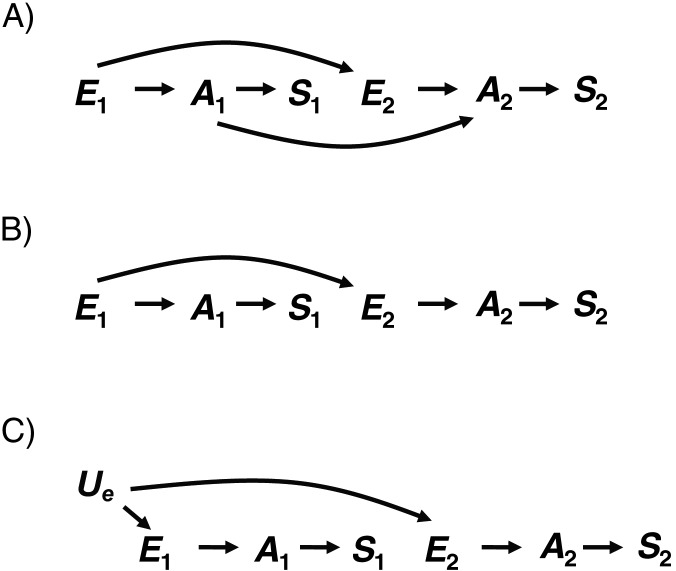Abstract
In a 1993 paper (Am J Epidemiol. 1993;137(1):1–8), Weinberg considered whether a variable that is associated with the outcome and is affected by exposure but is not an intermediate variable between exposure and outcome should be considered a confounder in etiologic studies. As an example, she examined the common practice of adjusting for history of spontaneous abortion when estimating the effect of an exposure on the risk of spontaneous abortion. She showed algebraically that such an adjustment could substantially bias the results even though history of spontaneous abortion would meet some definitions of a confounder. Directed acyclic graphs (DAGs) were introduced into epidemiology several years later as a tool with which to identify confounders. The authors now revisit Weinberg's paper using DAGs to represent scenarios that arise from her original assumptions. DAG theory is consistent with Weinberg's finding that adjusting for history of spontaneous abortion introduces bias in her original scenario. In the authors' examples, treating history of spontaneous abortion as a confounder introduces bias if it is a descendant of the exposure and is associated with the outcome conditional on exposure or is a child of a collider on a relevant undirected path. Thoughtful DAG analyses require clear research questions but are easily modified for examining different causal assumptions that may affect confounder assessment.
Keywords: bias (epidemiology), causality, confounding factors (epidemiology), reproductive history
In her 1993 paper, “Toward a Clearer Definition of Confounding,” Weinberg focused on clarifying the definition of a confounder in etiologic studies (1). Specifically, she noted a situation where a variable that did not confound the estimated effect would qualify as a confounder according to commonly applied criteria of the time: The variable was associated with exposure, was associated with the outcome among the unexposed, and was not an intermediate factor between the exposure and the outcome. As an illustrative example, she examined the common practice of adjusting for history of spontaneous abortion when estimating the causal effect of an exposure on the risk of spontaneous abortion. She showed, by example, that under simplified but biologically plausible assumptions, controlling for history of spontaneous abortion could substantially bias the estimated risk ratio.
Since the publication of Weinberg's paper, the use of directed acyclic graphs (DAGs) to identify confounders has become increasingly common. Concurrently, it has become more widely recognized that a variable could appear to be a confounder by some conventional definitions but adjustment for that variable could induce bias (2–9). Numerous published texts provide introductions to DAGs (2–7, 10). In this paper, we assume a basic familiarity with DAGs while revisiting Weinberg's original work, using DAGs to evaluate and expand upon her examples.
ORIGINAL ASSUMPTIONS
History of spontaneous abortion is predictive of pregnancy loss and is often adjusted for in studies of spontaneous abortion (11–13), presumably under the assumption that it serves as a proxy for unmeasured confounders rather than supposing that it causally affects the risk of subsequent spontaneous abortions. Weinberg described a hypothetical study for estimating the effect of an exposure (E) (e.g., smoking) on the risk of spontaneous abortion in a current pregnancy (S2) (1). The exposure was a risk factor for prior spontaneous abortion (S1), but S1 was not an intermediate variable between E and S2. However, S1 was associated with the outcome among the unexposed, a commonly applied criterion used to determine whether a variable is a confounder when the target population is the exposed group. Thus, S1 has often been treated as a confounder.
For simplification, Weinberg limited the example to second pregnancies and assumed that a woman's exposure status remained the same across pregnancies (1). Further, she assumed that E increased the risk of an occult abnormality (A) and that A also was the same across pregnancies. Finally, she assumed that the outcomes (S1 and S2) were independent, conditional on A, and that Pr[Si|E, A] = Pr[Si|A] for pregnancies i = 1, 2.
