Abstract
The authors examined the impact of cumulative neighborhood risk of psychosocial stress on allostatic load (AL) among adolescents as a mechanism through which life stress, including neighborhood conditions, may affect health and health inequities. They conducted multilevel analyses, weighted for sampling and propensity score-matched, among adolescents aged 12–20 years in the National Health and Nutrition Examination Survey (1999–2006). Individuals (first level, n = 11,886) were nested within families/households (second level, n = 6,696) and then census tracts (third level, n = 2,191) for examination of the contextual effect of cumulative neighborhood risk environment on AL. Approximately 35% of adolescents had 2 or more biomarkers of AL. A significant amount of variance in AL was explained at the neighborhood level. The likelihood of having a high AL was approximately 10% higher for adolescents living in medium-cumulative-risk neighborhoods (adjusted odds ratio (OR) = 1.09, 95% confidence interval (CI): 1.08, 1.09), 28% higher for those living in high-risk neighborhoods (adjusted OR = 1.28, 95% CI: 1.27, 1.30), and 69% higher for those living in very-high-risk neighborhoods (adjusted OR = 1.69, 95% CI: 1.68, 1.70) as compared with adolescents living in low-risk areas. Effect modification was observed by both individual- and neighborhood-level sociodemographic factors. These findings offer support for the hypothesis that neighborhood risks may culminate in a range of biologically mediated negative health outcomes detectable in adolescents.
Keywords: adolescent; allostasis; risk; risk factors; stress, physiological; stress, psychological
While health disparities are often noted among adult populations, the root of adult disparities probably lies early in development, as variations in health status have been observed in children (1). Differential exposure to physical, social, and psychosocial stressors, which are common in low socioeconomic status and minority populations, likely play an important role in producing and maintaining health disparities (2–7). Although it is known that stress exposure is associated with increased morbidity and mortality across health indices, little is known about the mechanisms through which stressors are translated into biologic risk, including when biologic effects can first be observed (8–10). Further, cumulative stress exposure may be more important than any single exposure (8, 11–14).
Accumulating evidence suggests an important role of biologically mediated pathways through which stress exposure during early childhood “gets under the skin” and results in gradients of health outcomes across socioeconomic and racial/ethnic lines (15, 16). The cumulative impact of stressful proximal and distal social exposures, or cumulative risk, has been proposed as an explanatory mechanism for a socioeconomic position–health gradient (1, 8). In this paper, we explore the impact of both positive and negative distal community environments on allostatic load (AL), independent of family or household characteristics. AL is defined as the cumulative wear and tear on physiologic processes due to recurrent or chronic stress (17, 18). The AL model proposes that dysregulation of physiologic systems designed to balance the organism's responses to environmental demands is a key mediator between adversity and negative health outcomes.
Lower neighborhood socioeconomic position in the United States has been associated with significantly greater AL, independent of individual-level SES (19, 20), with the strongest association being observed among African Americans (21). Stemming in part from disparities in neighborhood factors (5), blacks may have greater dysregulation in the hypothalamic pituitary axis than whites (22, 23).
Beyond socioeconomic status, few studies have examined additional neighborhood stressors and their cumulative impact on AL, particularly among children (8, 24). Using a large national sample of adolescents from the National Health and Nutrition Examination Survey (NHANES) in a 3-level multilevel model who were propensity score-matched on the basis of neighborhood risk, we examined 1) the impact of cumulative risk at the neighborhood level on AL in adolescents, 2) its differential impact by individual and neighborhood sociodemographic factors, and 3) the pathway from socioeconomic neighborhood polarization to AL through cumulative risk. Signs of neighborhood disorder can be indicative of a breakdown of social control and community social capital. Although debate exists, social capital remains an important component in deciphering the complex interactions associated with health disparities (25–27). We examined concentrated advantage as a marker of social capital linked at the level of cumulative social and physical risk in a neighborhood.
MATERIALS AND METHODS
Study design and population
Data were obtained from continuous biennial NHANES cross-sectional survey cycles for the period 1999–2006. The NHANES captures health information through a survey, a medical examination, and laboratory testing. One or more persons per household are selected, and data are collected from each participant through a face-to-face household interview. Participants are invited to provide biologic specimens and to undergo a physical examination in the medical examination component (28). We utilized data from adolescent respondents aged 12–20 years. Georeferenced NHANES data (29) were linked to neighborhood environmental data. The average sample size for continuous NHANES surveys is approximately 3,000 adolescents.
The following exclusion criteria were applied to the initial adolescent sample. Adolescents were excluded if they 1) did not fast for 6 hours prior to physical examination (n = 586), 2) were currently pregnant (n = 293) (30), or 3) were current smokers (n = 2,160; 14% of the total adolescent sample) (31). The final sample size, including persons with georeferenced data and nonmissing values on AL indicators, was 11,866. There were 2,191 census tracts, and the number of adolescents ranged from 1 to 194 per tract (mean = 8). Less than 15% of tracts had fewer than 3 adolescents. Small group size does not affect results when the group number is large, as was the case in the present analyses (32).
This study was approved by the Tulane and Louisiana State University institutional review boards and the National Center for Health Statistics Research Data Center (Centers for Disease Control and Prevention).
Individual-level measures
Similar to previous NHANES research (21), our primary outcome, AL, was based on a summary score from 10 biomarkers: waist circumference (cm), triglyceride concentrations (mg/dL), fasting glucose concentration (mg/dL), insulin resistance, high density lipoprotein (HDL) cholesterol concentration (mg/dL), low density lipoprotein cholesterol concentration (mg/dL), glycosylated hemoglobin level (%), hypertension, asthma diagnosis, and C-reactive protein concentration (mg/dL). Asthma diagnosis was added as an immune marker (33). Respondents were defined as being “at risk” with regard to each biomarker if their value was greater than 1 standard deviation above the mean for their age and sex or if they had a value at/above the adult cutoff value. The exception was hypertension, which was defined as being greater than or equal to the 90th percentile for age, sex, and height for systolic or diastolic blood pressure or a confirmed diagnosis of hypertension. Adult cutoff values included ≤35 mg/dL for HDL cholesterol, ≥102 cm for males and ≥88 cm females for waist circumference, ≥150 mg/dL for triglyceride level, and ≥100 mg/dL for glucose level or a confirmed diagnosis of diabetes. Homeostasis model assessment was used to evaluate insulin resistance according to the following formula: fasting serum insulin level (μU/mL) × fasting plasma glucose level (mmol/L)/22.5.
