Abstract
Few studies have addressed changes in physical activity participation over time among the elderly. The authors hypothesized that there were distinct trajectories of physical activity level over time and identifiable predictors of such trajectories, as well as that the maintenance of regular physical activity, even below recommended levels, was associated with lower mortality risk. Using longitudinal data (1994–2009) from 433 initially high-functioning older women aged 70–79 years at baseline, a joint latent class and survival mixture model identified 4 activity trajectory classes: always active (16.6%), fast declining (19.2%), stable moderate (32.3%), and always sedentary (31.9%). Obesity, coronary artery disease, chronic obstructive pulmonary disease, depressive symptoms, low self-efficacy, mobility disability, and low energy were associated with sedentary behavior and/or a fast decline in activity. Women in the fast declining and always sedentary classes had hazard ratios for death of 2.34 (95% confidence interval: 1.20, 4.59) and 3.34 (95% confidence interval: 1.72, 6.47), respectively, compared with the always active class; no mortality difference was found between the stable moderate and always active groups (hazard ratio = 1.24, 95% confidence interval: 0.63, 2.47). Our findings suggest that physical activity does not have to be vigorous to be beneficial and that the gain may be the greatest among women who reported the lowest levels of activity.
Keywords: aging, exercise, healthy people programs, lifestyle, motor activity, risk factors, survival, walking
There is compelling evidence of the benefits of physical activity for older adults. Regular exercise and leisure-time physical activities are consistently associated with antiinflammatory effects (1), regulation of anabolic hormones (2), and protection from cognitive impairment (3–5), geriatric syndromes (e.g., falls, frailty) (6, 7), decline in physical functioning (8–10), and certain diseases (11). However, despite the wide-ranging health benefits of physical activity, the majority of older US adults are inactive; only 16% of individuals aged 65–74 years report participating in the recommended 30 minutes or more of moderate physical activity on 5 or more days per week (12).
Few studies have addressed long-term changes in physical activity participation over time in older adults (13, 14). A cross-sectional measurement is limited in capturing an individual's true average activity over a given interval. We hypothesized that the maintenance of regular physical activity over time, even if below the recommended level, is a marker of greater physiologic reserve and status and therefore also should be associated with a lower risk of mortality. To test this hypothesis, we used longitudinal data from the Women's Health and Aging Study (WHAS) II to 1) characterize patterns of change in physical activity level over time in a representative sample of initially high-functioning older women, 2) assess the associations between patterns of change in physical activity level and all-cause mortality, and 3) identify predictors of change in physical activity.
MATERIALS AND METHODS
Study population
WHAS II is a prospective cohort study of 436 women aged 70–79 years who are representative of the two-thirds highest functioning women living in the community. Study eligibility criteria and recruitment are reported elsewhere (15). Interviews and physical examinations were conducted at baseline and at 6 follow-up visits (approximately 18 months apart except for the interval between the third and the fourth visit, which was, on average, 3 years). A median follow-up time of 12 years resulted between 1994 and 2009. The study was approved by the Johns Hopkins University Institutional Review Board. The analytic sample consisted of 433 women for whom we had data on physical activity levels at baseline. In longitudinal analyses, 95% and 67% of the 433 women contributed 2 or more and 5 or more measurements of physical activity, respectively, before study dropout or death.
Measures of physical activity
Physical activity level was assessed using a shortened version of the Minnesota Leisure Time Activities Questionnaire (16), which includes 4 exercise activities (walking for exercise, dancing, bowling and exercise (e.g., strengthening activities)) and 2 lifestyle activities (strenuous household (e.g., scrubbing) and outdoor (e.g., gardening) chores). Level of participation in each activity was assessed by self-reported frequency and duration of participation during the past 2 weeks.
We compared 3 categorical measures of physical activity by using both published and population-based cutoffs. First, we used the time-based criteria established by the Centers for Disease Control and Prevention and the American College of Sports Medicine: inactive (0 minute/week), moderately active (>0 to <150 minutes/week), and very active (≥150 minutes/week) (termed CDC/ACSM criteria). Second, as in the Health, Aging, and Body Composition Study (7), we classified a woman as inactive if she expended fewer than 1,000 kcal/week in exercise activity and 0 kcal/week in lifestyle activity, as lifestyle active if she expended more than 0 kcal/week in lifestyle activity and less than 1,000 kcal/week in exercise activity, and as exercise active if she expended 1,000 kcal/week or more in exercise activity (termed HABC criteria). Third, we calculated kilocalorie expenditure per kilogram of body weight per day (kcal/day/kg) over a 2-week period by multiplying activity-specific metabolic equivalents of task value by the average number of minutes per day spent in the activity and summed across the 6 activities. Using the cutoffs defined by the National Health Interview Survey (17), we then classified activity level into 3 categories: inactive (<1.5 kcal/day/kg), moderately active (≥1.5 and <3 kcal/day/kg), and very active (≥3 kcal/day/kg) (termed NHIS criteria).
Total mortality
Data on all-cause mortality were obtained through follow-up interviews with proxies, obituaries, and matching with the National Death Index, with the most recent update completed on January 28, 2009. Thirty-four percent of the 433 women (n = 149) died during the follow-up period, with an incidence rate of 31 per 1,000 person-years.
Covariates
Covariates included age, race classified as white versus nonwhite, years of education by self-report, and body mass index (weight (kg)/height (m)2) categorized as underweight (<18.5), normal weight (18.5–24.9), overweight (25–29.9), or obese (≥30). Self-report of the presence or absence of 14 major chronic diseases at baseline was adjudicated by physicians based on predefined criteria (18) (Table 1). The number of “definite” diseases out of 14 was used as a measure of disease burden. Smoking status was classified based on self-report as current, former, or never smoker. Depressive symptoms were assessed using the 30-item Geriatric Depression Scale (19). Mobility disability was defined as any self-reported difficulty in 1 or more of the following 4 tasks: walking 2–3 blocks, climbing 10 steps, getting in or out of bed/chairs, or doing heavy housework. A participant was considered to be living alone if the household size was 1. Self-efficacy was ascertained by asking, “Do you, in general, feel helpless?” with possible responses of strongly disagree, disagree, and agree to strongly agree, corresponding to high, medium, low self-efficacy, respectively. Energy level was rated by participants on a Likert scale from 0 to 10, where 0 denoted no energy and 10 denoted “the most energy that you have ever had.”