THE ORIGINAL DAG
We can use DAGs to represent possible underlying causal relations compatible with the statistical assumptions from Weinberg's paper (1). In order to draw appropriate conclusions from a DAG, it is necessary to assume that the DAG represents the true causal relations and that it is not missing any variable that causes any pair of variables in the graph (5, 7). We consider several DAGs that represent variations in the scenario considered by Weinberg. Our interest is in determining whether different DAGs compatible with the scenario described by Weinberg lead us to the same conclusions she reached originally (1) and whether biologically plausible revisions would cause us to revise those conclusions.
The construction and interpretation of a DAG differs depending on the causal contrast of interest and the hypothesized causal relations. First, we will consider an exposure that occurs prior to both pregnancies (e.g., in utero exposure of the pregnant woman to smoking). Formally, let E represent a dichotomous exposure where 1 indicates exposure and 0 indicates no exposure, and let S2 represent a dichotomous outcome where 1 indicates that the second pregnancy ended in a loss and 0 indicates that it ended in a birth. Assuming that the target population is the exposed group, let S2e=1 represent the outcome of the second pregnancy if all exposed women were exposed and S2e=0 represent the outcome of the second pregnancy if all exposed women had instead been unexposed. Then the causal risk ratio would be Pr[S2e=1 = 1]/Pr[S2e=0 = 1]. In the data, only Pr[S2e=1 = 1] would be observed (indicated Pr[S2 = 1|E = 1]). Pr[S2e=0 = 1] would be estimated from the experience of the unexposed group, Pr[S2 = 1|E = 0]. If the (observed) experience of the unexposed group differed from the (unobserved) experience the exposed group would have had if they had been unexposed, then the estimated effect parameter would be biased. Confounding could exist in the unadjusted estimate or could be introduced by inappropriate adjustment for covariates. In this article, we evaluate several DAGs to determine whether adjustment for S1 introduces bias. Formally, we assess whether Pr[S2e=1 = 1]/Pr[S2e=0 = 1] = Pr[S2 = 1|E = 1, S1]/Pr[S2 = 1|E = 0, S1].
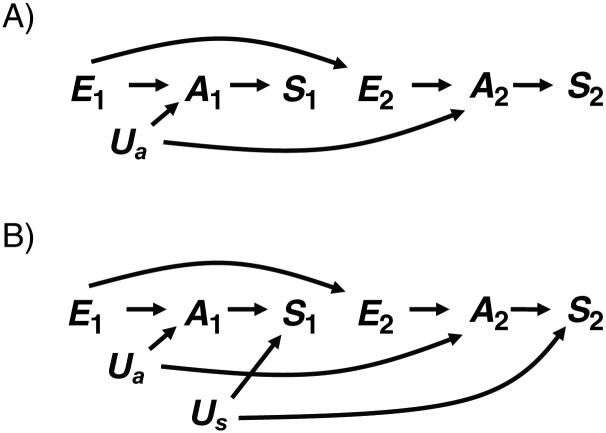
DAG 1A (Figure 1A) is consistent with Weinberg's assumptions and is equivalent to Figure 1b in her original paper (1). It assumes that E causes an underlying abnormality A, which in turn affects risk of spontaneous abortion in both pregnancies (S1 and S2). In this DAG, there is no confounding, so Pr[S2e=1 = 1]/Pr[S2e=0 = 1] = Pr[S2 = 1|E = 1]/Pr[S2 = 1|E = 0]. Nevertheless, S1 would be considered a confounder on the basis of some conventional criteria. S1 is associated with S2 through an open path that does not directly involve exposure (S1 ← A → S2); S1 is associated with E because S1 is a descendant of E; and S1 is not a causal intermediate or mediator between E and S2. Given these conditions, the unadjusted effect estimate for E would be expected to differ from the estimate adjusted for S1, but based on DAG 1A, controlling for S1 would introduce bias (i.e., Pr[S2e=1 = 1]/Pr[S2e=0 = 1] ≠ Pr[S2 = 1|E = 1, S1]/Pr[S2 = 1|E = 0, S1]) as Weinberg stated (1), because S1 and S2 are affected by a shared parent (A), which is an intermediate between E and S2. In a sense, S1 is a proxy for the intermediate A, so adjusting for S1 is like imperfectly adjusting for an intermediate and produces a form of bias that has been called overadjustment bias (14). The equations from the original paper are directly relevant to this DAG (1).
Figure 1.
A) Directed acyclic graph (DAG) 1A, in which a single exposure (E) causes a single underlying abnormality (A) that causes both outcomes (S1 and S2). B) DAG 1B, in which a shared cause (Us) of S1 and S2 is added to DAG 1A.