AL was examined as a continuous score and as high AL versus low AL, with high AL being defined as having 2 or more risk factors. Approximately 35% of adolescents had 2 or more biomarkers of AL. Average AL was 1.0 (standard deviation (SD), 1.12), and the range was 0–7; no adolescent had all 10 biomarkers.
Sociodemographic characteristics included age, sex, education, and a combined race/ethnicity indicator (non-Hispanic white, non-Hispanic black, Hispanic/Latino, or other). Diet as measured by the US Department of Agriculture's Healthy Eating Index (the sum of 10 dietary components, weighted equally), physical activity (engaging in no activity, at least moderate activity but less than vigorous activity, and at least vigorous activity during the last month), and current illness or cold were also included as covariates.
The Healthy Eating Index was comprised of 5 components that assessed the nutrient adequacy of the diet by using the 5 major food groups of the original Food Guide Pyramid: fruit, vegetables, grains, milk, and meat (US Department of Agriculture (http://www.cnpp.usda.gov/FGP.htm)). Four components assessed aspects of the diet that should be limited or consumed in moderation: total fat, expressed as a percentage of total calories; saturated fat, expressed as a percentage of total calories; cholesterol; and sodium. The 10th component was a measure of variety in food choices regardless of food group. Standards were set for scoring each of the components, relating to the Food Guide Pyramid and average estimated energy requirements for each of 11 age-sex groups. Scores for each component ranged from 0 to 10; thus, the total maximum score was 100, with a total score of more than 80 being considered “good,” scores of 51–80 indicating “needs improvement,” and scores of less than 51 being considered “poor.”
Household-level measures
Household-level measures comprised numerous primary confounders and potential mediators, including household poverty:income ratio, categorized as <1 (below the federal poverty threshold), 1–<2 (1–<2 times the poverty threshold), or ≥2 (≥2 times the poverty threshold); the AL score of the primary adult respondent, based on the same markers as those utilized for adolescents; parental educational level; parental marital status; duration of residence (years) in the household/neighborhood; and household density/crowding.
Neighborhood-level measures
Neighborhood-level data were generated using the geographic identifiers for each adolescent. Given available national and NHANES data sources, “neighborhood” was defined as US census tract. Given the nature of NHANES georeferencing, data for the entire United States at the census-tract level were merged into local NHANES data at secure research data centers. At no time was any identifying census tract information available to the authors. Sources for neighborhood data included US Census 2000, geographic data from ArcGIS (ESRI, Inc., Redlands, California) and US Census TIGER/Line Shapefiles (http://www.census.gov/geo/www/tiger/tgrshp2010/tgrshp2010.html), and North American Industry Classification (NAICS) System Standard Industrial Classification (SIC) data (34) and crime risk data from ESRI community data sources. All definitions of neighborhood were based on the Census 2000 TIGER/Line Shapefiles. Data on neighborhood-level sociodemographic characteristics were obtained from Census 2000. Primary sociodemographic characteristics included percentage of persons living below the federal poverty level, percentage of vacant homes, percentage of households with female heads, percent working class, percentage of adults in the tract with a college degree or higher, and education index of concentration at the extremes (ICE). ICE, an indicator of social capital, was calculated as the number of residents in a census tract with a college degree (as a measure of socioeconomic advantage), calculated as: ICE = (number of college graduates − number with no high school diploma)/total population) × 100. ICE scores range from −100 (extremely disadvantaged—all high school dropouts) to 100 (extremely advantaged—all college graduates) (35).
Data on neighborhood alcohol availability, physical activity facilities, and food environment (number of supermarkets, etc.) were based on NAICS codes provided by infoUSA (Infogroup Inc., Papillion, Nebraska) and obtained from ESRI, Inc. The NAICS is the federal US standard for classifying business establishments for the purpose of collecting, analyzing, and publishing statistical data related to the US business economy. infoUSA offers commercial databases on businesses with information regarding business openings and closings, updated on a weekly basis. Selected characteristics of the businesses are verified monthly by telephone interviews, and SIC codes are assigned to each business. SIC codes were supplemented with codes with an additional 2-digit number developed by infoUSA to further detail business type. SIC codes were utilized to classify off- and on-premise alcohol outlets used for calculation of the neighborhood alcohol environment and for classification of the availability of recreational facilities for physical activity. Given the temporal frame of the georeferenced continuous NHANES data (1999–2006), the project temporally aligned collected data with historical data from infoUSA on the length of time specific business establishments were present in a tract. Neighborhood alcohol environment measures included off-premise alcohol data—specifically, liquor store density and convenience store density—as well as on-premise outlet density, which included bars/pubs, clubs, and restaurants with on-site consumption. Physical activity environment included all recreational facilities in the tract, such as gyms and parks. Body mass index (BMI)-unhealthy density was based on the number of fast-food restaurants, pizzerias, convenience stores, unhealthy snack-food concessions, candy stores/confectioners, and bodegas per capita (36). The measure was calculated as the density of such establishments per 1,000 residents in the census tract.
Information on crime risk for each neighborhood was obtained from ESRI and comprised a series of standardized indexes (relative to national risk) for a range of serious crimes, based on categories from the Federal Bureau of Investigation's Uniform Crime Reporting Program. The total crime risk index, which included murder, rape, robbery, assault, burglary, theft, and motor vehicle theft, ranged from 1 to 1,036 (mean = 138.76 (SD, 134)).
A cumulative neighborhood risk index was constructed through factor analysis by selecting 9 out of 17 neighborhood conditions that loaded highly on factors that represented social or physical neighborhood risk: total crime risk index, on-premise alcohol density, liquor store density, low supermarket density, BMI-unhealthy density, low density of physical activity facilities, percentage below the poverty level, percentage of vacant homes, and percentage of female-headed households. To decrease collinearity between resulting factors, only variables that loaded above 0.55 were retained. Each variable was then standardized with a mean of 0 and a standard deviation of 1, categorized so that 1 standard deviation above the mean represented being “at risk,” and values were then summed. The cumulative risk index was categorized as low (0 risk factors), medium (1–2 risk factors), high (3–4 risk factors), or extremely high (>4 risk factors) and was examined with regard to physical (i.e., alcohol, food, and physical activity environment, vacant homes) and social (crime, female-headed households, poverty) characteristics.