Table 1.
Summary of Baseline Characteristics by Physical Activity Trajectory Profiles Derived From the Joint Latent Class and Survival Mixture Analysis, Women's Health and Aging Study II, 1994–2009
| Characteristic | Physical Activity Trajectory Profilea |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Sample (n = 433)b |
Always Active (n = 72, 16.6%) |
Stable Moderate (n = 140, 32.3%) |
Fast Declining (n = 83, 19.2%) |
Always Sedentary (n = 138, 31.9%) |
||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Age, yearsc | 73.9 (2.8) | 74.1 (3.1) | 73.3 (2.7) | 74.3 (2.6) | 74.2 (2.8) | |||||
| Race | ||||||||||
| White | 352 | 81.3 | 64 | 88.9 | 108 | 77.1 | 70 | 84.3 | 110 | 79.7 |
| Black | 80 | 18.7 | 7 | 9.7 | 32 | 22.9 | 13 | 15.7 | 28 | 20.3 |
| Other | 1 | 0.2 | 1 | 1.4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Years of educationc | 12.5 (3.3) | 13.2 (3.2) | 12.5 (3.1) | 12.0 (3.6) | 12.5 (3.4) | |||||
| Living alone | 222 | 51.3 | 38 | 52.8 | 61 | 43.6 | 48 | 57.8 | ||
| Body mass indexd,e | ||||||||||
| <18.5 | 14 | 3.2 | 5 | 6.9 | 3 | 2.1 | 2 | 2.4 | 4 | 2.9 |
| 18.5–24.9 | 154 | 35.6 | 32 | 44.4 | 48 | 34.3 | 27 | 32.5 | 47 | 34.1 |
| 25–29.9 | 164 | 37.9 | 28 | 38.9 | 54 | 38.6 | 40 | 48.2 | 42 | 30.4 |
| ≥30 | 99 | 22.9 | 7 | 9.7 | 34 | 24.3 | 14 | 16.9 | 44 | 31.9 |
| Self-reported health | ||||||||||
| Excellent | 69 | 15.9 | 14 | 19.4 | 29 | 20.7 | 9 | 10.8 | 17 | 12.3 |
| Very good | 145 | 33.5 | 31 | 43.1 | 50 | 35.7 | 33 | 39.8 | 31 | 22.5 |
| Good | 173 | 40.0 | 25 | 34.7 | 46 | 32.9 | 32 | 38.6 | 70 | 50.7 |
| Fair/poor | 46 | 10.7 | 2 | 2.8 | 15 | 10.7 | 9 | 10.8 | 20 | 14.5 |
| No. of diseasesc,f | 1.6 (1.0) | 1.3 (0.9) | 1.5 (1.0) | 1.5 (1.0) | 1.8 (1.1) | |||||
| Coronary artery disease | 66 | 15.2 | 4 | 5.6 | 20 | 14.3 | 10 | 12.1 | 32 | 23.2 |
| Chronic obstructive pulmonary disease | 100 | 23.1 | 9 | 12.5 | 28 | 20.0 | 23 | 27.7 | 40 | 29.0 |
| Diabetes | 33 | 7.6 | 5 | 6.9 | 10 | 7.1 | 3 | 3.6 | 15 | 10.9 |
| Osteoarthritis | 288 | 66.5 | 48 | 66.7 | 89 | 63.6 | 56 | 67.5 | 95 | 68.8 |
| Cancer | 36 | 8.3 | 7 | 9.7 | 9 | 6.4 | 7 | 8.4 | 13 | 9.4 |
| Physical activityg | ||||||||||
| Inactive | 185 | 42.7 | 11 | 15.3 | 43 | 30.7 | 22 | 26.5 | 109 | 79.0 |
| Moderately active | 99 | 22.9 | 17 | 23.6 | 61 | 43.6 | 5 | 6.0 | 16 | 12.0 |
| Active | 149 | 34.4 | 44 | 61.1 | 36 | 25.7 | 56 | 67.5 | 13 | 9.4 |
| Types of physical activity | ||||||||||
| Walk for exercise | 243 | 56.1 | 54 | 75.0 | 78 | 55.7 | 49 | 59.0 | 62 | 44.9 |
| Exercise | 85 | 19.6 | 23 | 31.9 | 31 | 22.1 | 19 | 22.9 | 12 | 8.7 |
| Dancing | 64 | 14.8 | 21 | 29.2 | 21 | 15.0 | 12 | 14.5 | 10 | 7.2 |
| Bowling | 24 | 5.5 | 9 | 12.5 | 6 | 4.3 | 9 | 10.8 | 0 | 0 |
| Strenuous household chores | 305 | 70.4 | 53 | 73.6 | 114 | 81.4 | 69 | 83.1 | 69 | 50.0 |
| Strenuous outdoor chores | 127 | 29.3 | 31 | 43.1 | 41 | 29.3 | 29 | 34.9 | 26 | 18.8 |
| Smoking status | ||||||||||
| Never smoker | 237 | 54.7 | 43 | 59.7 | 79 | 56.4 | 48 | 57.8 | 67 | 48.6 |
| Former smoker | 152 | 35.1 | 22 | 30.6 | 46 | 32.9 | 30 | 36.1 | 54 | 39.1 |
| Current smoker | 44 | 10.2 | 7 | 9.7 | 15 | 10.7 | 5 | 6.0 | 17 | 12.3 |
| Geriatric Depression Scale scoreh | 3 (1–6) | 2 (1–3) | 3 (1–5) | 3 (1–5.5) | 4 (2–7) | |||||
| Geriatric Depression Scale score ≥10 | 36 | 8.3 | 0 | 0 | 14 | 10.0 | 5 | 6.0 | 17 | 12.3 |
| Mobility disability | 133 | 30.7 | 13 | 18.1 | 39 | 27.9 | 17 | 20.5 | 64 | 46.4 |
| Self-efficacy | ||||||||||
| High | 262 | 60.5 | 53 | 73.6 | 87 | 62.1 | 48 | 57.8 | 74 | 53.6 |
| Medium | 119 | 27.5 | 14 | 19.4 | 40 | 28.6 | 21 | 25.3 | 44 | 31.9 |
| Low | 52 | 12.0 | 5 | 6.9 | 13 | 9.3 | 14 | 16.9 | 20 | 14.5 |
| Energy leveli | 8 (7–9) | 8.5 (7–10) | 8 (7–9) | 8 (7–9) | 7 (6–8) | |||||
a Estimated from the joint latent class and survival model.
b Three of the 436 women were missing data on activity measures and were therefore excluded from the analysis.
c Values are mean (standard deviation).
d Percentages may not add up to 100 because of missing data.
e Weight (kg)/height (m)2.
f Presence of definite diseases, including angina pectoris/myocardial infarction, congestive heart failure, peripheral artery disease, hip fractures, osteoarthritis of the hip, knee and/or hand, Parkinson's disease, rheumatoid arthritis, osteoporosis, stroke, pulmonary diseases, diabetes mellitus, cancer, spinal stenosis, and disc disease.
g Based on the National Health Interview Survey criteria.
hValues are median (interquartile range).