One of the advantages of DAG analyses is that one can easily illustrate increasingly complex situations. DAG 1B (Figure 1B) builds on DAG 1A by introducing an unmeasured risk factor for spontaneous abortion (Us). This addition changes Weinberg's simplifying assumption that S1 and S2 are independent conditional on A (1) and is more realistic. Under DAG 1B, Pr[S2 = 1|E = 1]/Pr[S2 = 1|E = 0] is still an unconfounded estimate of Pr[S2e=1 = 1]/Pr[S2e=0 = 1], and Weinberg's conclusion that adjusting for S1 introduces bias still holds, because adjustment for S1 would be equivalent to adjusting for a proxy of an intermediate. There are further complications in DAG 1B, because S1 is also a collider on a path from E to S2 (E → A → S1 ← Us → S2), so adjusting for S1 would open this path.
ADDING MULTIPLE EXPOSURES
The DAGs shown in Figure 1 indicate that E and A both occur prior to or during the first pregnancy but affect both pregnancies. For some exposures that do not change (e.g., in utero exposures of the study participant) this might be appropriate, but for an exposure such as participant smoking, which can change, it is important to specify the exact research question. Are we interested in the effect of smoking during the first pregnancy on the outcome of the second pregnancy, or are we interested in the effect of smoking during the second pregnancy on that pregnancy? If the exposure of interest were smoking during the second pregnancy, the DAG would have to be modified to reflect the fact that smoking during the second pregnancy cannot affect the first pregnancy.
The DAGs shown in Figure 2 represent the same exposure (such as participant smoking) at 2 points in time through 2 nodes: E1 and E2 (designating smoking during the first and second pregnancies, respectively). In these DAGs, the exposure during each pregnancy produces its own underlying abnormality (such as poor response of the endometrium to pregnancy hormones (1)), which affects only the contemporaneous pregnancy. The new causal risk ratio of interest is 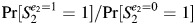 .
.
Figure 2.
A) Directed acyclic graph (DAG) 2A, in which exposure during the first pregnancy (E1) causes an underlying abnormality during the first pregnancy (A1) and exposure during the second pregnancy (E2). E2 causes an underlying abnormality during the second pregnancy (A2). In addition, an unknown covariate (Ua) causes both A1 and A2, and A1 and A2 cause the corresponding outcomes (S1 and S2, respectively). B) DAG 2B, in which a shared cause (Us) of S1 and S2 is added to DAG 2A.
Our revised DAGs require us to modify Weinberg's assumptions slightly. The original simplifying assumption that S1 and S2 are independent conditional on A is no longer unambiguous, because the abnormality may occur at 2 different points in time. However, we can be consistent with the spirit of the assumption in our revised DAG if S1 and S2 are independent conditional on A1 and A2. This is the case in DAG 2A, where S1 and S2 are not associated through any open paths that do not involve A1 and A2 and where conditioning on either would block the otherwise open paths S1 ← A1 ← Ua → A2 → S2 and S1 ← A1 ← E1 → E2 → A2 → S2. In addition, we now assume Pr[Si|Ai, Ei] = Pr[Si|Ai] as an extension of the corresponding original assumption (1). This is represented by the fact that all paths from Ei to Si go through Ai. Weinberg's assumptions that exposure status and abnormality status are the same for both pregnancies (E1 = E2 and A1 = A2) are not required to evaluate the revised DAGs and are neither indicated nor contradicted.
In DAG 2A, Pr[S2 = 1|E2 = 1]/Pr[S2 = 1|E2 = 0] is an unconfounded estimate of 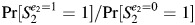 because the only backdoor path from E2 to S2 (E2 ← E1 → A1 ← Ua → A2 → S2) is blocked by the collider A1. As before, S1 is not a confounder of the estimated effect of E2 on S2, but some conventional criteria would still define it as such. S1 is associated with the outcome (S2) among the unexposed because of the open path between them that does not include E2 (S1 ← A1 ← Ua → A2 → S2). This path exists because Ua is a shared parent of A1 and A2. For concreteness, we can think of Ua as a genetic factor that makes some women liable to develop the abnormality every time they become pregnant. In DAG 2A, S1 is associated with the exposure of interest (via the open path E2 ← E1 → A1 → S1) but is not on the causal path of interest (from E2 to S2). The association between S1 and E2 is due to the assumption in DAG 2A that exposure status during the first pregnancy directly affects exposure status during the second pregnancy (e.g., smoking is addictive, and therefore smokers tend to continue smoking). That is to say, S1 and E2 are associated because they share E1 as a cause.
because the only backdoor path from E2 to S2 (E2 ← E1 → A1 ← Ua → A2 → S2) is blocked by the collider A1. As before, S1 is not a confounder of the estimated effect of E2 on S2, but some conventional criteria would still define it as such. S1 is associated with the outcome (S2) among the unexposed because of the open path between them that does not include E2 (S1 ← A1 ← Ua → A2 → S2). This path exists because Ua is a shared parent of A1 and A2. For concreteness, we can think of Ua as a genetic factor that makes some women liable to develop the abnormality every time they become pregnant. In DAG 2A, S1 is associated with the exposure of interest (via the open path E2 ← E1 → A1 → S1) but is not on the causal path of interest (from E2 to S2). The association between S1 and E2 is due to the assumption in DAG 2A that exposure status during the first pregnancy directly affects exposure status during the second pregnancy (e.g., smoking is addictive, and therefore smokers tend to continue smoking). That is to say, S1 and E2 are associated because they share E1 as a cause.