Statistical analysis
Descriptive univariate, bivariate, and multivariate analyses were all weighted for sampling design and survey nonresponse. Analyses used SAS, version 9 (SAS Institute Inc., Cary, North Carolina), including PROC MIXED and GLIMMIX for hierarchical models. Three-level hierarchical regression models included individuals (first level; n = 11,866) nested within families/households (second level; n = 6,696) nested within census tracts (third level; n = 2,191) in order to examine cumulative neighborhood risk effects on AL, to evaluate potential mediation of cumulative risk in the relation between ICE and AL, and to identify effect modification. Such models allow for partitioning of variance estimates at all levels (37, 38), expressed as the intraclass correlation coefficient (ICC), thereby accounting for variance in individual-level outcomes that can be attributed to differences between neighborhoods. To examine clustering in AL and biomarkers based on unconditional means models, we utilized PROC MIXED for linear outcomes with the traditional ICC calculation. When PROC GLIMMIX examined high AL versus low AL (i.e., ≥2 biomarkers), the pseudo-ICC was calculated by following Snijders and Boskers' formula (38) based on an underlying continuous variable with Vindividual = П2/3. However, because the pseudo-ICC for nonlinear models may not be appropriate in all analyses, we also calculated the median odds ratio (37). All individual-level variables were mean-centered.
RESULTS
Respondent characteristics are presented in Table 1. Males, older adolescents, minorities, adolescents who engaged in no physical activity or only moderate physical activity, those living below the federal poverty line, and those living in a rural census tract had a significantly higher AL. A greater Healthy Eating Index was associated with a lower likelihood of high AL, while a greater adult AL was associated with higher AL. Results were similar for dichotomous and continuous AL scores.
Table 1.
Characteristics of Adolescents Aged 12–20 Years According to Allostatic Load, National Health and Nutrition Examination Survey, 1999–2006a
| High AL (≥2 Biomarkers) (n = 4,141) |
Lower AL (<2 Biomarkers) (n = 7,725) |
Total (n = 11,866) |
||||
|---|---|---|---|---|---|---|
| Row % | Mean | Row % | Mean | Column % | Mean | |
| Sex | ||||||
| Male | 37.4 | 62.6 | 49.6 | |||
| Female | 33.9 | 66.1 | 50.4 | |||
| Age, years | ||||||
| 12–14 | 34.3 | 65.7 | 42.1 | |||
| 15–17 | 31.8 | 68.2 | 33.2 | |||
| 18–20 | 38.6 | 61.4 | 24.7 | |||
| Race/ethnicity | ||||||
| Non-Hispanic white | 31.4 | 68.6 | 62.2 | |||
| Non-Hispanic black | 36.5 | 63.5 | 13.7 | |||
| Hispanic/Latino | 35.7 | 64.3 | 17.5 | |||
| Other | 32.6 | 67.4 | 6.6 | |||
| Education | ||||||
| Less than 9th grade | 32.8 | 67.2 | 82.8 | |||
| 9th–12th grade | 47.8 | 52.2 | 8.4 | |||
| High school graduate/ GED or more | 27.7 | 72.3 | 8.9 | |||
| Poverty status | ||||||
| PIR <1 (below federal poverty line) | 36.4 | 63.6 | 23.0 | |||
| PIR 1–<2 | 35.0 | 65.0 | 23.4 | |||
| PIR ≥2 | 30.0 | 70.0 | 53.6 | |||
| Urbanicity | ||||||
| Urban | 33.9 | 66.1 | 28.6 | |||
| Rural | 37.4 | 62.6 | 71.3 | |||
| Healthy Eating Index scoreb | 58.7 | 60.5 | 59.5 | |||
| Past-month physical activity | ||||||
| None | 35.3 | 64.7 | 35.2 | |||
| Moderate | 34.2 | 65.8 | 15.5 | |||
| Vigorous | 30.7 | 69.3 | 49.3 | |||
| Household adult AL scorec | 1.2 | 0.6 | 0.9 | |||
Abbreviations: AL allostatic load; GED, General Educational Development; PIR, poverty:income ratio.
a Proportions and means based on nonmissing values.
b Healthy Eating Index scores ranged from 0 to 100, with a total score of more than 80 considered “good,” scores of 51–80 indicating “needs improvement,” and scores less than 51 considered “poor.”
c The AL score for adults was similar to that for children and was calculated on the basis of a summary score for being classified as at-risk with regard to 10 biomarkers: waist circumference (cm), triglycerides (mg/dL), fasting glucose (mg/dL), insulin resistance, high density lipoprotein cholesterol (mg/dL), low density lipoprotein cholesterol (mg/dL), glycosylated hemoglobin (%), hypertension, asthma diagnosis, and C-reactive protein (mg/dL).
Table 2 illustrates the substantial variability in neighborhoods with an average cumulative neighborhood risk of 3.23 (SD, 1.87), ranging from 1 to 8. Classified by risk category, 9% of adolescents lived in low-risk neighborhoods, 42.5% lived in medium-risk neighborhoods, 36.5% lived in high-risk neighborhoods, and 12% lived in very-high-risk neighborhoods. Of adolescents residing in neighborhoods classified as having a very high cumulative risk, 15% were non-Hispanic white, while 85% were minority (52% non-Hispanic black, 31% Hispanic/Latino, and 2% other). Only 5.5% of non-Hispanic whites resided in very-high-risk neighborhoods, compared with 20.3% of non-Hispanic black adolescents and 9.4% of Hispanic adolescents.
Table 2.
