group, the age-, height-, and height2-adjusted HF estimate of the HR was ∼2.5–3 times higher in the lowest quartile of FEV1 than in the highest (Table 2).
Table 2.
Hazard ratios of heart failure contrasting the lowest and highest quartiles of forced expiratory volume in 1 s by incremental level of covariate adjustments (N = 13 360)
| Gender | Smoking status/model | White participants (N = 10 293, n = 927) | Black participants (N = 3367, n = 442) | All participants (N = 13 360, n = 1369) |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Women | Currenta | 9.87 (3.01–32.34) | 3.56 (1.50–8.49) | 4.94 (2.50–9.76) |
| Formera | 4.35 (1.65–11.50) | 2.99 (1.13–7.80) | 4.27 (2.13–8.53) | |
| Noa | 3.88 (2.03–7.42) | 3.01 (1.63–5.56) | 4.01 (2.57–6.27) | |
| Model 1b | 4.80 (3.00–7.68) | 2.98 (1.91–4.66) | 4.12 (2.98–5.70) | |
| Model 2c | 3.91 (2.40–6.35) | 2.11 (1.33–3.34) | 2.91 (2.14–3.97) | |
| Model 3d | 3.61 (2.21–5.88) | 2.00 (1.25–3.19) | 2.44 (1.79–3.34) | |
| Model 4e | 3.44 (2.03–5.83) | 1.61 (0.95–2.72) | 2.19 (1.54–3.12) | |
| Men | Currenta | 2.51 (1.31,–4.81) | 2.71 (1.25–5.88) | 2.42 (1.48–3.97) |
| Formera | 6.72 (4.07–11.08) | 2.91 (1.23–6.88) | 5.60 (3.65–8.60) | |
| Noa | 3.04 (1.46–6.31) | 2.44 (0.94–6.33) | 2.94 (1.65–5.22) | |
| Model 1b | 4.11 (2.89–5.84) | 2.55 (1.57–4.13) | 3.51 (2.65–4.66) | |
| Model 2c | 3.03 (2.12–4.33) | 2.23 (1.37–3.59) | 2.23 (1.69–2.93) | |
| Model 3d | 2.98 (2.07–4.28) | 2.11 (1.30–2.41) | 1.92 (1.44–2.55) | |
| Model 4e | 2.68 (1.83–3.92) | 1.72 (0.96–3.07) | 1.80 (1.32–2.44) |
Data are from the Atherosclerosis Risk in Communities (ARIC) study baseline examination (1987–89) in a subsample without prevalent HF and not missing information on important covariates, followed up to 31 December 2004.
FEV1 quartiles were race and gender specific, and the models contained quartiles as indicator variables to estimate the effect size without assuming log-linearity in FEV1 quartiles. Estimates are reported only contrasting the lowest with the highest quartile of FEV1.
N, study sample size; n, incident heart failure cases; FEV1, forced expiratory volume in 1 s.
Each of the hazard ratios (HRs) and their 95% confidence interval (95% CIs) is from a separate model. For smoking categories, subset analysis was done by running separate models for each smoking stratum for each race and gender.
aHRs and 95% CI, adjusted for age, height × height2.
Further models with adjustment for incremental level of covariates were:
bModel 1: in addition to age, height, and height × height, this model additionally adjusts for current smoking status by using two binary variables: current smoking and former smoking, and cigarette-years of smoking.
cModel 2: in addition to model 1 covariates, this model adjusts for prevalent coronary heart disease, diabetes, hypertension, LDL-cholesterol, and HDL-cholesterol.
dModel 3: in addition to model 2 covariates, this model adjusts for fibrinogen levels, white blood count, von Willebrand's factor, serum albumin, and body mass index.
eModel 4: in addition to model 3, this model adjusts for maximum inspiratory pressure measured during visit 2 (3 years after baseline visit), with fewer observations (whites n = 9027, blacks n = 2820).
In multivariable models adjusted for age, height, prevalent CHD, and traditional cardiovascular risk factors (model 2), the HRs (95% CI) comparing the lowest with the highest quartiles of FEV1 were 2.91 (2.14–3.97) for women and 2.23 (1.69–2.93) for men (Table 2). On further adjustment for covariates including prevalent CHD and traditional cardiovascular risk factors, body mass index, white blood cell count, fibrinogen, von Willebrand's factor, and serum albumin (model 3) or heart rate, the magnitude of the HRs was attenuated slightly, although they remained statistically significant for all race and gender group (Table 2).