In DAG 2A, S1 is neither a confounder nor a child of an intermediate. It meets some conventional criteria for confounding because it is a child of the collider A1. Adjusting for S1 will open a backdoor path and bias the effect estimate (i.e., 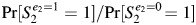 ≠ Pr[S2 = 1|E2 = 1, S1]/Pr[S2 = 1|E2 = 0, S1]). Thus, as Weinberg described in the original paper (1), adjusting for S1 is not appropriate. The bias introduced by conditioning on S1 could be removed by conditioning on E1 or Ua, provided that DAG 2A is correct and complete. However, if the DAG were missing variables, such as a shared cause of S1 and S2 (DAG 2B), bias could be introduced by adjusting for S1 that would not be removed by adjusting for Ua. In addition, adjusting for E1, a close correlate of the exposure, would affect statistical efficiency.
≠ Pr[S2 = 1|E2 = 1, S1]/Pr[S2 = 1|E2 = 0, S1]). Thus, as Weinberg described in the original paper (1), adjusting for S1 is not appropriate. The bias introduced by conditioning on S1 could be removed by conditioning on E1 or Ua, provided that DAG 2A is correct and complete. However, if the DAG were missing variables, such as a shared cause of S1 and S2 (DAG 2B), bias could be introduced by adjusting for S1 that would not be removed by adjusting for Ua. In addition, adjusting for E1, a close correlate of the exposure, would affect statistical efficiency.
In DAG 2B, the assumption that S1 and S2 are independent conditional on A1 and A2 is relaxed by adding an unmeasured shared cause of the pregnancy outcomes (Us). This creates 2 backdoor paths from E2 to S2 (E2 ← E1 → A1 ← Ua → A2 → S2 and E2 ← E1 → A1 → S1 ← Us → S2), but both paths are unconditionally blocked by colliders (A1 and S1, respectively). Thus, as before, the unadjusted estimate would be an unbiased estimate of the causal contrast, while adjusting for S1 would open backdoor paths between E2 and S2, which would therefore introduce bias.
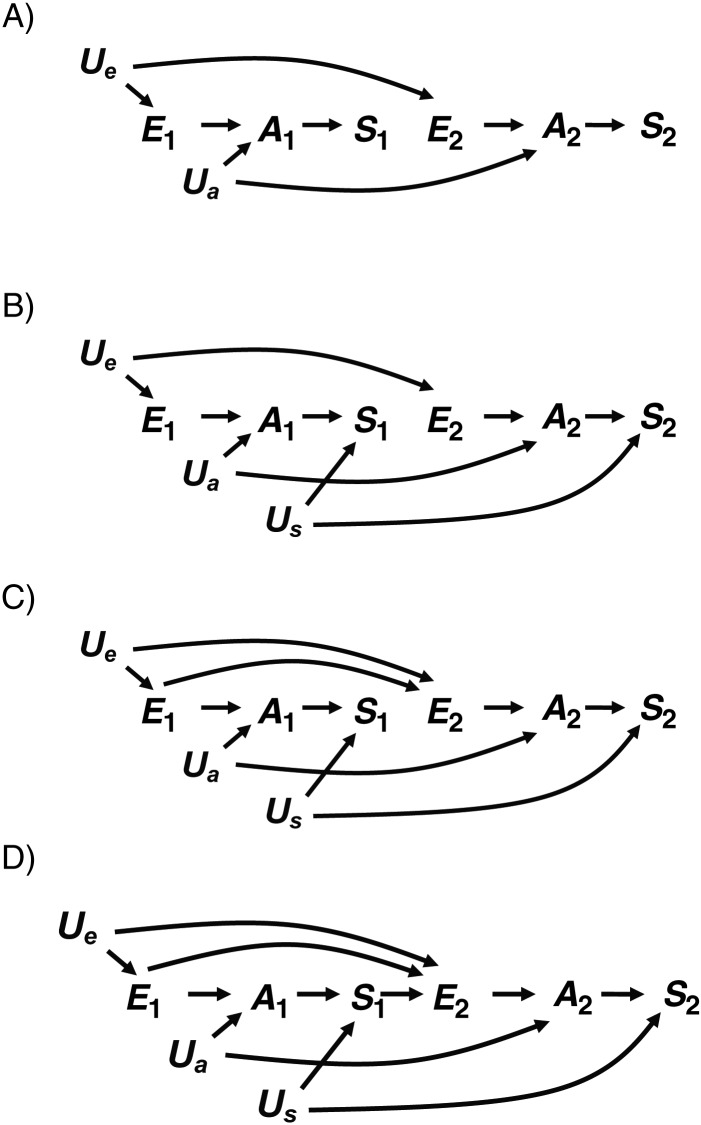
DAG 3A (Figure 3) is the same as DAG 2A and DAG 3B is the same as DAG 2B, except that in Figure 3 the association between E1 and E2 is due to the shared cause Ue (e.g., societal factors that determine smoking behavior) instead of E1's directly causing E2 (i.e., we no longer consider smoking addictive). In both DAG 3A and DAG 3B, as before, the unadjusted estimate for the effect of E2 on S2 is unbiased. In DAG 3A, we need not condition on any covariate, because the only backdoor path between E2 and S2 (E2 ← Ue → E1 → A1 ← Ua → A2 → S2) is blocked by the collider A1. Once again, S1 is the child of the collider A1, so adjusting for S1 would also open a backdoor path and introduce bias. In DAG 3B, it is inappropriate to adjust for S1 because it is a collider as well as a child of a collider. This is also true in DAG 3C, which combines DAGs 2B and 3B by allowing E2 both to be affected by E1 and to have a shared cause with E1.
