Abstract
Not all obese adults have cardiometabolic abnormalities. It is unknown whether this is true in children and, if true, whether children who have metabolically healthy overweight/obesity (MHO) will also have favorable cardiometabolic profiles in adulthood. These aspects were examined in 1,098 individuals who participated as both children (aged 5–17 years) and adults (aged 24–43 years) in the Bogalusa Heart Study between 1997 and 2002 in Bogalusa, Louisiana. MHO was defined as being in the top body mass index quartile, while low density lipoprotein cholesterol, triglycerides, mean arterial pressure, and glucose were in the bottom 3 quartiles, and high density lipoprotein cholesterol was in the top 3 quartiles. Forty-six children (4.2%) had MHO, and they were more likely to retain MHO status in adulthood compared with children in other categories (P < 0.0001). Despite markedly increased obesity in childhood and in adulthood, these same MHO children and adults showed a cardiometabolic profile generally comparable to that of nonoverweight/obese children (P > 0.05 in most cases). Moreover, there was no difference in carotid intima-media thickness in adulthood between MHO children and nonoverweight/obese children. Further, carotid intima-media thickness in adulthood was lower in MHO children than in metabolically abnormal, overweight/obese children (P = 0.003). In conclusion, the MHO phenotype starts in childhood and continues into adulthood.
Keywords: carotid intima-media thickness, longitudinal studies, metabolism, obesity, risk factors
Obesity has reached epidemic proportions in children as well as adults over the last few decades in developed countries and more recently in developing countries (1–4). Obesity is associated with increased risk of insulin resistance, dyslipidemia, elevated blood pressure, and inflammation (5, 6), which is linked to increased risk of type 2 diabetes and cardiovascular disease, among others (7, 8).
However, there exists substantial heterogeneity in the prevalence of cardiometabolic risk factors in obese individuals (9, 10); that is, not all obese individuals present adverse metabolic profiles (11–15). In a national representative survey, Wildman et al. (12) reported that, overall, 16.6% of the obese had no cardiometabolic abnormalities for the risk factors considered (blood pressure, triglycerides, fasting plasma glucose, C-reactive protein, homeostasis model assessment of insulin resistance, and high density lipoprotein cholesterol). Obese individuals who do not have an adverse cardiometabolic profile have been referred to as having “metabolically healthy obesity.” Because there has been no consensus on the definition of metabolically healthy obesity, the prevalence of metabolically healthy obesity varies depending on how metabolically healthy obesity is defined (16).
Observations on metabolically healthy obesity have been made only in adult populations and are mostly cross-sectional. Whether metabolically healthy obesity also exists in childhood and, if so, whether metabolically healthy obesity persists into adulthood are not known. Such information will provide insight into our understanding of the evolution of the metabolically healthy obesity phenotype from childhood to adulthood.
We hypothesized that metabolically healthy obesity exists in children and that metabolically healthy obese children may not necessarily present an adverse cardiometabolic risk profile in adulthood. The Bogalusa Heart Study, a community-based, black-white, longitudinal study, provides a unique opportunity to examine metabolically healthy obesity in children and its cardiometabolic profile in adulthood.
MATERIALS AND METHODS
Study population
Between 1973 and 2002, 7 cross-sectional surveys of children aged 4–17 years and 7 surveys of young adults aged 18–43 years who participated earlier as children and remained accessible were conducted in the biracial (65% whites, 35% blacks) community of Bogalusa, Louisiana. The participation rates ranged from 80% to 92% for children and from 60% to 65% for young adults. During the 2000–2002 survey of young adults (n = 1,203, aged 24–43 years), B-mode ultrasound examination of the carotid artery was conducted in 1,148 subjects. Of those, 1,098 individuals who were previously examined for traditional cardiovascular risk factors at least 2 times in childhood (ages 4–17 years) and had all of the risk factors measured formed the cohort in this study. The average follow-up period was 24.2 (range: 14.1–28.6) years. Characteristics of the study cohort are presented in Table 1.
Table 1.