Characteristics of Respondents' (Adolescents Aged 12–20 Years) Neighborhoods (2,191 US Census Tracts), National Health and Nutrition Examination Survey, 1999–2006a
| Mean (SD) | % | Range | |
|---|---|---|---|
| Race/ethnicity, mean % | |||
| Non-Hispanic black | 21.39 (29.82) | 0.00–99.41 | |
| Non-Hispanic white | 60.56 (29.87) | 0.00–99.42 | |
| Asian | 3.17 (6.89) | 0.00–36.90 | |
| Hispanic/Latino | 12.00 (20.00) | 0.00–91.43 | |
| Mean % male | 46.31 (4.07) | 30.83–76.10 | |
| Mean % married | 48.71 (13.33) | 1.60–85.90 | |
| Mean % female-headed households | 43.12 (14.59) | 5.37–84.62 | |
| Education, mean % | |||
| Less than high school | 27.67 (15.57) | 0.00–84.45 | |
| College degree or higher | 18.08 (14.29) | 0–92.31 | |
| Mean % living below federal poverty level | 33.05 (16.72) | 3.96–84.20 | |
| Mean socioeconomic position indexb | 0.79 (2.43) | −4.17 to 11.87 | |
| Mean % unemployed | 4.67 (3.27) | 0.00–46.91 | |
| Mean % of vacant homes | 12.45 (7.07) | 2.31–41.92 | |
| Mean % of residents on public assistance | 5.69 (5.51) | 0.00–64.63 | |
| Mean ICEc | −9.57 (28.49) | −82.71 to 92.30 | |
| Characteristics of the built and social environment (per 1,000 residents) | |||
| No. of on-premise alcohol outletsd per capita | 0.16 (0.39) | 0–7.89 | |
| No. of liquor stores per capita | 0.10 (0.48) | 0–6.16 | |
| No. of convenience stores per capita | 0.48 (0.53) | 0–4.27 | |
| No. of supermarkets per capita | 0.10 (0.13) | 0–1.35 | |
| BMIe-unhealthy densityf per capita | 2.42 (2.79) | 0–46.18 | |
| No. of physical activity facilities per capita | 0.18 (0.30) | 0–3.15 | |
| Mean total crime riskg | 345.59 (161.29) | 9.50–791.50 | |
| Mean cumulative neighborhood risk score | 3.23 (1.87) | 1.00–8.00 | |
| Low cumulative risk (0 risk factors) | 9.0 | ||
| Medium cumulative risk (1–2 risk factors) | 42.5 | ||
| High cumulative risk (3–4 risk factors) | 36.5 | ||
| Very high cumulative risk (>4 risk factors) | 12.0 |
Abbreviations: BMI, body mass index; ICE, index of concentration at the extremes; SD, standard deviation.
a Based on nonmissing values.
b Based on percentage of residents with less than a high school education, percent unemployed, percent living below the federal poverty level, percent working class, and median household income.
c ICE = (number of college graduates – number with no high school diploma/total population) × 100.
d Bars/pubs, clubs, and restaurants with on-site alcohol consumption.
e Weight (kg)/height (m)2.
f Based on the number of fast-food restaurants, pizzerias, convenience stores, unhealthy snack-food concessions, candy stores/confectioners, and bodegas per capita, calculated as the density of such establishments per 1,000 residents.
g Crime risk was calculated using a series of standardized indexes (relative to national risk) for a range of serious crimes, based on categories from the Federal Bureau of Investigation's Uniform Crime Reporting Program. The total crime risk index, which included murder, rape, robbery, assault, burglary, theft, and motor vehicle theft, ranged from 1 to 1,036 (mean = 138.76 (SD, 134)).
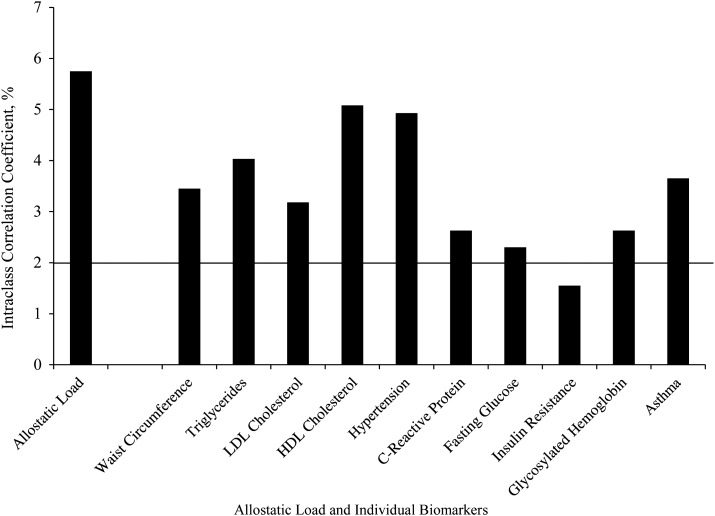
While lower than the level of household clustering, we observed significant clustering of AL at the neighborhood level, with an ICC of 5.75%, suggesting that persons from the same neighborhood are likely to have similar ALs in comparison with persons from other neighborhoods (see Figure 1). This finding suggests that some variance in adolescent AL may be explained by neighborhood-level factors such as cumulative risk. Significant clustering above an ICC of 2% was observed for each biomarker, with HDL cholesterol exhibiting the highest level of clustering and insulin resistance the lowest level of clustering by neighborhood.
Figure 1.
Clustering (intraclass correlation coefficient) of allostatic load and biomarkers (on original, continuous scales; see text for units) in neighborhoods, National Health and Nutrition Examination Survey, 1999–2006. (HDL, high density lipoprotein; LDL, low density lipoprotein).
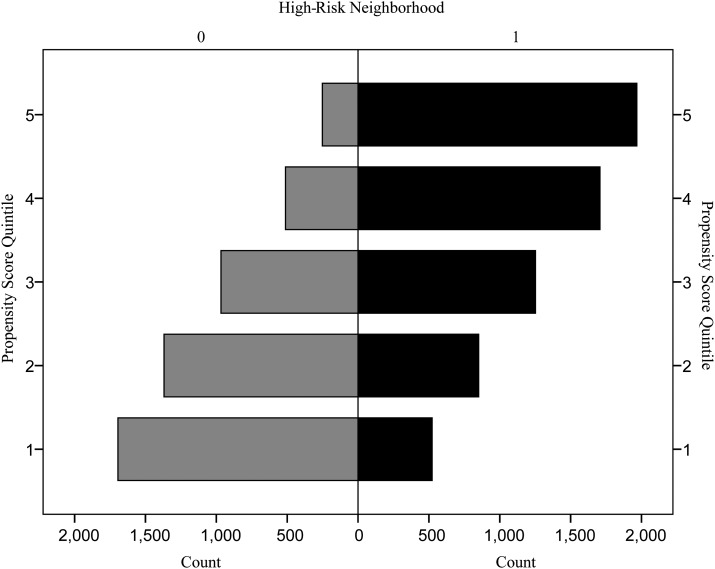
To address potential selection bias or endogeneity, we frequency-matched the sample based on respondents' propensity for living in a very-high- or high-cumulative-risk neighborhood versus a medium- or low-cumulative-risk neighborhood. Figure 2 demonstrates propensity score overlap, yet adolescents living in high-risk neighborhoods had a greater propensity for living in such an area (as expected). Accounting for such differences in the multilevel analyses was therefore justified.