Data analysis
We began by comparing prevalence estimates of activity status by study visit and by classification system (i.e., NHIS, HABC, and CDC/ACSM). We used a Cox proportional hazards model to analyze the association between baseline physical activity level and all-cause mortality among women who reported any activity at baseline (n = 401) after adjustment for age, race, and educational level. We modeled the baseline activity level as a continuous variable in kcal/day/kg with both a linear and quadratic term to account for nonlinear trend.
To determine which of the 3 classification systems for activity status was most appropriate for capturing meaningful changes in activity level over time while taking into account measurement error due to self-report, we first analyzed transitions between different physical activity states using stationary and first-order Markov models for each classification system (20, 21). Death was treated as an absorbing state, with zero probability of all other activity states after death has occurred. From this model, we estimated for each classification system a matrix of conditional probabilities of transiting from state j (e.g., moderately active) at time t − 1 to state k (e.g., inactive) at time t and then used this transition matrix to calculate the Shannon conditional entropy (22, 23), which is a measure of uncertainty of an outcome at time t given the value of the same outcome at time t − 1. A larger value of conditional entropy for a transition matrix indicates greater overall unpredictability in changes in activity status over time.
We identified patterns of change in physical activity over time using a latent class analysis of the within-subject repeated measurements of activity status. The latent class analysis hypothesizes the existence of subpopulations of older women with distinct patterns of activity trajectories. The analysis then aims to determine the number of subpopulations (“trajectory classes”) and estimate for each subpopulation its prevalence in the overall population and the proportions who were inactive, moderately active, and very active at each visit within each trajectory class. We identified 4 trajectory classes based on Lo-Mendell-Rubin adjusted likelihood ratio test (24) and scientific plausibility and meaningfulness of the resulting trajectory patterns.
To account for missing data, the latent class analysis model was fit using the full information maximum likelihood estimator that is unbiased under the assumption of data missing at random (25). However, missing data on physical activity level due to death is arguably informative rather than missing at random, so to address this, we applied a mixture survival model (26) that combines the latent class analysis of the physical activity trajectories and the time-to-event analysis of all-cause mortality in relation to the trajectory classes. The baseline hazard of mortality was modeled as a nonparametric step function varying by trajectory classes.
After fitting the mixture survival model, we estimated the person-specific probabilities of being in each of the classes given their observed profile of activity status over time and then assigned each subject to the class with the highest probability. Next, we used Cox proportional hazards model to assess the associations between the activity trajectory classes and all-cause mortality after adjusting for baseline age, race, and educational level. Finally, we identified predictors of trajectory classes by regressing an individual's class membership against baseline demographic and health characteristics separately and jointly using multinomial logistic models. MPLUS, version 6 (Muthén & Muthén, Los Angeles, California) and Stata, version 9.2 (StataCorp LP, College Station, Texas) were used for model fitting.
RESULTS
Table 1 summarizes the demographic and health characteristics of the study sample at baseline. Of the 433 women included in this study, 19% were nonwhite, 61% were either overweight or obese, 31% reported mobility disability, and 8% reported 10 or more depressive symptoms. On average, the cohort was 74 years of age and had 12.5 years of education and 1.6 chronic diseases.
Physical activity participation at baseline
At baseline, 60% of the women met the CDC/ACSM recommended level of moderate intensity physical activity of 150 minutes/week. They engaged in various activities: 56% of women reported walking for exercise, 70% reported performing strenuous household chores, 29% reported performing strenuous outdoor chores, and 20% reported performing regular exercises. Dancing (15%) and bowling (6%) were the least frequent activities.
The proportions of women classified as inactive, moderately active, and very active varied substantially across the 3 different classification systems. For example, 42.7% of women were inactive by the NHIS criteria, whereas only 20.2% and 7.4% were inactive by the HABC and CDC/ACSM criteria, respectively. The estimates of conditional entropy were 1.65, 1.11, and 0.90 for the CDC/ACSM, HABC, and NHIS criteria, respectively. We therefore selected the measure with the smallest entropy, that is, the NHIS criteria, for the longitudinal analysis. By the NHIS criteria, 34%, 23%, and 43% were classified as being active, moderately active, and inactive at baseline, respectively.
Baseline level of physical activity and all-cause mortality
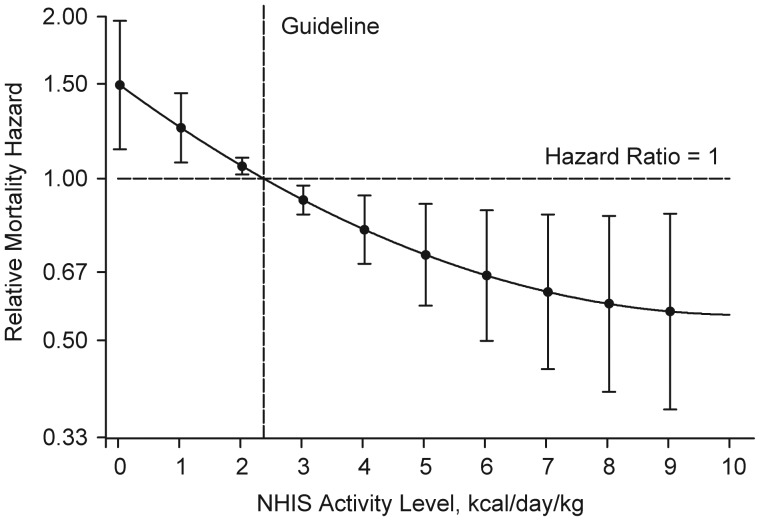
Women who reported no activity participation at baseline (n = 32, 7.4%) were twice as likely to die over the 12-year follow-up period than were women who reported any physical activity after adjustment for age, race, and educational level (hazard ratio (HR) = 2.15, 95% confidence interval (CI): 1.31, 3.53). Moreover, there was a nonlinear relation between baseline levels of activity modeled as a continuous variable (in kcal/day/kg) and mortality risk (Figure 1). Compared with women who met the 150 minutes/week guideline, which is equivalent to approximately 1,000 kcal/week for an individual weighing 60 kg (or 2.38 kcal/day/kg), those who reported 75 minutes of activity per week (i.e., 50% of the guideline-recommended level) had a 20% increase in mortality risk (HR = 1.21, 95% CI: 1.07, 1.38). However, even at this suboptimal level of 75 minutes/week or about 10 minutes/day, mortality risk fell by 16% compared with women performing only half of that amount (i.e., 38 minutes/week) (HR = 1.16, 95% CI: 1.05, 1.29).