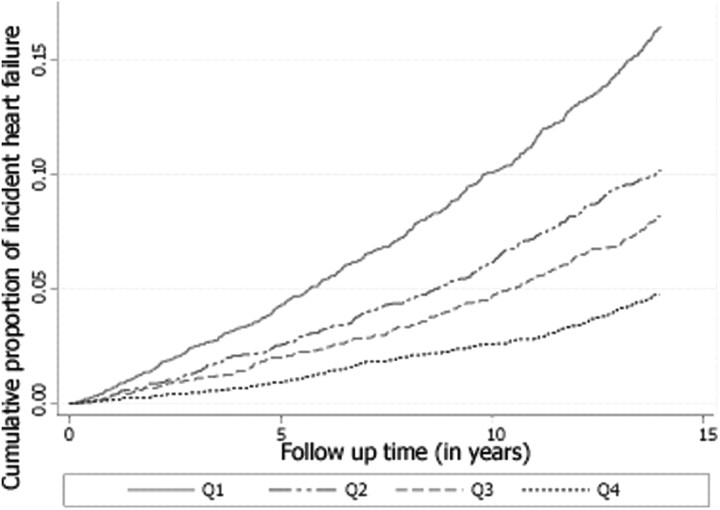
The multivariable adjusted cumulative risk of incident HF by quartiles of FEV1 is shown in Figure 3. A graded increase in the risk of HF for decreasing quartiles of FEV1 is seen throughout the study period.
Figure 3.
Multivariable adjusted cumulative-events estimates for incident heart failure by quartiles of forced expiratory volume in 1 s, i.e. Q1–Q4. Adjusted for age, gender, race, height, height × height, prevalent chronic heart disease, diabetes, hypertension, cigarette smoking status, cigarette-years of smoking, LDL-cholesterol, HDL-cholesterol, and body mass index. The curves are statistically not similar, using log rank test (P < 0.001). Data are from the Atherosclerosis Risk in Communities (ARIC) study baseline examination (1987–89) in a subsample without prevalent HF and not missing information on important covariates, followed up to 31 December 2004.
Airflow obstruction and incident heart failure
In multivariable adjusted models, the HRs for HF comparing those with FEV1/FVC <70% with those with FEV1/FVC ≥70% were 1.44 (1.20–1.74) and 1.40 (1.13–1.72) for women and men, respectively (Table 3). Of note, moderately severe airflow obstruction as defined by spirometry was associated with incident HF but not mild airflow obstruction. Self-reported COPD was similar to spirometry-defined COPD in its association with incident HF. For example, the HR (95% CI) comparing those with a self-reported diagnosis of COPD with those without was 1.84 (1.47–2.31) for women and 1.44 (1.08–1.91) for men.
Table 3.
Hazard ratios of heart failure for airway obstruction, and self-reported diagnosis of respiratory disease (N = 13 130)
| Gender | Model 1a | Model 2b | |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | ||
| Men | COPD vs. no COPD by spirometry | 1.61 (1.36–1.91) | 1.44 (1.20–1.74) |
| Mild COPD vs. no COPD by spirometry | 0.90 (0.69–1.18) | 0.98 (0.74–1.29) | |
| Moderate/severe COPD vs. no COPD by spirometry | 2.30 (1.90–2.77) | 1.83 (1.49–2.26) | |
| Chronic bronchitisc | 2.07 (1.60–2.69) | 1.42 (1.03–1.98) | |
| Self-reported diagnosis of bronchitis | 1.65 (1.20–2.27) | 1.44 (1.04–2.00) | |
| Self-reported diagnosis of emphysema | 2.22 (1.51–3.25) | 1.85 (1.23–2.77) | |
| Self-reported diagnosis of COPDd | 1.70 (1.30–2.20) | 1.44 (1.08–1.91) | |
| Self-reported diagnosis of asthma | 1.06 (0.74–1.51) | 1.15 (0.80–1.64) | |
| Women | COPD vs. no COPD by spirometry | 1.40 (1.15–1.71) | 1.40 (1.13–1.72) |
| Mild COPD vs. no COPD by spirometry | 0.90 (0.67–1.20) | 1.07 (0.79–1.45) | |
| Moderate/severe COPD vs. no COPD by spirometry | 2.09 (1.65–2.65) | 1.73 (1.34–2.22) | |
| Chronic bronchitisc | 2.11 (1.54–2.99) | 1.74 (1.34–2.19) | |
| Self-reported diagnosis of bronchitis | 2.27 (1.83–2.82) | 1.84 (1.84–5.28) | |
| Self-reported diagnosis of emphysema | 4.41 (2.72–7.16) | 3.12 (2.11–5.93) | |
| Self-reported diagnosis of COPDd | 2.40 (1.94–2.96) | 1.84 (1.47–2.31) | |
| Self-reported diagnosis of asthma | 1.60 (1.17–2.17) | 1.56 (1.14–2.13) |
Data are from the Atherosclerosis Risk in Communities (ARIC) study baseline examination (1987–89) in a subsample without prevalent HF and not missing information on important covariates, followed up to 31 December 2004.