Figure 2.
Propensity score matching (propensity for living in a high-cumulative-risk neighborhood vs. a low-cumulative-risk neighborhood according to residence in a high- vs. low-cumulative-risk neighborhood), National Health and Nutrition Examination Survey, 1999–2006. “High Risk Neighborhood” = actual residence in a very-high- or high-cumulative-risk neighborhood (1) versus a medium- or low-cumulative-risk neighborhood (0). Propensity score quintiles rank the propensity for living in a high-cumulative-risk neighborhood.
Table 3 presents crude and adjusted results of 3-level multilevel logistic regression analysis. The empty model (model A) demonstrated that 6.23% of the variance in high AL was explained at the neighborhood level. Including cumulative neighborhood risk in this empty model (model B) explained 65% of the group-level variance, as shown by declines in the ICC (6.23%–2.72%) and the median odds ratio (2.20–1.47). The median odds ratio of 1.47 suggests more than a doubling of risk for high AL when going from an area of low cumulative risk to an area of very high cumulative risk. Cumulative neighborhood risk was also significantly associated with AL, with evidence of a dose-response relation. Compared with adolescents living in low-risk neighborhoods, adolescents living in medium-risk neighborhoods were 1.16 times as likely to have a high AL (95% confidence interval (CI): 1.16, 1.17); those in high-risk neighborhoods were 37% more likely to have a high AL (crude odds ratio (OR) = 1.37, 95% CI: 1.36, 1.37); and adolescents living in very-high-cumulative-risk neighborhoods were 1.84 times more likely to have a high AL (95% CI: 1.83, 1.85). Examining neighborhood risk conditions separately revealed that poverty, crime, and off-premise alcohol density had the strongest associations with AL.
Table 3.
Factors Associated With Allostatic Load (≥2 Biomarkers) in Hierarchical Logistic Regression Models (1,958 Census Tracts, 8,851 Adolescents), National Health and Nutrition Examination Survey, 1999–2006a
| Variable | Model A: Empty Model |
Model B: Model With Cumulative Neighborhood Risk |
Model C: Model
With ICE + Other Covariates |
Model D: Model With Cumulative Neighborhood Risk + Other Covariates |
Model E: Model With Cumulative Risk and ICE (Potential Mediation) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | % | OR | 95% CI | % | OR | 95% CI | % | OR | 95% CI | % | OR | 95% CI | % | |
| Individual-level variables | |||||||||||||||
| Female sex | 0.87 | 0.86, 0.88 | 0.87 | 0.86, 0.88 | 0.80 | 0.80, 0.81 | |||||||||
| Age, years | 1.07 | 1.06, 1.08 | 1.05 | 1.05, 1.05 | 1.04 | 1.04, 1.05 | |||||||||
| Race/ethnicity and PIR | |||||||||||||||
| Non-Hispanic white and PIR ≥1 (referent) | 1.00 | 1.00 | 1.00 | ||||||||||||
| Minority and PIR ≥1 | 1.04 | 1.03, 1.04 | 1.02 | 1.01, 1.02 | 1.02 | 1.01, 1.02 | |||||||||
| Non-Hispanic white and PIR <1 | 1.15 | 1.14, 1.17 | 1.22 | 1.21, 1.23 | 1.22 | 1.21, 1.23 | |||||||||
| Minority and PIR <1 | 1.11 | 1.10, 1.12 | 1.12 | 1.11, 1.13 | 1.12 | 1.11, 1.13 | |||||||||
| Physical activity (none, moderate, or vigorous) | 0.91 | 0.91, 0.92 | 0.90 | 0.89, 0.91 | 0.90 | 0.89, 0.91 | |||||||||
| Healthy Eating Index scoreb | 0.99 | 0.98, 0.99 | 0.98 | 0.97, 0.99 | 0.98 | 0.97, 0.99 | |||||||||
| Currently having an infection | 1.19 | 1.19, 1.20 | 1.31 | 1.30, 1.32 | 1.31 | 1.30, 1.32 | |||||||||
| Survey cycle (cycle 1 (1999–2000) to cycle 4 (2005–2006)) | 1.11 | 1.11, 1.12 | 1.11 | 1.11, 1.12 | 1.11 | 1.11, 1.12 | |||||||||
| Household-level variables | |||||||||||||||
| Adult respondent education (<HS, HS/GED, >HS) | 0.71 | 0.70, 0.72 | 0.72 | 0.71, 0.73 | 0.72 | 0.71, 0.73 | |||||||||
| Adult respondent marital status (married vs. not married) | 0.51 | 0.50, 0.52 | 0.65 | 0.65, 0.66 | 0.65 | 0.65, 0.66 | |||||||||
| No. of years of residence in neighborhood | 1.01 | 1.01, 1.02 | 1.01 | 1.01, 1.02 | 1.01 | 1.01, 1.02 | |||||||||
| Adult respondent allostatic load score | 1.12 | 1.11, 1.13 | 1.13 | 1.12, 1.13 | 1.13 | 1.12, 1.13 | |||||||||
| Census tract-level variables | |||||||||||||||
| Cumulative neighborhood risk | |||||||||||||||
| Low (referent) (0 risk factors) | 1.00 | 1.00 | 1.00 | ||||||||||||
| Medium (1–2 risk factors) | 1.16 | 1.16, 1.17 | 1.09 | 1.08, 1.09 | 1.05 | 1.01, 1.09 | |||||||||
| High (3–4 risk factors) | 1.37 | 1.36, 1.37 | 1.28 | 1.27, 1.30 | 1.26 | 1.25, 1.28 | |||||||||
| Very high (>4 risk factors) | 1.84 | 1.83, 1.85 | 1.69 | 1.68, 1.70 | 1.36 | 1.35, 1.36 | |||||||||
| ICEc | 0.96 | 0.93, 0.98 | 0.97 | 0.95, 0.99 | |||||||||||
| Urbanicity (urban census tract vs. rural census tract) | 0.83 | 0.82, 0.83 | 0.80 | 0.79, 0.81 | 0.79 | 0.79, 0.80 | |||||||||
| Random effectsd | |||||||||||||||
| Household ICC | 41.35 | 40.09 | 25.34 | 25.52 | 25.53 | ||||||||||
| Neighborhood ICC | 6.23 | 2.71 | 4.61 | 2.10 | 2.09 | ||||||||||
| Neighborhood median OR | 2.20 | 1.47 | 1.92 | 1.45 | 1.42 | ||||||||||
Abbreviations: CI, confidence interval; GED, General Educational Development; HS, high school; ICC, intraclass correlation coefficient; ICE, index of concentration at the extremes; OR, odds ratio; PIR, poverty:income ratio.