Figure 3.
A) Directed acyclic graph (DAG) 3A, in which an unknown variable (Ue) causes exposure during the first (E1) and second (E2) pregnancies. Each exposure causes the corresponding underlying abnormality (A1 and A2). In addition, an unknown variable (Ua) causes both underlying abnormalities, and each underlying abnormality causes the corresponding outcome (S1 and S2). B) DAG 3B, in which a shared cause (Us) of S1 and S2 is added to DAG 3A. C) DAG 3C, which builds on DAG 3B by allowing E1 to affect E2 directly. D) DAG 3D, which builds on DAG 3C by allowing the first outcome (S1) to affect the second exposure (E2).
DAG 3D is similar to DAG 3C, but now S1 is allowed to affect the exposure of interest (E2). This could occur if a woman modified her behavior based on the outcome of her first pregnancy. For example, a smoker who had a spontaneous abortion during her first pregnancy might quit smoking prior to her second pregnancy. This would violate Weinberg's original assumption that exposure was the same across pregnancies (1). In DAG 3D, there are unconditionally open backdoor paths through S1 (e.g., E2 ← S1 ← Us → S2). Thus, this is the first example in which the unadjusted estimate is biased (i.e., 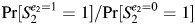 ≠ Pr [S2 = 1|E2 = 1]/Pr[S2 = 1|E2 = 0]). If Us and Ua were measured, the simplest way to block the backdoor paths would be to condition on them. If they were unmeasured, then conditioning on S1 would be necessary, but S1 is a collider on other backdoor paths (E2 ← E1 → A1 → S1 ← Us → S2 and E2 ← Ue → E1 → A1 → S1 ← Us → S2). Therefore, additional adjustment (e.g., adjusting for E1) would be needed to block the paths opened by conditioning on S1.
≠ Pr [S2 = 1|E2 = 1]/Pr[S2 = 1|E2 = 0]). If Us and Ua were measured, the simplest way to block the backdoor paths would be to condition on them. If they were unmeasured, then conditioning on S1 would be necessary, but S1 is a collider on other backdoor paths (E2 ← E1 → A1 → S1 ← Us → S2 and E2 ← Ue → E1 → A1 → S1 ← Us → S2). Therefore, additional adjustment (e.g., adjusting for E1) would be needed to block the paths opened by conditioning on S1.
DAGS THAT FURTHER ALTER THE ORIGINAL ASSUMPTIONS
Figure 4 presents DAGs that substantially modify Weinberg's original assumptions (1) despite being similar to the preceding DAGs. DAG 4A is similar to DAG 2A, except that A1 causes A2. This modification means that A1 is no longer a collider, so there is an open backdoor path from E2 to S2 (E2 ← E1 → A1 → A2 → S2). An unbiased estimate for the effect of E2 on S2 would require adjustment for E1 or A1. The simplest way to understand this is to note that under this DAG, E1 is a confounder because it causes both the exposure of interest (E2) and the outcome (S2). Theoretically, if neither E1 nor A1 were measured, adjusting for S1 could serve as a proxy for A1. However, the backdoor path would still remain open to the degree that S1 was not perfectly correlated with A1 (7). For DAG 4A, the effect estimate adjusted for S1 might be less biased than the unadjusted estimate. This conclusion is incompatible with Weinberg's original calculations, because we have modified her assumptions.
Figure 4.
A) Directed acyclic graph (DAG) 4A, in which exposure during the first pregnancy (E1) causes an underlying abnormality during the first pregnancy (A1) and exposure during the second pregnancy (E2). E2 causes an underlying abnormality during the second pregnancy (A2). In addition, A1 causes A2. Each underlying abnormality causes the corresponding outcome (S1 and S2). B) DAG 4B, which removes the effect of A1 on A2 that was present in DAG 4A. C) DAG 4C, which alters DAG 4B so that E1 and E2 have a shared cause (Ue) instead of E1 causing E2.