Using spirometry, any COPD was defined as FEV1/FVC <70%, mild COPD was defined as FEV1/FVC <70% and FEV ≥80% (predicted), moderate/severe COPD was defined as FEV1/FVC <70% and FEV1 <80% (predicted), and no COPD as FEV1/FVC ≥70%.
CI, confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HR, hazard ratio.
aModel 1 is adjusted for age and race.
bModel 2 is adjusted for age, smoking status, cigarette-years of smoking, hypertension, diabetes, LDL-cholesterol, HDL-cholesterol, and body mass index.
cChronic bronchitis was defined as productive cough for at least 3 months in two contiguous years.
dSelf-reported diagnosis of COPD is the presence of self-reported diagnosis of either bronchitis or emphysema.
On testing the associations of several definitions of respiratory disease with incident HF, a self-reported physician diagnosis of emphysema had the strongest association with incident HF in both men and women, whereas self-reported physician diagnosis of asthma had a weak association in women, and no association in men (Table 3).
Discussion
We observed a strong inverse association between baseline lung function and incident HF, and a monotonic, direct association between airflow obstruction and incident HF. These associations were seen across groups defined by race, gender, and smoking status. Notably, the relationship was also seen in never smokers and persisted after adjustment for smoking status and cigarette-years of smoking, indicating that our results are not primarily confounded by smoking.
Low forced expiratory volume in 1 s and incident heart failure
These results in a population-based cohort are consistent with clinical observations, but also with four previous reports from population-based cohorts, indicating that a low FEV1 was strongly predictive of HF, independent of many traditional and non-traditional risk markers.9–11,16 A recently published study reported an ∼25% increased risk of HF per standard decrease in FEV1 or FVC; however, the strength of the association was much lower when comparing quartile 1 with quartile 4 of FEV1, suggesting that CHD may be the primary driver of this association in this cohort of younger men. Similarly, among older adults who were relatively healthy, a strong association of 21% higher odds per 10% decrease in predicted FEV1 was reported.10 The present study extends these results by the length and completeness of its follow-up, with replication in African Americans, women, and never smokers.
Airflow obstruction and incident heart failure
We also found a consistent association of incident HF with interviewer-ascertained chronic bronchitis, self-reported COPD, self-reported emphysema, and spirometry-defined airflow obstruction (FEV1/FVC <70%), but not with self-reported asthma. Our results are similar to those of a large retrospective case–control study based on claims data, where COPD was associated with an almost three-fold higher rate of HF hospitalization,17 although the magnitude of the reported association was considerably bigger than seen in our study. Although no association was seen between low FEV1/FVC (<60% vs. higher) and HF in the original Framingham cohort,18 perhaps owing to low power, recent studies have reported associations of a magnitude similar to the present study despite many differences in the age, gender, race, and co-morbidities between the cohorts, as well as different definitions of airflow obstruction.9–11
Potential confounders/intermediaries
The associations quantified as HRs were of greater magnitude in women and in whites than in men and African Americans, respectively. The lower absolute risk of HF in women and in whites is one explanation (thus similar risk differences), as well as a likely higher frequency of diastolic HF in these groups than in African Americans.