Figure 1.
Relation between National Health Interview Survey (NHIS) physical activity level at baseline and 10-year all-cause mortality rate adjusted for age, race, and educational level among women in the Women's Health and Aging Study II (1994–2009) who reported any activity at baseline (n = 401). Shown are relative hazard estimates for mortality linked to specific activity levels compared with the hazard linked to the Centers for Disease Control and Prevention/American College of Sports Medicine recommended 150 minutes of moderate-intensity physical activity per week (equivalent to approximately 1,000 kcal/week for an individual weighing 60 kg or 2.38 kcal/day/kg, as denoted by the vertical dashed line). The curve represents the age-, race-, and educational level-adjusted relative mortality hazards across activity levels, and the bars indicate their 95% confidence intervals. The y-axis was transformed so that, for example, the graphic display of an increase in risk of the magnitude of 2 (hazard ratio = 2) would be equivalent to that of a decrease in risk of the same magnitude (hazard ratio = 0.5) in terms of scale size.
Change in physicality activity over time
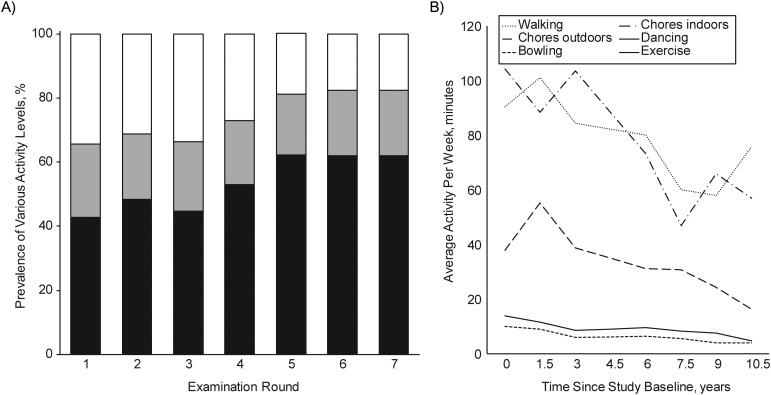
Using the NHIS criteria, we found that there was, in general, a decline in the proportion of women who remained very active over time, from 34% at baseline (visit 1) to 18% at visit 7; over this period, the prevalence of inactivity increased from 43% at baseline to 62% at visit 7 (Figure 2A). With regard to specific activities, participation declined most in walking and household chores, with an average rate of decline of 3–5 minutes/year, followed by strenuous outdoor chores at a rate of 2.7 minutes/year (Figure 2B). The changes in the other activities were minimal.
Figure 2.
A) Prevalence of being very active (white), moderately active (grey), and inactive (black) by the National Health Interview Survey criteria over time in the Women's Health and Aging Study II, 1994–2009. B) Average minutes per week of physical activity participation by activity type and follow-up time (year) in the Women's Health and Aging Study II, 1994–2009.
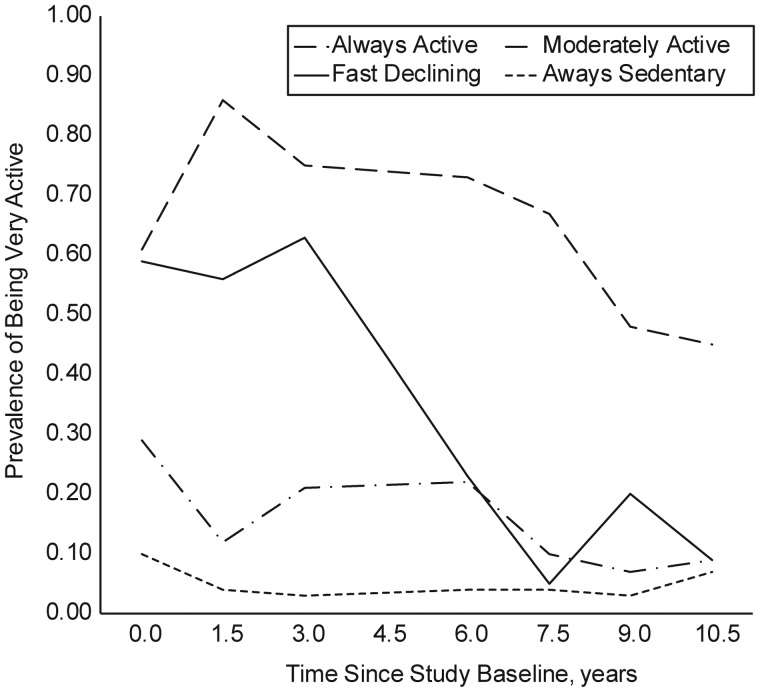
The joint latent class and survival mixture model identified 4 activity trajectory classes over the 12-year follow-up period: 1) most active at baseline and staying active (“always active”), 2) active at baseline but becoming inactive (“fast declining”), 3) moderately active throughout (“stable moderate”), and 4) least active at baseline and staying inactive (“always sedentary”). The estimates of class prevalence were 16.6%, 19.2%, 32.3%, and 31.9% for classes 1–4, respectively (Figure 3).
Figure 3.