Characteristics (Mean (SD)) of the Study Cohort: The Bogalusa Heart Study, Bogalusa, Louisiana, 1973–2002
| Variable | White |
Black |
Pdifference |
|||
|---|---|---|---|---|---|---|
| Men (n = 351) | Women (n = 408) | Men (n = 131) | Women (n = 208) | Race | Sex | |
| Childhood | ||||||
| Age, years | 11.93 (3.79) | 11.74 (3.80) | 11.92 (3.77) | 11.51 (3.74) | >0.05 | >0.05 |
| Body mass indexa | 19.28 (4.36) | 18.69 (3.62) | 19.26 (4.51) | 19.32 (4.56) | >0.05 | >0.05 |
| HDL cholesterol, mg/dL | 57.81 (19.24) | 59.13 (17.25) | 64.40 (18.31) | 63.86 (17.42) | <0.001 | >0.05 |
| LDL cholesterol, mg/dL | 83.98 (23.38) | 87.47 (25.82) | 83.60 (24.60) | 89.33 (25.29) | >0.05 | >0.05 |
| Triglycerides, mg/dL | 73.45 (41.68) | 73.36 (33.14) | 62.15 (28.56) | 62.14 (22.74) | <0.001 | >0.05 |
| Systolic blood pressure, mm Hg | 103.77 (11.44) | 101.70 (9.89) | 103.29 (12.00) | 101.83 (11.37) | >0.05 | >0.05 |
| Diastolic blood pressure, mm Hg | 62.74 (9.95) | 63.43 (9.50) | 62.73 (9.63) | 63.78 (10.58) | >0.05 | >0.05 |
| Glucose, mg/dL | 89.11 (8.35) | 86.69 (8.24) | 87.89 (10.09) | 85.25 (9.86) | 0.02 | <0.001 |
| Adulthood | ||||||
| Age, years | 36.19 (4.26) | 35.96 (4.39) | 36.35 (4.36) | 35.38 (4.75) | >0.05 | >0.05 |
| Body mass indexa | 29.32 (5.93) | 28.37 (7.05) | 29.87 (7.44) | 31.59 (8.51) | <0.001b | <0.05c |
| Waist, cm | 99.59 (15.46) | 87.23 (16.39) | 98.24 (18.24) | 94.16 (18.32) | <0.001b | <0.05 |
| HDL cholesterol, mg/dL | 41.21 (12.00) | 50.34 (12.89) | 49.27 (15.88) | 51.61 (12.81) | <0.001d | <0.001c |
| LDL cholesterol, mg/dL | 128.80 (33.93) | 124.55 (32.39) | 124.79 (44.07) | 115.14 (31.98) | >0.05 | >0.05 |
| Triglycerides, mg/dL | 164.56 (131.04) | 122.13 (71.56) | 130.33 (108.62) | 89.52 (39.26) | <0.001 | <0.001 |
| Systolic blood pressure, mm Hg | 118.34 (10.97) | 111.20 (11.21) | 128.47 (16.63) | 118.57 (14.86) | <0.001 | <0.001 |
| Diastolic blood pressure, mm Hg | 80.22 (7.99) | 75.03 (8.49) | 86.76 (12.32) | 79.41 (10.61) | <0.001 | <0.001 |
| Insulin, µU/mL | 13.41 (10.37) | 11.55 (8.21) | 12.55 (9.37) | 15.94 (19.84) | <0.001b | <0.001e |
| Glucose, mg/dL | 88.85 (23.78) | 82.66 (15.91) | 90.63 (32.38) | 88.90 (32.54) | <0.01 | <0.01 |
| Carotid IMT, mm | 0.85 (0.19) | 0.76 (0.13) | 0.89 (0.22) | 0.80 (0.16) | <0.001 | <0.001 |
Abbreviations: HDL, high density lipoprotein; IMT, intima-media thickness; LDL, low density lipoprotein; SD, standard deviation.
a Body mass index: weight (kg)/height (m)2.
b Only in women.
c Only in whites.
d Only in men.
e Different directions in blacks versus whites.
Written, informed consent was obtained from parents or guardians in childhood and from the participants in adulthood. The protocol was approved by the Institutional Review Board of the Tulane University Health Sciences Center.
Examinations
All examinations followed essentially the same protocols. Subjects were instructed to fast for 12 hours before the screening, with compliance ascertained by interview on the morning of the examination. Height, weight, and waist circumference were measured twice, and the mean values were used. Body mass index (BMI) was calculated as weight (kg)/height (m)2.
Replicate blood pressure measurements were obtained on the right arm of the subjects in a relaxed, sitting position. Arm measurements, length and circumference, were made during the examination to ensure proper cuff size. Systolic and diastolic blood pressure levels were analyzed as the first, fourth (in children), and fifth (in adults) Korotkoff phases by using mercury sphygmomanometers. Blood pressure levels were reported as the mean of 6 replicate readings, 3 taken by each of 2 randomly assigned and trained observers.