Several traditional cardiovascular risk factors are associated with both low lung function and HF, and thus could confound the associations reported here. Both FVC and FEV1 have been shown to have an inverse association with blood glucose levels, blood pressure, serum cholesterol, body mass index, and left ventricular mass.19,20 Our estimates were somewhat attenuated after adjustment for these putative confounding variables, but they remained statistically significant (Tables 2 and 3). Kannel et al. suggested that decreased muscle strength may partially account for the inverse association of FEV1 with HF.18 Statistical adjustment for maximum inspiratory pressure, a measure of respiratory muscle strength, indeed attenuated the magnitude of our results.
It has been proposed that lower lung volumes may be seen in HF patients with large heart size due to lung impingement.21 This is unlikely to be common in a population sample of middle-aged individuals without HF at study baseline; moreover, statistical adjustment for left ventricular mass estimated by the Cornell voltage did not change the magnitude of our estimates. Overweight and obesity were also considered as possible confounders. However, no clear patterns of association between ponderosity and lung function or respiratory disease were seen in these data, and statistical adjustment for body mass index did not change our results appreciably.
Levels of systemic markers of inflammation are higher in those with low lung volumes, and links between inflammation and HF have been proposed. These high levels may reflect either a spill of inflammatory markers from inflammation localized to the lungs or an upstream up-regulation of the inflammatory cascade.22 We observed some attenuation of the HR estimates in our study after adjustment for baseline levels of inflammatory/haemostatic markers, which lends some support to the inflammation–HF hypothesis. However, it is far from established whether inflammation increases the risk of HF beyond its association with CHD. Exercise training appears to improve physical function in both COPD and HF—primarily by improving skeletal muscle function—but possibly by reducing inflammation as well.23 Whether exercise can prevent HF in COPD patients needs evaluation.
Some of the traits mentioned above as covariates and potential intermediaries may be associated with HF directly or indirectly through their association with CHD. For instance, low FVC is associated with subclinical atherosclerosis24 and incident ischaemic heart disease.5 Empirically, excluding those with prevalent CHD at baseline did not visibly change the results (Supplementary material online, Table S1).
Other potential mechanisms
An increase in pulmonary vascular resistance and pulmonary artery pressure, and lower pulmonary vessel endothelial function and endothelial proliferation in pulmonary vasculature is seen with alveolar hypoxia and in individuals with even mild/moderate COPD.22,25 It seems reasonable to think that such changes may manifest in early right ventricular structural26 and functional abnormalities that could ultimately lead to a deterioration of left ventricular function and clinical HF due to interventricular dependence.27 Further, animal studies show increased right-sided heart injury with increased end-expiratory volume as seen in acute exacerbation of COPD.28 However, this has been a debatable issue as many studies have not seen differences in right ventricular structure or function with mild COPD.29 Several other mechanisms such as systemic inflammation, autonomic dysfunction, oxidative stress, and chronic muscle wasting have been implicated to result in new-onset HF in individuals with COPD.
Strengths/limitations/future research
The strengths of our findings include their prospective and population-based nature, their internal consistency—including among non-smokers—the sizeable magnitude of HR estimates even after multivariable adjustment, and the imperviousness of the results to various sensitivity analyses. Several limitations of this study should also be mentioned. The most important among them is the lack of validation and detailed characterization of the HF events. Further, respiratory symptoms and diagnoses were self-reported and not validated. Perhaps less worrisome, smoking status was self-reported and not validated with biomarkers, thus some residual confounding due to smoking could not be ruled out, and neither post-bronchodilator lung function nor total lung volume were measured. Due to use of pre-bronchodilator response, the specificity of diagnosis of COPD should be lower, and this, if anything, will dilute the effect estimate towards null. This is also clear as the estimate for moderate COPD is higher than for mild COPD. We also examined the potential confounding bias introduced by heart rate, and the use of beta-blockers, adrenergic medication use, steroid intake, and xanthine use, but did not see evidence of appreciable confounding (data not shown).
Misclassification of hospitalized COPD exacerbations as HF is conceivable given the many overlapping signs and symptoms,30 and that the HF events in this study were not validated by chart review. An ICD code 428.x has a positive predictive value of 94.3% when compared with Framingham criteria,31 and 83.0% compared with a physician diagnosis and pulmonary oedema on chest X-ray.32 To evaluate whether our findings may be a result of misclassification of incident HF, sensitivity analyses were conducted as stated in the Methods. The estimated associations did not change appreciably following the exclusion of COPD at baseline, or by censoring those with concurrent COPD and HF (Supplementary material online, Table S1). Lastly, a decreased lung function may contribute to early exacerbation of asymptomatic ventricular dysfunction and possibly to lead-time bias. Given the strength of the estimates observed and the almost constant HR estimates over time, these biases, if present, are unlikely to have an effect that would alter the inferences presented here.