Results from the mixture survival model: estimated prevalence of being very active by the National Health Interview Survey criteria within each of the 4 physical activity trajectory classes, the Women's Health and Aging Study II, 1994–2009.
Physical activity trajectory and all-cause mortality
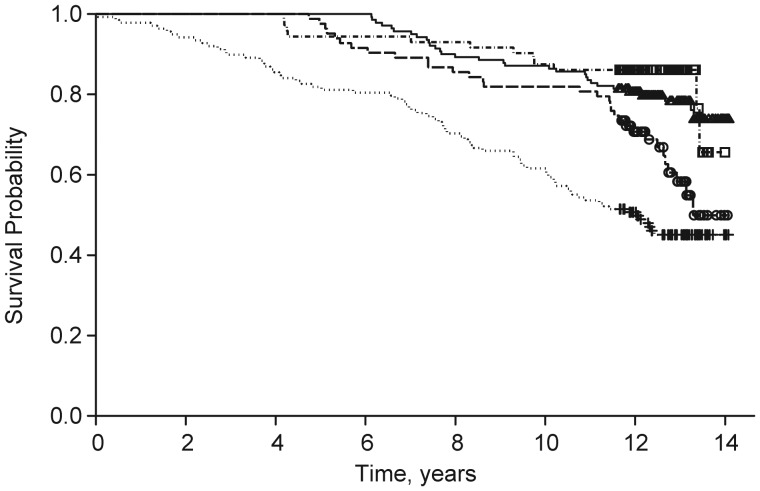
There was a stepwise association between low or decreasing levels of physical activity and increasing risk of mortality (Figure 4). After adjustment for age, race, educational level, and NHIS activity status at baseline, the mortality hazards for women in the fast declining and always sedentary classes were 2.34 (95% CI: 1.20, 4.59) and 3.34 (95% CI: 1.72, 6.47) times higher than for women in the always active class, respectively; the mortality hazard was not significantly different between the stable moderate and the always active classes (HR = 1.24, 95% CI: 0.63, 2.47).
Figure 4.
Kaplan-Meier survival curve by physical activity trajectory classes in the Women's Health and Aging Study II, 1994–2009. Shown are curves for the always active (dot-dashed line, squares), fast declining (dashed line, circles), stable moderate (solid line, triangles), and always sedentary (dotted line, cross) groups, with the squares, circles, triangles, and crosses representing censoring time within each of the 4 classes, respectively.
Predictors of physical activity trajectories
Our multinomial logistic regression analyses (adjusted for age, race, and educational level) demonstrated that obesity, having self-reported fair or poor health, and mobility disability were associated with 3.97 (95% CI: 1.54, 10.26), 7.28 (95% CI: 1.40, 37.82), and 3.88 (95% CI: 1.94, 7.78) times higher odds, respectively, of being always sedentary than that of being always active (Table 2). Women with coronary artery disease and chronic obstructive pulmonary disease (COPD) were 5 (HR = 5.04, 95% CI: 1.69, 15.04) and 3 (HR = 2.97, 95% CI: 1.35, 6.57) times more likely to remain inactive than to stay active, and those with COPD were also 3 times (HR = 2.77, 95% CI: 1.18, 6.50) more likely to experience a fast decline in activity than to stay active. Every additional disease was associated with 70% increased odds of being always sedentary versus always active (HR = 1.70, 95% CI: 1.26, 2.30). Every additional depressive symptom was associated with a 19% increased odds of being stable moderate (HR = 1.19, 95% CI: 1.06, 1.34) or fast declining (HR = 1.19, 95% CI: 1.06, 1.34) and a 28% increased odds of being always sedentary (HR = 1.28, 95% CI: 1.14, 1.43) compared with that of being always active. Every unit increase in energy level was associated with a 23%, 19%, and 35% reduction in the odds of being stable moderate (HR = 0.77, 95% CI: 0.63, 0.93), fast declining (HR = 0.81, 95% CI: 0.66, 0.99), and always sedentary (HR = 0.65, 95% CI: 0.53, 0.78), respectively, compared with that of being always active. Women with low self-efficacy were about 3 times more likely to be fast decliners (HR = 3.14, 95% CI: 1.04, 9.46) or remain inactive (HR = 2.91, 95% CI: 1.02, 8.31) than to stay active. Living alone was not associated with trajectory patterns.
Table 2.
Relative Risks for Every Unit Increase or Category Change in the Predictors: Results From Multinomial Logistic Regressions at Baseline (n = 433a), Women's Health and Aging Study II, 1994–2009
| Predictor | Physical Activity Trajectory Profileb |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Stable Moderate (n = 140, 32.3%) |
Fast Declining (n = 83, 19.2%) |
Always Sedentary (n = 138, 31.9%) |
|||||||
| RR | 95% CI | P Valuec | RRd | 95% CI | P Value | RRd | 95% CI | P Value | |
| Age, years | |||||||||
| 70–74 | 1 | Referent | 1 | Referent | 1 | Referent | |||
| 75–80 | 0.60 | 0.33, 1.08 | 0.09 | 0.77 | 0.41, 1.47 | 0.42 | 1.05 | 0.59, 1.87 | 0.87 |
| Race | |||||||||
| White | 1 | Referent | 1 | Referent | 1 | Referent | |||
| Nonwhite | 2.06 | 0.87, 4.87 | 0.10 | 1.14 | 0.43, 3.02 | 0.80 | 1.88 | 0.79, 4.46 | 0.16 |
| Years of education | 0.96 | 0.87, 1.05 | 0.35 | 0.89 | 0.81, 0.99 | 0.03 | 0.96 | 0.88, 1.05 | 0.36 |
| Living alone | 0.67 | 0.37, 1.19 | 0.17 | 1.15 | 0.61, 2.19 | 0.66 | 1.04 | 0.58, 1.84 | 0.90 |
| Body mass indexe | |||||||||
| <18.5 | 0.37 | 0.08, 1.68 | 0.20 | 0.46 | 0.08, 2.62 | 0.38 | 0.55 | 0.14, 2.21 | 0.40 |
| 18.5–24.9 | 1 | Referent | 1 | Referent | 1 | Referent | |||
| 25–29.9 | 1.22 | 0.64, 2.33 | 0.55 | 1.57 | 0.77, 3.20 | 0.22 | 1.03 | 0.53, 1.99 | 0.94 |
| ≥30 | 2.51 | 0.95, 6.59 | 0.06 | 1.83 | 0.62, 5.39 | 0.27 | 3.97 | 1.54, 10.26 | 0.004 |
| Self-reported health | |||||||||
| Excellent | 1 | Referent | 1 | Referent | 1 | Referent | |||
| Very good | 0.76 | 0.35, 1.68 | 0.50 | 1.63 | 0.61, 4.33 | 0.33 | 0.80 | 0.34, 1.92 | 0.62 |
| Good | 0.81 | 0.36, 1.86 | 0.62 | 1.76 | 0.64, 4.83 | 0.27 | 2.20 | 0.93, 5.19 | 0.07 |
| Fair/poor | 2.87 | 0.55, 14.91 | 0.21 | 5.69 | 0.95, 33.90 | 0.06 | 7.28 | 1.40, 37.82 | 0.02 |
| No. of diseasesf | 1.24 | 0.92, 1.69 | 0.16 | 1.24 | 0.89, 1.73 | 0.21 | 1.70 | 1.26, 2.30 | 0.001 |