Serum lipid and lipoprotein analyses
During 1973–1986, cholesterol and triglyceride levels were measured by the use of chemical procedures with a Technicon AutoAnalyzer II (Technicon Instrument Corporation, Tarrytown, New York) according to the Laboratory Manual of the Lipid Research Clinics Program (17). Since 1987, these variables were determined by using the Abbott VP instrument (Abbott Laboratories, North Chicago, Illinois) by enzymatic procedures (18). Both chemical and enzymatic procedures met the performance requirements of the Lipid Standardization Program of the Centers for Disease Control and Prevention, Atlanta, Georgia, which routinely monitors the accuracy of measurements of total cholesterol, triglycerides, and high density lipoprotein (HDL) cholesterol concentrations. Measurements on the Centers for Disease Control and Prevention-assigned quality control samples showed no consistent bias over time within or between surveys. Serum lipoprotein cholesterols were analyzed by using a combination of heparin-calcium precipitation and agar–agarose gel electrophoresis procedures (19). A commercial radioimmunoassay kit was used for measuring plasma immunoreactive insulin levels (Pharmacia Diagnostics, Piscataway, New Jersey). Plasma glucose levels were measured as part of a sequential multichannel analysis with computer-20 (SMA20) chemistry profile by a glucose oxidase method.
Carotid ultrasonography
Trained sonographers performed ultrasound examinations with a Toshiba Sonolayer SSH160A (Toshiba Medical, Tokyo, Japan), a 7.5-MHz linear array transducer on subjects in the supine position with the head slightly extended and turned to the opposite direction of the carotid artery being studied. Images were recorded at the common carotid, carotid bulb (bifurcation), and internal carotid arteries bilaterally according to previously developed protocols for the Atherosclerosis Risk in Communities Study (20). Images were recorded on super-video home system (S-VHS) tapes and read by certified readers from the Division of Vascular Ultrasound Research using a semiautomatic ultrasound image processing program developed by the California Institute of Technology Jet Propulsion Laboratory (Pasadena, California) according to strict protocols (20, 21). The mean of the maximum carotid intima-media-thickness (CIMT) readings of 3 right and 3 left far walls for common, bulb, and internal segments was used.
Statistical methods
Data analyses were performed by using SAS, version 9.2 (SAS Institute, Inc., Cary, North Carolina), software. Average values of all measurements in childhood were used as the childhood values; values at the last examination were used as the adulthood values. To define metabolically healthy overweight/obesity (MHO), we first regressed these values on age, race, and sex, and the residuals were then standardized by inverse-normal transformation. MHO was defined as BMI in the top quartile, while low density lipoprotein cholesterol, triglycerides, mean arterial pressure, and glucose were in the bottom 3 quartiles, and HDL cholesterol was in the top 3 quartiles, for both childhood and adulthood; metabolically abnormal overweight/obesity was defined as BMI in the top quartile but not MHO. Metabolically healthy nonoverweight/obesity was defined as BMI, low density lipoprotein cholesterol, triglycerides, mean arterial pressure, and glucose in the bottom 3 quartiles and HDL cholesterol in the top 3 quartiles; metabolically abnormal nonoverweight/obesity was defined as BMI in the bottom 3 quartiles but not belonging to the previous group. For the definition of MHO in adulthood, subjects with type II diabetes and taking blood pressure medications were considered to be in the top quartile of glucose and mean arterial pressure, respectively, in adulthood; subjects taking lipid-lowering drugs were considered to be in the top quartile of triglycerides and low density lipoprotein cholesterol and in the bottom quartile of HDL cholesterol. We also defined MHO in adulthood, according to the latest definition of metabolic syndrome (22), as waist circumference ≥102 cm for men or 88 cm for women, triglycerides ≥150 mg/dL or taking lipid-lowering drugs, systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg, or taking blood pressure-lowering drugs, plasma glucose ≥100 mg/dL or being diabetic, and HDL cholesterol less than 40 mg/dL for men or 50 mg/dL for women or taking lipid-lowering drugs. Metabolically abnormal obesity, metabolically healthy nonobesity, and metabolically abnormal nonobesity were defined accordingly. Because findings were similar for the 2 definitions of MHO, we focused on findings from quartile splits only. Triglycerides, insulin, and CIMT were log transformed to increase normality before further data processing.
The chi-square test was used to examine tracking of MHO status from childhood to adulthood. The general linear model was used to examine the differences in cardiometabolic risk factors in childhood as well as in adulthood among children with different obesity status.
RESULTS
In childhood, 46 (4.2%) had MHO; 228 (20.8%) were metabolically abnormal, overweight/obese; 514 (46.8%) were metabolically abnormal, nonoverweight/obese; and 310 (28.2%) were metabolically healthy, nonoverweight/obese. Of the 46 children, 24 (52.2%) became nonoverweight/obese as adults, 9 (37.5%) of whom were metabolically healthy, and 16 (34.8%) became metabolically abnormal, overweight/obese. Although only 6 (13%) retained MHO status in adulthood, MHO children were 2.7–9.3 times more likely to retain the MHO status as adults compared with children in other categories (P < 0.0001) (Table 2). Of note, 67.4% (31/46) of those who were classified as MHO in childhood became metabolically abnormal in adulthood; 52.3% (162/310) of those who were classified as metabolically healthy nonoverweight/obese in childhood became metabolically abnormal. In addition, 62.0% (against 25% expected) of children in the top BMI quartile remained in the top BMI quartile in adulthood, indicating strong BMI tracking, even after an average period of 24.2 years.