Studies are needed that can address the associations reported here with further characterization of HF into systolic and diastolic phenotypes. Whether improvement (or a slower decline) in lung function with smoking cessation,33 increased physical activity,34 or other novel interventions is related to future risk of HF similarly need attention. In a population-based study, computed tomography (CT) scan-defined emphysema and spirometry-defined airflow obstruction were linearly associated with impaired ventricular filling and reduced cardiac output, but not with ejection fraction.8 A recently reported huge burden on unrecognized HF in patients with COPD35 further suggests that more attention to HF in individuals with COPD is warranted. An enhanced understanding of the pathophysiological changes in heart structure and function in the context of COPD would be similarly important.
In conclusion, in this large population-based closed cohort study with long-term follow-up, we observe that low FEV1, self-reported COPD, and airway obstruction are associated with incident HF.
Supplementary material
Supplementary material is available at European Journal of Heart Failure online.
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding
The National Heart, Lung, and Blood Institute [contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022 for the Atherosclerosis Risk in Communities study; HL077612 (R.G.B.)].
Conflict of interest: none declared.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 3.Rutten FH, Cramer MJ, Lammers JW, Grobbee DE, Hoes AW. Heart failure and chronic obstructive pulmonary disease: an ignored combination? Eur J Heart Fail. 2006;8:706–711. doi: 10.1016/j.ejheart.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 5.Yilmaz R, Gencer M, Ceylan E, Demirbag R. Impact of chronic obstructive pulmonary disease with pulmonary hypertension on both left ventricular systolic and diastolic performance. J Am Soc Echocardiogr. 2005;18:873–881. doi: 10.1016/j.echo.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Funk GC, Lang I, Schenk P, Valipour A, Hartl S, Burghuber OC. Left ventricular diastolic dysfunction in patients with COPD in the presence and absence of elevated pulmonary arterial pressure. Chest. 2008;133:1354–1359. doi: 10.1378/chest.07-2685. [DOI] [PubMed] [Google Scholar]
- 7.Sabit R, Bolton C, Edwards J, Edwards P, Cockcroft J, Fraser A, Shale D. Sub-clinical bi-ventricular dysfunction in chronic obstructive pulmonary disease (COPD) Thorax. 2007;62(Supplement 3):142. [Google Scholar]
- 8.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal RA, Lima JA, Shahar E, Smith LJ, Watson KE. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engstrom G, Melander O, Hedblad B. Population-based study of lung function and incidence of heart failure hospitalisations. Thorax. 2010;65:633–638. doi: 10.1136/thx.2010.135392. [DOI] [PubMed] [Google Scholar]
- 10.Georgiopoulou VV, Kalogeropoulos AP, Psaty BM, Rodondi N, Bauer DC, Butler AB, Koster A, Smith AL, Harris TB, Newman AB, Kritchevsky SB, Butler J. Lung function and risk for heart failure among older adults: the Health ABC Study. Am J Med. 2011;124:334–341. doi: 10.1016/j.amjmed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam CS, Lyass A, Kraigher-Krainer E, Massaro JM, Lee DS, Ho JE, Levy D, Redfield MM, Pieske BM, Benjamin EJ, Vasan RS. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011;124:24–30. doi: 10.1161/CIRCULATIONAHA.110.979203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Standardization of spirometry—1987 update. Statement of the American Thoracic Society. Am Rev Respir Dis. 1987;136:1285–1298. doi: 10.1164/ajrccm/136.5.1285. [DOI] [PubMed] [Google Scholar]
- 13.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 14.Vollmer WM, Johnson LR, McCamant LE, Buist AS. Methodologic issues in the analysis of lung function data. J Chronic Dis. 1987;40:1013–1023. doi: 10.1016/0021-9681(87)90115-9. [DOI] [PubMed] [Google Scholar]