| Coronary artery disease | 2.96 | 0.96, 9.15 | 0.06 | 2.19 | 0.65, 7.40 | 0.21 | 5.04 | 1.69, 15.04 | 0.004 |
| Chronic obstructive pulmonary disease | 1.83 | 0.81, 4.14 | 0.15 | 2.77 | 1.18, 6.50 | 0.02 | 2.97 | 1.35, 6.57 | 0.007 |
| Diabetes | 0.86 | 0.27, 2.68 | 0.79 | 0.42 | 0.10, 1.86 | 0.25 | 1.40 | 0.48, 4.10 | 0.54 |
| Osteoarthritis | 1.01 | 0.55, 1.87 | 0.97 | 1.15 | 0.58, 2.29 | 0.68 | 1.20 | 0.64, 2.23 | 0.57 |
| Cancer | 0.67 | 0.24, 1.89 | 0.45 | 0.85 | 0.28, 2.58 | 0.78 | 0.96 | 0.36, 2.53 | 0.93 |
| Smoking status | |||||||||
| Never smoker | 1 | Referent | 1 | Referent | 1 | Referent | |||
| Former smoker | 1.15 | 0.61, 2.19 | 0.66 | 1.34 | 0.67, 2.70 | 0.41 | 1.65 | 0.87, 3.12 | 0.12 |
| Current smoker | 1.12 | 0.42, 2.99 | 0.83 | 0.65 | 0.19, 2.24 | 0.50 | 1.61 | 0.61, 4.24 | 0.34 |
| Geriatric Depression Scale score | 1.19 | 1.06, 1.34 | 0.003 | 1.19 | 1.05, 1.34 | 0.005 | 1.28 | 1.14, 1.43 | <0.001 |
| Mobility disability | 1.81 | 0.88, 3.71 | 0.11 | 1.14 | 0.51, 2.58 | 0.75 | 3.88 | 1.94, 7.78 | <0.001 |
| Self-efficacy | |||||||||
| High | 1 | Referent | 1 | Referent | 1 | Referent | |||
| Medium | 1.84 | 0.90, 3.74 | 0.09 | 1.58 | 0.72, 3.49 | 0.26 | 2.27 | 1.12, 4.60 | 0.02 |
| Low | 1.74 | 0.58, 5.22 | 0.33 | 3.14 | 1.04, 9.46 | 0.04 | 2.91 | 1.02, 8.31 | 0.04 |
| Energy levelg | 0.77 | 0.63, 0.93 | 0.006 | 0.81 | 0.66, 0.99 | 0.04 | 0.65 | 0.53, 0.78 | <0.001 |
Abbreviations: CI, confidence interval; RR, relative risk.
a Three of the 436 women were missing data on activity measures and were therefore excluded from the analysis
b Estimated from the joint latent class and survival model.
c Two-sided P value.
d The always active class was the reference category.
e Weight (kg)/height (m)2.
f Presence of definite diseases, including angina pectoris/myocardial infarction, congestive heart failure, peripheral artery disease, hip fractures, osteoarthritis of the hip, knee and/or hand, Parkinson's disease, rheumatoid arthritis, osteoporosis, stroke, pulmonary diseases, diabetes mellitus, cancer, spinal stenosis, and disc disease.
g Energy level was rated on a Likert scale from 0–10, where 0 denoted no energy and 10 denoted the most energy that you have ever had.
When all the covariates in Table 2 except specific diseases and self-efficacy (a component of Geriatric Depression Scale) were included in a multivariable model, the effect sizes for Geriatric Depression Scale score remained essentially unchanged (results not shown). The associations of obesity, number of diseases, mobility disability, and energy level were attenuated but remained significant for having a greater odds of being always sedentary versus always active (results not shown).
DISCUSSION
We identified 4 distinct trajectories of change in leisure-time physical activity levels over 12 years in community-dwelling older women. To our knowledge, this is the first longitudinal epidemiologic study that focused specifically on characterizing physical activity participation over time and examining predictors of such change in community-dwelling, higher-functioning older women. Although similar trajectory patterns were reported by Barnett et al. (27), their results were limited to adults 18–60 years of age.
The observed curvilinear relation between lower levels of physical activity and higher mortality risk suggests that although any amount of increase could be beneficial, the gain was the greatest among women reporting the lowest levels of activity. This finding is further supported by our longitudinal finding that the stable moderate and always active groups had similar mortality risks. Our results therefore provide additional data supporting the call by the 2008 Physical Activity Guidelines for Americans for Sedentary Older Adults With Chronic Diseases and Disabilities to be as physically active as abilities and conditions allow (28). In addition, our study demonstrates that the ability to maintain an active or moderately active lifestyle was associated with the lowest mortality rate, which is consistent with our hypothesis that stability in activity level is a marker of greater physiologic reserve. The observation of increased mortality associated with fast decliners after adjustment for baseline activity status highlights the importance of monitoring changes in physical activity level over time.
Walking for exercise and indoor household chores were the 2 activities most likely to change among older women. Walking is known to be the most common physical activity among physically active adults (29), and regular walking of 8 blocks a week is protective against further mobility loss among disabled older women (30). Taken together, these findings suggest that walking, as an inexpensive and readily accessible form of exercise, may be a particularly effective and attainable goal for promoting physical activity, especially among functionally challenged older adults with limited exercise capacity. A prior report indicated that some disabled older women are able to maintain walking at a level consistent with guideline recommendations, suggesting greater potential for activity than is currently seen (31).