DAGs 4B and 4C are similar to previous DAGs, except that Ai is only directly affected by Ei. Although these DAGs would initially appear compatible with Weinberg's example (1), they violate the assumption that S1 and S2 are associated among the unexposed, because the only path between the two pregnancy outcomes goes through the exposure of interest. Consequently, S1 would not meet some conventional criteria for a confounder, nor would it be a confounder based on the DAG. If the DAG is correct, if all variables are measured perfectly, if the adjusted model is specified correctly, and if there is no effect-measure modification, adjusting for S1, although unnecessary, would not bias the effect estimate (i.e., 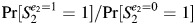 = Pr[S2 = 1|E2 = 1]/Pr[S2 = 1|E2 = 0] = Pr[S2 = 1|E2 = 1, S1]/Pr[S2 = 1|E2 = 0, S1]) because S1 is not a risk factor for the outcome. However, adjustment could affect the precision of the estimate (7, 14).
= Pr[S2 = 1|E2 = 1]/Pr[S2 = 1|E2 = 0] = Pr[S2 = 1|E2 = 1, S1]/Pr[S2 = 1|E2 = 0, S1]) because S1 is not a risk factor for the outcome. However, adjustment could affect the precision of the estimate (7, 14).
OTHER EXAMPLES FROM THE ORIGINAL PAPER
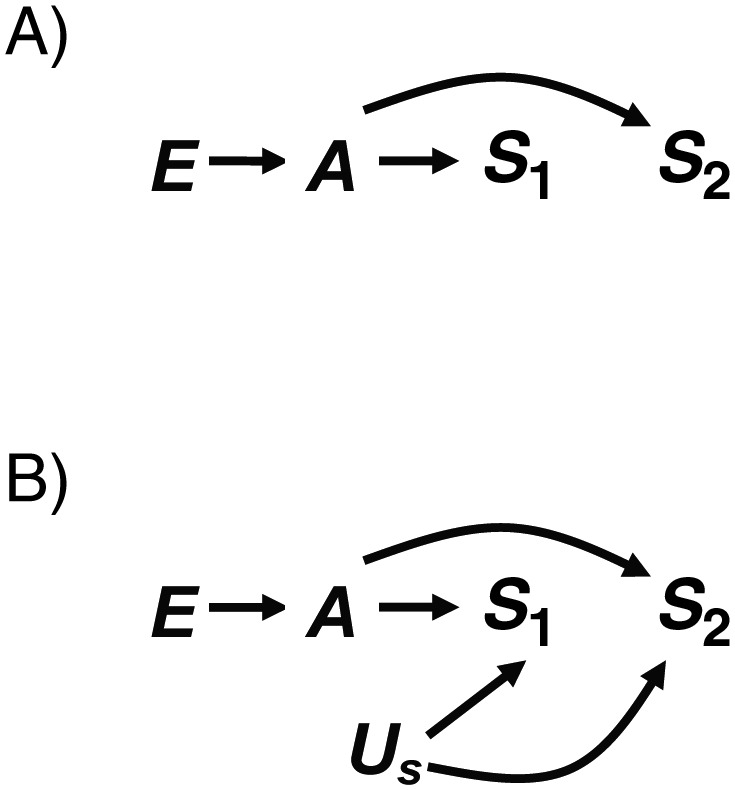
Weinberg considered other scenarios in which a variable could be mistaken for a confounder (1). She described a general situation where E caused 2 events that were independent given E, and the causal contrast of interest was Pr[S2e=1 = 1]/Pr[S2e=0 = 1]. The simplest case can be seen in DAG 5A (Figure 5), where the unadjusted estimate of the effect of E on S2 is not confounded. Nevertheless, as Weinberg reported, no bias would be introduced by adjusting for S1 because S1 is not associated with the outcome conditional on E (1, 14). However, in the more realistic scenario where there is a shared cause (Us) of S1 and S2, adjustment for S1 is no longer benign. DAG 5B is analogous to Figure 1c in the original paper (1). Here again there is no confounding, so Pr[S2e=1 = 1]/Pr[S2e=0 = 1] = Pr[S2 = 1|E = 1]/Pr[S2 = 1|E = 0]; but in this case, adjustment for S1 induces bias by opening the path E → S1 ← Us → S2. In DAG 5C, however, S1 is no longer a collider because there is no association between E and S1, so adjustment for S1 is unnecessary but would not introduce bias.
Figure 5.

A) Directed acyclic graph (DAG) 5A, in which exposure (E) causes both pregnancy outcomes (S1 and S2). B) DAG 5B, which adds a shared cause of S1 and S2 (Us) to DAG 5A. C) DAG 5C, in which E still causes S2 but no longer causes S1.