a All models were based on frequency-matching of propensity score, or propensity to live in a high- or very-high-cumulative-risk neighborhood (≥3 risk factors) versus a medium- (1–2 risk factors) or low- (0 risk factors) cumulative-risk neighborhood. Results were similar when allostatic load was examined as a continuous outcome.
b Healthy Eating Index scores ranged from 0 to 100, with a total score of more than 80 considered “good,” scores of 51–80 indicating “needs improvement,” and scores less than 51 considered “poor.”
c ICE = number of college graduates – number with no high school diploma/total population) × 100.
d Individual-level variance was calculated using the formula of Snijders and Boskers (38) on the basis of an underlying continuous variable with Vindividual = П2/3. Because of the limitations of the ICC with regard to nonlinear outcomes, the median OR (37) was also calculated.
Model C in Table 3 presents results from the multivariate, multilevel logistic model that included additional individual-, household- and neighborhood/tract-level predictors of AL—examining specifically the impact of ICE. Adolescents living in disadvantaged neighborhoods (with a lower concentration of college graduates) had a significantly higher likelihood of a high AL, with a 4% higher likelihood for each unit increase in ICE (adjusted OR = 0.96, 95% CI: 0.93, 0.98). Model D was similar to model C but examined the impact of cumulative risk after taking into account predictors and potential confounders. The likelihood of having a high AL remained approximately 10% higher for adolescents living in medium-risk neighborhoods (adjusted OR = 1.09, 95% CI: 1.08, 1.09), 28% higher for adolescents living in high-cumulative-risk neighborhoods (adjusted OR = 1.28, 95% CI: 1.27, 1.30), and 69% higher for adolescents living in very-high-risk neighborhoods (adjusted OR = 1.69, 95% CI: 1.68, 1.70) than for those living in low-risk areas. These covariates explained an additional 15% of neighborhood-level variance.
Including both cumulative risk and ICE in the model (Table 3, model E) revealed partial mediation by cumulative risk, evidenced by a decrease but not removal of the impact of ICE on AL. Both ICE and cumulative risk remained significantly associated with AL. Results were similar using linear regression (AL score) versus logistic regression for all analyses.
Significant effect modification was observed in the relation between cumulative risk and AL by race/ethnicity/poverty, sex, age, and ICE. The likelihood of high AL at each cumulative risk level was dependent on these individual and neighborhood sociodemographic factors (see Web Figure 1, which appears on the Journal's website (http://aje.oxfordjournals.org/)). There was limited interaction for sex and age, with fairly parallel lines across cumulative risk categories, except for adolescents in low-cumulative-risk environments (where high AL likelihood was greater for girls than for boys) and between medium- and high-cumulative-risk categories, where the probability of high AL became even greater for adolescents aged 15–17 years. However, slopes for adolescents aged 12–14 years and 15–17 years were greater than the slope for adolescents aged 18–20 years. With respect to race/ethnicity/poverty, persons living in poverty had a greater likelihood of high AL.
DISCUSSION
The present results help expand the broader literature on chronic stress in childhood. We found that greater cumulative neighborhood risk resulted in a higher AL, over and above household-level risk. These results are in agreement with the limited previous studies of AL in children (8, 24) and a demonstrate a positive and dose-response relation between cumulative neighborhood risk/stress exposure and AL. We have extended the previous evidence by 1) examining neighborhood risk over and above household risk in a multilevel framework, 2) including a broad array of social and physical stressful neighborhood conditions and examination of neighborhood sociodemographic pathways, and 3) providing significant external validity by testing the hypothesis in the NHANES study, which represents a large national sample of US adolescents. These investigative strengths and results demonstrated that a significant proportion of the variance in AL was explained at the neighborhood level, consistent with both allostasis (20) and cumulative risk (16) theories. Furthermore, because we accounted for reference-respondent AL (typically the adolescent's mother), the results illustrate that this is a unique effect beyond even biologically mediated household-level risk. The observed impact of cumulative risk on adolescent AL suggests that younger children are indeed vulnerable to the same neighborhood stress exposures as adults. Boys and adolescents living below the poverty level appear to be most at risk. Together, these findings suggest heightened vulnerability to neighborhood stressors in children and adolescents, perhaps due to the rapidness of developmental changes at these ages.
Differentiating between potentially modifiable socioecologic levels, such as household and community or neighborhood, is vital for targeted prevention efforts. While the household level explained a significantly greater proportion of variance in AL than the neighborhood level (as expected), the impact of neighborhood effects was not insignificant, and it persisted after we accounted for household clustering and household- and individual-level covariates. Targeting neighborhood change is feasible and may not only improve neighborhood quality but also exert a small, sustained improvement in the health of young people in those neighborhoods. From a public health perspective, such structural changes offer an effective alternative method for reducing health disparities (39). Doing so, however, requires knowledge of neighborhood effects on health, why these environments influence health, and how early in development the impact of stressful neighborhood conditions is observed.
McEwen and Seeman emphasized that “wear and tear” on the body is exerted slowly and persistently across time (18, 40, 41). In childhood and adolescence, a life stage in which physical health is at its peak (e.g., all-cause mortality is <1% of the population (42)), subtle alterations in allostatic mediators are primarily observed (43–45). Adverse health outcomes are anticipated years later as the cost of adjusting physiologic processes takes its toll and AL emerges (40). Nevertheless, ours and other investigations (11, 16, 17, 23, 24, 46, 47) have found early evidence for AL and other adverse health outcomes. Halting or slowing this process is of utmost policy importance, since continued exposure to stressful environments is expected to result in exponential accumulation of AL across the life span. The present investigation suggested that neighborhood factors may be fruitful targets for alleviating the cumulative impact of environmental stress exposures. Cumulative risk factors act both singularly and collectively to culminate in AL, potentially together and in interaction with household-level risk factors. Nevertheless, while cumulative impact may be more important than singular exposures in altering organismic functioning to shape health later in the life course, disentangling these exposures and how they relate to one another is relevant. While further investigation was beyond the scope of the present analysis, results suggest that neighborhood poverty, crime, and alcohol availability may play key roles in the production of AL. Our findings also suggest that such poor neighborhood environments may be linked to sociodemographic advantage at the neighborhood level, with greater educational advantage conferring less risk within a neighborhood.