Despite the persuasive evidence of health benefits of regular physical activity and exercise, most older adults are not physically active enough to achieve these benefits. Consistent with previous reports (32, 33), we found that obesity, self-reported fair or poor health, mobility disability, low self-efficacy, and having a greater number of diseases (coronary artery disease and COPD in particular) and depressive symptoms are strongly associated with both inactivity at baseline (results not shown) and a tendency to maintain a sedentary lifestyle. These findings provide guidance for factors to mitigate or prevent to help maintain physical activity.
The strengths of our study include longitudinal data, the community-based representative sample, the inclusion of initially high-functioning women, which allowed for better delineation of temporal relations, and latent variable modeling that took into account measurement error and missing data. In addition, we implemented a novel statistical approach to aid the selection of a subjective measure of physical activity for the study. The observed heterogeneity in different measures was striking and has not been addressed previously. By choosing a measure that minimized the degree of uncertainty associated with intraperson transitions in physical activity status, we not only achieved objectivity but also optimized our ability to identify meaningful trajectory patterns. The reason that the NHIS criteria outperformed the CDC/ACSM criteria could be 2-fold. First, the former accounts for not only duration and frequency but also intensity of activities. Second, the CDC/ACSM definition of inactivity as no activity at all may not represent the usual level of activity for older adults because interruption of activity participation for short period of time may be more frequent among older adults compared with younger adults because of older adults' increased vulnerability to acute health problems or weather related factors.
Despite these strengths, several limitations should be considered when interpreting our results. First, our analyses were restricted to high-functioning older women living in the community. Therefore, the results cannot be generalized to older men or to more disabled women. Second, questionnaire-based assessments of physical activity may be subject to recall bias (34–36). However, using subjective measures for ranking rather than quantifying physical activity levels among older adults may still be valid (37). The observed dose-response relation between activity trajectory and mortality in our study supports the utility of the self-report. Third, only 6 of the original 18 activities in the Minnesota Leisure Time Activities Questionnaire were assessed in the WHAS, which may lead to underestimation of the between-person heterogeneity in physical activity level. However, the WHAS subset has been shown to be an effective surrogate for the full Minnesota Leisure Time Activities Questionnaire in older women with high predictive accuracy and validity (38). Fourth, the identification of predictors of change in physical activity level was restricted to baseline covariates. Few studies have investigated the impact of change in risk factors on change in physical activity in older adults. More longitudinal work that accounts for time-varying risk factors is needed to improve our ability to delineate temporal relations with the ultimate goal of identifying causes of sedentary behavior and fast decline in physical activity. Finally, given that increasing illness and disability over time could be both risk factors for and consequences of sedentary behavior or decline in physical activity through negative feedback mechanisms, the observed association between decline in physical activity and mortality may be partially explained by the increased burden of illness and disability. Further work is needed to assess the degree of mediating and feedback effects such that the health benefits of physical activity intervention can be better quantified.
In summary, our findings have several practical implications for promoting physical activity in older women. First, physical activity does not have to be vigorous and time-consuming to be beneficial, but a moderate activity level should be maintained. Second, given that most of the decline in physical activity in older women was due to a decrease in walking and doing household chores rather than regular exercise, programs tailored to overcome individual (and perhaps environmental) barriers to the walking may yield the most benefits. Third, by delineating the impact of individual-level demographic, psychosocial, and health characteristics on physical activity engagement in older women, our study informs the development of future screening and intervention efforts to target older women who might benefit the most, namely those with chronic diseases such as obesity, coronary artery disease, or COPD, depressive symptoms, low self-efficacy, mobility disability, and low energy level.
ACKNOWLEDGMENTS
Author affiliations: Department of Medicine, Division of Geriatric Medicine and Gerontology, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Qian-Li Xue, Ravi Varadhan, Wenliang Yao); Center on Aging and Health, Johns Hopkins Medical Institutions, Baltimore, Maryland (Qian-Li Xue, Karen Bandeen-Roche, Sarah L. Szanton, Roland J. Thorpe, Rita R. Kalyani, Ravi Varadhan, Wenliang Yao); Department of Biostatistics, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Karen Bandeen-Roche); Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York (Thelma J. Mielenz, Linda P. Fried); Department of Community and Preventive Medicine, School of Medicine and Dentistry, University of Rochester, Rochester, New York (Christopher L. Seplaki); Department of Health Systems and Outcomes, School of Nursing, Johns Hopkins University, Baltimore, Maryland (Sarah L. Szanton); Department of Health Policy and Management, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Roland J. Thorpe); Johns Hopkins Center for Health Disparities Solutions, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Roland J. Thorpe); Department of Medicine Division of Endocrinology and Metabolism, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Rita R. Kalyani); Benjamin Leon, Jr., Family Center for Geriatric Research and Education and Department of Medicine, Herbert Wertheim College of Medicine, Florida International University, Miami, Florida (Paulo H. M. Chaves); Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, New York (Thuy-Tien L. Dam); Department of Medicine, Mount Sinai School of Medicine, New York, New York (Katherine Ornstein); and Department of Biostatistics, Mailman School of Public Health, Columbia University, New York, New York (Arindam RoyChoudhury).
This work was supported by the National Institutes of Health (grants R37-AG019905 to L. P. F. and grant K01-AG031332 to C. L. S.) and by the Johns Hopkins Older Americans Independence Center (grant P30-AG021334).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Conflict of interest: none declared.