Weinberg also discussed cases where spontaneous abortion might serve as a proxy for an unmeasured confounder (Figure 6). In Figure 5, Us is not a confounder because it is not unconditionally associated with E, but in Figure 6, Use is a confounder on a backdoor path from E to S2. DAG 6A represents a scenario described by Weinberg (1) in which E is unrelated to the risk of S2 and the unadjusted estimate would be biased because of the open backdoor path E ← Use → S2. Adjusting for Use would block the backdoor path. Although S1 is not a confounder, it could be used as a proxy for Use if Use were unmeasured. However, there would be residual confounding to the degree that Use and S1 were not perfectly correlated. In addition, stratifying on S1 (as Weinberg demonstrated (1)) could result in greater bias in one stratum than in another (7), causing spurious effect-measure modification. DAG 6B is the same as DAG 6A, except that E is allowed to have an effect on S2. This change would not alter the above conclusions about using S1 as a proxy for Use.
Figure 6.
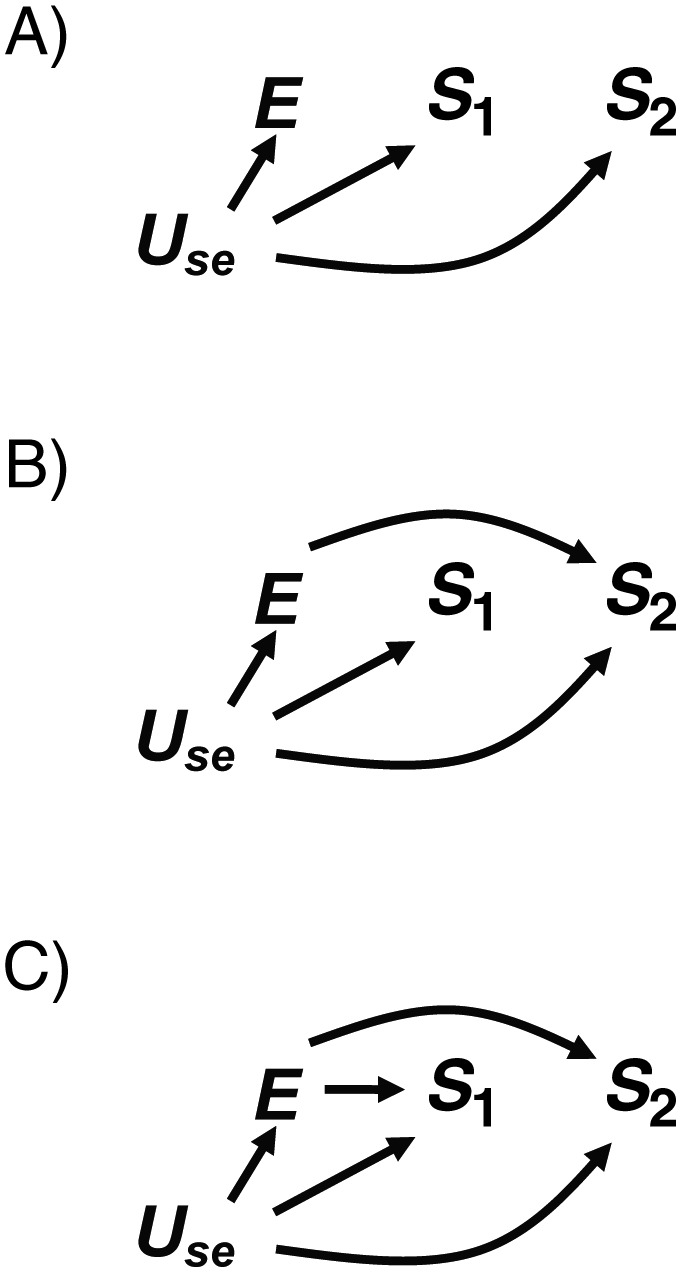
A) Directed acyclic graph (DAG) 6A, in which exposure (E) has no effect on the outcome (S2). An unmeasured confounder (Use) is a risk factor for both pregnancy outcomes (S1 and S2) and causes E. B) DAG 6B, which builds on DAG 6A by allowing E to be a risk factor for S2. C) DAG 6C, which builds on DAG 6B by allowing E to affect both pregnancy outcomes (S1 and S2).
DAG 6C resembles DAG 6B, except that now E affects both S1 and S2. Use is still a confounder, but S1 is now a collider, so its ability to serve as a proxy for Use is compromised. While adjusting for S1 as a proxy for Use might be intended to close the backdoor path E ← Use → S2, it would open the backdoor path E → S1 ← Use → S2. Whether the effect estimate adjusted for S1 would be more or less biased than the unadjusted estimate would depend on information not captured in the DAG, such as the strength of the effects represented by each arrow. Thus, for this example, some bias is unavoidable unless Use is measured.
DISCUSSION
We have used DAGs to revisit the examples Weinberg provided in her landmark 1993 paper, where a variable appeared to be a confounder using commonly applied criteria but adjustment for the variable actually introduced bias (1). Given her assumptions, our conclusions based on several possible DAGs matched those she derived with algebraic examples. To summarize the conclusions of the specific examples we explored, bias may be introduced when a descendant of the exposure is adjusted for if there is a path from that descendant to the outcome that does not involve the exposure (e.g., DAG 1A). Bias also may be introduced by adjusting for a variable that is a collider or a child of a collider on a noncausal path between the exposure and the outcome of interest (e.g., DAG 2A). Both of these situations often plausibly apply to history of spontaneous abortion. Thus, under several different causal structures, bias would be introduced by adjusting for this variable. Even when the true DAG is unknown, it is useful to draw and evaluate several different likely possibilities because, as is illustrated here, the conclusions may be the same under several different causal structures.