Limitations of this study must be recognized. We did not examine additional stress buffers (e.g., parks and social services), which could be a component mechanism behind risk expression related to stress mitigation or buffering the adolescent from the environment. Second, the data were cross-sectional; consequently, temporal relations could not be fully evaluated. Additionally, observed effects could be due to endogenous factors or structural confounding, although propensity score matching largely accounted for this. Our neighborhood definition was based on administrative census tract boundaries but may not have truly represented an adolescent's neighborhood with respect to having opportunities for social interaction, developing meaningful relationships, and experiencing both stressors and stress buffers and the many neighborhoods and contexts through which s/he travels daily. However, these limitations would largely work against finding an effect rather than explain away results.
An additional limitation was the lack of available biomarkers in NHANES data. We relied on measures selected from adult AL studies in order to describe adverse health outcomes even at a relatively early developmental stage, recognizing their limitations from a developmental standpoint (8, 23, 46). We also examined AL as a simple, unweighted summary score, although exploration of additional algorithms was performed (48, 49).
Nonetheless, our results add to the conceptualization of neighborhoods as critical arenas in which a child's stress process is developed. In our study, cumulative neighborhood risk for AL emerged above and beyond household- or individual-level effects and may have been partially affected by concentrated advantage in the neighborhood. The results highlight the fact that the outcomes of AL may be well under way in adolescents aged 12–20 years. Given the empirically demonstrated high concentrations of negative psychosocial environments in minority and lower-income neighborhoods, addressing these factors may be one approach to reducing health disparities.
ACKNOWLEDGMENTS
Author affiliations: Department of Global Community Health and Behavioral Sciences, School of Public Health and Tropical Medicine, Tulane University, New Orleans, Louisiana (Katherine P. Theall); Department of Psychology, College of Sciences, University of New Orleans, New Orleans, Louisiana (Elizabeth A. Shirtcliff); and Department of Psychiatry and Neurology, School of Medicine, Tulane University, New Orleans, Louisiana (Stacy S. Drury).
This study was supported by grants from the Centers for Disease Control and Prevention (grant 1K01SH000002 to K. P. T.), the National Institutes of Health (grant 1R01ES020447-01 to K. P. T. and grant K01-MH077687 to E. A. S.), and Tulane University (a Clinical and Translational Research Enhancement Core grant to S. S. D.).
The authors thank Dr. Chris Rodgers and all the staff of the National Center for Health Statistics Research Data Center, Dr. Richard Scribner, Jigar Chotalia, and Emily Mabile.
Conflict of interest: none declared.
REFERENCES
- 1.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 2.Kwate NO. Fried chicken and fresh apples: racial segregation as a fundamental cause of fast food density in black neighborhoods. Health Place. 2008;14(1):32–44. doi: 10.1016/j.healthplace.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 3.LaVeist TA, Wallace JM., Jr. Health risk and inequitable distribution of liquor stores in African American neighborhood. Soc Sci Med. 2000;51(4):613–617. doi: 10.1016/s0277-9536(00)00004-6. [DOI] [PubMed] [Google Scholar]
- 4.Lee C. Environmental justice: building a unified vision of health and the environment. Environ Health Perspect. 2002;110(suppl 2):141–144. doi: 10.1289/ehp.02110s2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massey DS. Residential segregation and neighborhood conditions in US metropolitan areas. In: Smelser NJ, Wilson WJ, Mitchell F, editors. American Becoming: Racial Trends and Their Consequences. Washington, DC: National Academy Press; 2001. pp. 391–434. [Google Scholar]
- 6.Yen IH, Syme SL. The social environment and health: a discussion of the epidemiologic literature. Annu Rev Public Health. 1999;20(1):287–308. doi: 10.1146/annurev.publhealth.20.1.287. [DOI] [PubMed] [Google Scholar]
- 7.Geronimus AT, Hicken MT, Pearson JA, et al. Do US black women experience stress-related accelerated biological aging?: a novel theory and first population-based test of black-white differences in telomere length. Hum Nat. 2010;21(1):19–38. doi: 10.1007/s12110-010-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans GW, English K. The environment of poverty: multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Dev. 2002;73(4):1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- 9.Seeman TE, McEwen BS. Impact of social environment characteristics on neuroendocrine regulation. Psychosom Med. 1996;58(5):459–471. doi: 10.1097/00006842-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Zhang TY, Bagot R, Parent C, et al. Maternal programming of defensive responses through sustained effects on gene expression. Biol Psychol. 2006;73(1):72–89. doi: 10.1016/j.biopsycho.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Evans GW, Kim P, Ting AH, et al. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Dev Psychol. 2007;43(2):341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- 12.Morales JR, Guerra NG. Effects of multiple context and cumulative stress on urban children's adjustment in elementary school. Child Dev. 2006;77(4):907–923. doi: 10.1111/j.1467-8624.2006.00910.x. [DOI] [PubMed] [Google Scholar]
- 13.Rutter M, Garmezy N, Rutter M. Stress, coping, development in children. In: Garmezy N, Rutter M, editors. Stress, Coping and Development: Some Issues. New York, NY: McGraw-Hill Publishing Company, Inc;; 1983. pp. 1–41. [Google Scholar]
- 14.Sameroff AJ, Seifer R, Baldwin A, et al. Stability of intelligence from preschool to adolescence: the influence of social and family risk factors. Child Dev. 1993;64(1):80–97. doi: 10.1111/j.1467-8624.1993.tb02896.x. [DOI] [PubMed] [Google Scholar]
- 15.Adler NE, Boyce WT, Chesney MA. Socioeconomic inequalities in health. No easy solution. JAMA. 1993;269(24):3140–3145. [PubMed] [Google Scholar]