- 15.Robins J. The control of confounding by intermediate variables. Stat Med. 1989;8:679–701. doi: 10.1002/sim.4780080608. [DOI] [PubMed] [Google Scholar]
- 16.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 17.Sidney S, Sorel M, Quesenberry CP, Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest. 2005;128:2068–2075. doi: 10.1378/chest.128.4.2068. [DOI] [PubMed] [Google Scholar]
- 18.Kannel WB, Seidman JM, Fercho W, Castelli WP. Vital capacity and congestive heart failure. The Framingham study. Circulation. 1974;49:1160–1166. doi: 10.1161/01.cir.49.6.1160. [DOI] [PubMed] [Google Scholar]
- 19.Kannel WB, Hubert H, Lew EA. Vital capacity as a predictor of cardiovascular disease: the Framingham study. Am Heart J. 1983;105:311–315. doi: 10.1016/0002-8703(83)90532-x. [DOI] [PubMed] [Google Scholar]
- 20.Enright PL, Kronmal RA, Smith VE, Gardin JM, Schenker MB, Manolio TA. Reduced vital capacity in elderly persons with hypertension, coronary heart disease, or left ventricular hypertrophy. The Cardiovascular Health Study. Chest. 1995;107:28–35. doi: 10.1378/chest.107.1.28. [DOI] [PubMed] [Google Scholar]
- 21.Olson TP, Beck KC, Johnson JB, Johnson BD. Competition for intrathoracic space reduces lung capacity in patients with chronic heart failure: a radiographic study. Chest. 2006;130:164–171. doi: 10.1378/chest.130.1.164. [DOI] [PubMed] [Google Scholar]
- 22.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 23.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 24.Schroeder EB, Welch VL, Evans GW, Heiss G. Impaired lung function and subclinical atherosclerosis. The ARIC Study. Atherosclerosis. 2005;180:367–373. doi: 10.1016/j.atherosclerosis.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Han MK, McLaughlin VV, Criner GJ, Martinez FJ. Pulmonary diseases and the heart. Circulation. 2007;116:2992–3005. doi: 10.1161/CIRCULATIONAHA.106.685206. [DOI] [PubMed] [Google Scholar]
- 26.Vonk-Noordegraaf A, Marcus JT, Holverda S, Roseboom B, Postmus PE. Early changes of cardiac structure and function in COPD patients with mild hypoxemia. Chest. 2005;127:1898–903. doi: 10.1378/chest.127.6.1898. [DOI] [PubMed] [Google Scholar]
- 27.Vonk Noordegraaf A, Marcus JT, Roseboom B, Postmus PE, Faes TJ, de Vries PM. The effect of right ventricular hypertrophy on left ventricular ejection fraction in pulmonary emphysema. Chest. 1997;112:640–645. doi: 10.1378/chest.112.3.640. [DOI] [PubMed] [Google Scholar]
- 28.Simpson JA, Brunt KR, Collier CP, Iscoe S. Hyperinflation-induced cardiorespiratory failure in rats. J Appl Physiol. 2009;107:275–282. doi: 10.1152/japplphysiol.91342.2008. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Roisin R, MacNee W. Pathophysiology of chronic obstructive pulmonary disease. Eur Respir Monogr. 2006;38:177. [Google Scholar]
- 30.Hawkins NM, Petrie MC, Jhund PS, Chalmers GW, Dunn FG, McMurray JJ. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail. 2009;11:130–139. doi: 10.1093/eurjhf/hfn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee DS, Donovan L, Austin PC, Gong Y, Liu PP, Rouleau JL, Tu JV. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43:182–188. doi: 10.1097/00005650-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Goff DC, Jr, Pandey DK, Chan FA, Ortiz C, Nichaman MZ. Congestive heart failure in the United States: is there more than meets the I(CD code)? The Corpus Christi Heart Project. Arch Intern Med. 2000;160:197–202. doi: 10.1001/archinte.160.2.197. [DOI] [PubMed] [Google Scholar]
- 33.Scanlon PD, Connett JE, Waller LA, Altose MD, Bailey WC, Buist AS. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med. 2000;161:381–390. doi: 10.1164/ajrccm.161.2.9901044. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007;175:458–463. doi: 10.1164/rccm.200607-896OC. [DOI] [PubMed] [Google Scholar]
- 35.Rutten FH, Cramer MJ, Grobbee DE, Sachs AP, Kirkels JH, Lammers JW, Hoes AW. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J. 2005;26:1887–1894. doi: 10.1093/eurheartj/ehi291. [DOI] [PubMed] [Google Scholar]