History of spontaneous abortion could serve as a proxy for an unmeasured confounder if it is not subject to the conditions just described (e.g., DAG 6B). However, residual bias would remain to the degree that reproductive history was imperfectly associated with the true unmeasured confounder, and this residual bias might be worse in one stratum than in another (7), causing apparent effect-measure modification. Thus, use of reproductive history as a proxy for an unmeasured confounder requires careful consideration.
The DAGs considered here also highlight the importance of defining the specific research question. In Figure 1, we considered an exposure that occurred prior to the first pregnancy outcome, whereas in Figures 2 and 3, we considered an exposure that occurred just prior to the current pregnancy outcome. The evaluation of a potential confounder needs to be made in the context of the specific research question and the associated DAG.
As DAG theory has percolated into epidemiology, there has been increasing awareness of the inadequacy of conventional criteria for appropriately identifying confounders, especially in the case where collider stratification bias may occur (3, 7, 9). DAGs help us build useful etiologic models by providing a transparent way to represent the causal relations between variables. They have simple rules that allow the identification of confounders, and they allow complexity that cannot be illustrated as concisely using algebraic proofs. However, DAGs do not provide information on the magnitude of the effects or the magnitude of the bias introduced by improper exclusion of confounders or inappropriate adjustment for nonconfounders. Quantitative analyses complement DAGs because they can be used to explore the magnitude of these biases under specific assumptions. Nevertheless, in order for a DAG (or an algebraic example) to be effective, the research question must be clearly defined. Even then, both approaches require simplifying assumptions. Despite their limitations, together quantitative sensitivity analyses and DAGs improve our ability to make causal inferences from observational data.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia (Penelope P. Howards); Division of Epidemiology, Statistics, and Prevention Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland (Enrique F. Schisterman); Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Charles Poole); Department of Epidemiology, Biostatistics, and Occupational Health, Faculty of Medicine, McGill University, Montreal, Quebec, Canada (Jay S. Kaufman); and Division of Intramural Research, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (Clarice R. Weinberg).
This investigation was supported in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Environmental Health Sciences (project ZIA ES040006-14), National Institutes of Health. J. S. K. was supported by the Canada Research Chairs program.
The authors thank the Helen Riaboff Whiteley Center, University of Washington (Friday Harbor, Washington), for providing a work environment conducive to writing.
Conflict of interest: none declared.
REFERENCES
- 1.Weinberg CR. Toward a clearer definition of confounding. Am J Epidemiol. 1993;137(1):1–8. doi: 10.1093/oxfordjournals.aje.a116591. [DOI] [PubMed] [Google Scholar]
- 2.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 3.Greenland S. Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology. 2003;14(3):300–306. [PubMed] [Google Scholar]
- 4.Hernán MA, Hernández-Díaz S, Werler MM, et al. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155(2):176–184. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 5.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 6.Pearl J. Causal diagrams for empirical research. Biometrika. 1995;82(4):669–710. [Google Scholar]
- 7.Glymour MM, Greenland S. Causal diagrams. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3rd. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. pp. 183–209. [Google Scholar]
- 8.Shpitser I, VanderWeele TJ, Robins JM. Proceedings of the 26th Conference on Uncertainty and Artificial Intelligence. Corvallis, WA: AUAI Press; 2010. On the validity of covariate adjustment for estimating causal effects; pp. 527–536. [Google Scholar]
- 9.Hernández-Díaz S, Schisterman EF, Hernán MA. The birth weight “paradox” uncovered? Am J Epidemiol. 2006;164(11):1115–1120. doi: 10.1093/aje/kwj275. [DOI] [PubMed] [Google Scholar]
- 10.Pearl J. Causality: Models, Reasoning, and Inference. 2nd. New York, NY: Cambridge University Press; 2009. [Google Scholar]
- 11.Nelson DB, McMahon K, Joffe M, et al. The effect of depressive symptoms and optimism on the risk of spontaneous abortion among innercity women. J Womens Health (Larchmt) 2003;12(6):569–576. doi: 10.1089/154099903768248276. [DOI] [PubMed] [Google Scholar]
- 12.Swan SH, Waller K, Hopkins B, et al. A prospective study of spontaneous abortion: relation to amount and source of drinking water consumed in early pregnancy. Epidemiology. 1998;9(2):126–133. [PubMed] [Google Scholar]
- 13.Weng X, Odouli R, Li DK. Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am J Obstet Gynecol. 2008;198(3):e1–e8. doi: 10.1016/j.ajog.2007.10.803. [DOI] [PubMed] [Google Scholar]
- 14.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]