REFERENCES
- 1.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 2.Moran S, Chen YM, Ruthie A, et al. Alterations in IGF-I affect elderly: role of physical activity. Eur Rev Aging Phys Act. 2007;4(2):77–84. [Google Scholar]
- 3.Weuve J, Kang JH, Manson JE, et al. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292(12):1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 4.Yaffe K, Barnes D, Nevitt M, et al. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161(14):1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 5.Laurin D, Verreault R, Lindsay J, et al. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 6.Pereira CLN, Vogelaere P, Baptista F. Role of physical activity in the prevention of falls and their consequences in the elderly. Eur Rev Aging Phys Act. 2008;5(1):51–58. [Google Scholar]
- 7.Peterson MJ, Giuliani C, Morey MC, et al. Physical activity as a preventative factor for frailty: the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci. 2009;64(1):61–68. doi: 10.1093/gerona/gln001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seeman TE, Berkman LF, Charpentier PA, et al. Behavioral and psychosocial predictors of physical performance: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 1995;50(4):M177–M183. doi: 10.1093/gerona/50a.4.m177. [DOI] [PubMed] [Google Scholar]
- 9.Brach JS, FitzGerald S, Newman AB, et al. Physical activity and functional status in community-dwelling older women: a 14-year prospective study. Arch Intern Med. 2003;163(21):2565–2571. doi: 10.1001/archinte.163.21.2565. [DOI] [PubMed] [Google Scholar]
- 10.Miller ME, Rejeski WJ, Reboussin BA, et al. Physical activity, functional limitations, and disability in older adults. J Am Geriatr Soc. 2000;48(10):1264–1272. doi: 10.1111/j.1532-5415.2000.tb02600.x. [DOI] [PubMed] [Google Scholar]
- 11.Monninkhof EM, Elias SG, Vlems FA, et al. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18(1):137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- 12.United States Department of Health and Human Services. Healthy People 2010: Understanding and Improving Health. Washington, DC: US Government Printing Office; 2000. [Google Scholar]
- 13.Bennett KM. Gender and longitudinal changes in physical activities in later life. Age Ageing. 1998;27(suppl 3):24–28. doi: 10.1093/ageing/27.suppl_3.24. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Fisher J, Brownson RC. A multilevel analysis of change in neighborhood walking activity in older adults. J Aging Phys Act. 2005;13(2):145–159. doi: 10.1123/japa.13.2.145. [DOI] [PubMed] [Google Scholar]
- 15.Fried LP, Bandeen-Roche K, Chaves PH, et al. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55(1):M43–M52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 16.Taylor HL, Jacobs DR, Jr, Schucker B, et al. Questionnaire for the assessment of leisure-time physical activities. J Chronic Dis. 1978;31(12):741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 17.National Health Interview Survey. Adult Physical Activity Information: Comparison of Two Recodes: Core and Supplement Leisure-Time Physical Activity Questions. Atlanta, GA: Centers for Disease Control and Prevention; 2011. (http://www.cdc.gov/nchs/nhis/physical_activity/pa_comparison.htm. ). (Accessed November 13, 2011) [Google Scholar]
- 18.Guralnik J, Fried LP, Simonsick EM, et al. The Women's Health and Aging Study: Health and Social Characteristics of Older Women With Disability. Bethesda, MD: National Institute on Aging; 1995. NIH publication no. 95-4009. [Google Scholar]
- 19.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982–1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 20.Thorpe RJ, Jr, Weiss C, Xue QL, et al. Transitions among disability levels or death in African American and white older women. J Gerontol A Biol Sci Med Sci. 2009;64(6):670–674. doi: 10.1093/gerona/glp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diggle P, Heagerty PJ, Liang KY, et al. Analysis of Longitudinal Data. New York, NY: Oxford University Press, Inc; 2002. [Google Scholar]
- 22.Shannon CE. Prediction and entropy of printed English. Bell Syst Tech J. 1951;30(1):50–64. [Google Scholar]
- 23.Arndt C. Information Measures: Information and Its Description in Science and Engineering. Berlin, Germany: Springer; 2001. [Google Scholar]
- 24.Lo YT, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88(3):767–778. [Google Scholar]
- 25.Rubin DB. Inference and missing Data. Biometrika. 1976;63:581–592. [Google Scholar]
- 26.Asparouhov T, Masyn K, Muthen B. Continuous time survival in latent variable models. 2006 Presented at the Annual Meeting of the American Statistical Association, ASA Section on Biometrics, Seattle, Washington, August 8. [Google Scholar]
- 27.Barnett TA, Gauvin L, Craig CL, et al. Distinct trajectories of leisure time physical activity and predictors of trajectory class membership: a 22 year cohort study. Int J Behav Nutr Phys Act. 2008;5:57. doi: 10.1186/1479-5868-5-57. doi:10.1186/1479-5868-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 29.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 30.Simonsick EM, Guralnik JM, Volpato S, et al. Just get out the door! Importance of walking outside the home for maintaining mobility: findings from the women's health and aging study. J Am Geriatr Soc. 2005;53(2):198–203. doi: 10.1111/j.1532-5415.2005.53103.x. [DOI] [PubMed] [Google Scholar]
- 31.Jerome GJ, Glass TA, Mielke M, et al. Physical activity participation by presence and type of functional deficits in older women: The Women's Health and Aging Studies. J Gerontol A Biol Sci Med Sci. 2006;61(11):1171–1176. doi: 10.1093/gerona/61.11.1171. [DOI] [PubMed] [Google Scholar]
- 32.Trost SG, Owen N, Bauman AE, et al. Correlates of adults' participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34(12):1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 33.Satariano WA, Haight TJ, Tager IB. Reasons given by older people for limitation or avoidance of leisure time physical activity. J Am Geriatr Soc. 2000;48(5):505–512. doi: 10.1111/j.1532-5415.2000.tb04996.x. [DOI] [PubMed] [Google Scholar]
- 34.Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(2 suppl):S1–S14. [PubMed] [Google Scholar]
- 35.Lichtman SW, Pisarska K, Berman ER, et al. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med. 1992;327(27):1893–1898. doi: 10.1056/NEJM199212313272701. [DOI] [PubMed] [Google Scholar]
- 36.Lamonte MJ, Ainsworth BE. Quantifying energy expenditure and physical activity in the context of dose response. Med Sci Sports Exerc. 2001;33(6 suppl):S370–S378. doi: 10.1097/00005768-200106001-00006. [DOI] [PubMed] [Google Scholar]
- 37.Colbert LH, Matthews CE, Havighurst TC, et al. Comparative validity of physical activity measures in older adults. Med Sci Sports Exerc. 2011;43(5):867–876. doi: 10.1249/MSS.0b013e3181fc7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckel SP, Bandeen-Roche K, Chaves PH, et al. Surrogate screening models for the low physical activity criterion of frailty. Aging Clin Exp Res. 2011;23(3):209–216. doi: 10.1007/bf03324962. [DOI] [PMC free article] [PubMed] [Google Scholar]