- 16.Lupie SJ, King S, Meaney MJ, et al. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13(3):653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- 17.Geronimus AT, Hicken M, Keene D, et al. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896(1):30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 19.Bird CE, Seeman T, Escarce JJ, et al. Neighbourhood socioeconomic status, and biological ‘wear and tear’ in a nationally representative sample of US adults. J Epidemiol Community Health. 2010;64(10):860–865. doi: 10.1136/jech.2008.084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King KE, Morenoff JD, House JS. Neighborhood context and social disparities in cumulative biological risk factors. Psychosom Med. 2011;73(7):572–579. doi: 10.1097/PSY.0b013e318227b062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merkin SS, Basurto-Dávila R, Karlamangla A, et al. Neighborhoods and cumulative biological risk profiles by race/ethnicity in a national sample of U.S. adults: NHANES III. Ann Epidemiol. 2009;19(3):194–201. doi: 10.1016/j.annepidem.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chong RY, Uhart M, McCaul ME, et al. Whites have a more robust hypothalamic-pituitary-adrenal axis response to a psychological stressor than blacks. Psychoneuroendocrinology. 2008;33(2):246–254. doi: 10.1016/j.psyneuen.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skinner ML, Shirtcliff EA, Haggerty KP, et al. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Dev Psychopathol. 2011;23(4):1167–1186. doi: 10.1017/S095457941100054X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Worthman CM, Panter-Brick C. Homeless street children in Nepal: use of allostatic load to assess the burden of childhood adversity. Dev Psychopathol. 2008;20(1):233–255. doi: 10.1017/S0954579408000114. [DOI] [PubMed] [Google Scholar]
- 25.Poortinga W. Social capital: an individual or collective resource for health? Soc Sci Med. 2006;62(2):292–302. doi: 10.1016/j.socscimed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Szreter S, Woolcock M. Health by association? Social capital, social theory, and the political economy of public health. Int J Epidemiol. 2004;33(4):650–667. doi: 10.1093/ije/dyh013. [DOI] [PubMed] [Google Scholar]
- 27.Woolcock M. Social capital and economic development: toward a theoretical synthesis and policy framework. Theory Soc. 1998;27(2):151–208. [Google Scholar]
- 28.Research Data Center, National Center for Health Statistics. National Health and Nutrition Examination Survey 2005–2006. Documentation, Codebook, and Frequencies: Geocoding Variables. Hyattsville, MD: National Center for Health Statistics; 2009. [Google Scholar]
- 29.Entringer S, Buss C, Shirtcliff EA, et al. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress Int J Biol Stress. 2009;13(3):258–268. doi: 10.3109/10253890903349501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Entringer S, Buss C, Shirtcliff EA, et al. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress. 2010;13(3):258–268. doi: 10.3109/10253890903349501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiolero A, Faeh D, Paccaud F, et al. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87(4):801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 32.Theall KP, Scribner R, Broyles S, et al. Impact of small group size on neighbourhood influences in multilevel models. J Epidemiol Community Health. 2011;65(8):688–695. doi: 10.1136/jech.2009.097956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowd JB, Zajacova A, Aiello A. Early origins of health disparities: burden of infection, health, and socioeconomic status in U.S. children. Soc Sci Med. 2009;68(4):699–707. doi: 10.1016/j.socscimed.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Economic Classification Policy Committee, US Census Bureau. NAICS: New Data for a New Economy. Washington, DC: Bureau of the Census, US Department of Commerce; 1998. [Google Scholar]
- 35.Finch BK, Phuong Do D, Heron M, et al. Neighborhood effects on health: concentrated advantage and disadvantage. Health Place. 2010;16(5):1058–1060. doi: 10.1016/j.healthplace.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rundle A, Neckerman KM, Freeman L, et al. Neighborhood food environment and walkability predict obesity in New York City. Environ Health Perspect. 2009;117(3):442–447. doi: 10.1289/ehp.11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merlo J, Chaix B, Ohlsson H, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60(4):290–297. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snijders T, Boskers R. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. London, United Kingdom: Sage Publications; 1999. [Google Scholar]
- 39.Cohen DA, Scribner RA, Farley TA. A structural model of health behavior: a pragmatic approach to explain and influence health behaviors at the population level. Prev Med. 2000;30(2):146–154. doi: 10.1006/pmed.1999.0609. [DOI] [PubMed] [Google Scholar]
- 40.McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 41.McEwen BS. From molecules to mind. Stress, individual differences, and the social environment. Ann N Y Acad Sci. 2001;935:42–49. [PubMed] [Google Scholar]
- 42.Miniño AM. Mortality Among Teenagers Aged 12–19 Years: United States, 1999–2006. Hyattsville, MD: National Center for Health Statistics; 2010; (NCHS data brief no. 37) [PubMed] [Google Scholar]
- 43.Essex MJ, Shirtcliff EA, Burk LR, et al. Influence of early life stress on later hypothalamic-pituitary-adrenal axis functioning and its covariation with mental health symptoms: a study of the allostatic process from childhood into adolescence. Dev Psychopathol. 2011;23(4):1039–1058. doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hastings PD, Shirtcliff EA, Klimes-Dougan B, et al. Allostasis and the development of internalizing and externalizing problems: changing relations with physiological systems across adolescence. Dev Psychopathol. 2011;23(4):1149–1165. doi: 10.1017/S0954579411000538. [DOI] [PubMed] [Google Scholar]
- 45.Lupien SJ, Ouellet-Morin I, Hupbach A, et al. Beyond the stress concept: allostatic load—a developmental biological and cognitive perspective. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology. 2nd. Hoboken, NJ: John Wiley & Sons, Inc; 2006. pp. 578–628. [Google Scholar]
- 46.Johnston-Brooks CH, Lewis MA, Evans GW. Chronic stress and illness in children: the role of allostatic load. Psychosom Med. 1998;60(5):597–603. doi: 10.1097/00006842-199809000-00015. [DOI] [PubMed] [Google Scholar]
- 47.Zimmermann US, Blomeyer D, Laucht M, et al. How gene-stress-behavior interactions can promote adolescent alcohol use: the roles of predrinking allostatic load and childhood behavior disorders. Pharmacol Biochem Behav. 2007;86(2):246–262. doi: 10.1016/j.pbb.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 48.Aldenderfer M, Blashfield R. Cluster Analysis: Quantitative Applications in the Social Sciences. Thousand Oaks, CA: Sage Publications; 1984. [Google Scholar]
- 49.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]