Table 2.
Tracking of Obesity Status According to Quartile Splits From Childhood to Adulthood: The Bogalusa Heart Study, Bogalusa, Louisiana, 1973–2002*
| Childhooda | Adulthooda |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MHNO |
MANO |
MAO |
MHO |
Total |
||||||
| No. | %b | No. | %b | No. | %b | No. | %b | No. | %c | |
| MHNO | 138 | 44.5 | 135 | 43.6 | 27 | 8.7 | 10 | 3.2 | 310 | 28.2 |
| MANO | 157 | 30.5 | 300 | 58.4 | 50 | 9.7 | 7 | 1.4 | 514 | 46.8 |
| MAO | 20 | 8.8 | 60 | 26.3 | 137 | 60.1 | 11 | 4.8 | 228 | 20.8 |
| MHO | 9 | 19.6 | 15 | 32.6 | 16 | 34.8 | 6 | 13.0 | 46 | 4.2 |
| Total | 324 | 29.5 | 510 | 46.4 | 230 | 21.0 | 34 | 3.1 | 1,098 | 100.0 |
Abbreviations: MANO, metabolically abnormal nonoverweight/obesity; MAO, metabolically abnormal overweight/obesity; MHNO, metabolically healthy nonoverweight/obesity; MHO, metabolically healthy overweight/obesity.
* P < 0.0001 according to χ2 test.
a MHO was defined as body mass index in the top quartile, while low density lipoprotein cholesterol, triglycerides, mean arterial pressure, and plasma glucose were in the bottom 3 quartiles, and high density lipoprotein cholesterol was in the top 3 quartiles in both childhood and adulthood; medication use in adulthood was taken into account.
b “%” indicates proportions in the row.
c “%” indicates proportions in the column.
If cutoff points for metabolic syndrome components were used to define MHO in adults, 5.2% (57/1,098) had MHO as adults. Results for tracking (or otherwise changing) of MHO status from childhood to adulthood were similar to those observed if quartile splits were used in adulthood (Table 3).
Table 3.
Tracking of Obesity Status From Childhood (According to Quartile Splits) to Adulthood (According to Cutoff Points for Proposed Metabolic Syndromea): The Bogalusa Heart Study, Bogalusa, Louisiana, 1973–2002*
| Childhoodb | Adulthoodc |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MHNO |
MANO |
MAO |
MHO |
Total |
||||||
| No. | %d | No. | %d | No. | %d | No. | %d | No. | %e | |
| MHNO | 119 | 38.4 | 109 | 35.1 | 65 | 21.0 | 17 | 5.5 | 310 | 28.2 |
| MANO | 136 | 26.5 | 217 | 42.2 | 145 | 28.2 | 16 | 3.1 | 514 | 46.8 |
| MAO | 18 | 7.9 | 23 | 10.1 | 170 | 74.6 | 17 | 7.4 | 228 | 20.8 |
| MHO | 5 | 10.9 | 7 | 15.2 | 27 | 58.7 | 7 | 15.2 | 46 | 4.2 |
| Total | 278 | 25.3 | 356 | 32.4 | 407 | 37.1 | 57 | 5.2 | 1,098 | 100.0 |
Abbreviations: MANO, metabolically abnormal nonoverweight/obesity; MAO, metabolically abnormal overweight/obesity; MHNO, metabolically healthy nonoverweight/obesity; MHO, metabolically healthy overweight/obesity (for children) or metabolically healthy obesity (for adults).
* P < 0.0001 according to χ2 test.
a According to Alberti et al. (Circulation. 2009;120(16):1640–1645) (22)).
b MHO was defined as body mass index in the top quartile, while low density lipoprotein cholesterol, triglycerides, mean arterial pressure, and plasma glucose were in the bottom 3 quartiles, and high density lipoprotein cholesterol was in the top 3 quartiles.
c MHO was defined as waist circumference ≥102 cm for men or 88 cm for women, triglycerides ≥150 mg/dL, systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg, plasma glucose ≥100 mg/dL, and HDL cholesterol <40 mg/dL for men or 50 mg/dL for women; medication use was taken into account.
d “%” indicates proportions in the row.
e “%” indicates proportions in the column.
Despite substantially higher BMI, MHO children had similar cardiometabolic risk factors compared with metabolically healthy, nonoverweight/obese children except for HDL cholesterol (Table 4). Further, MHO children had more favorable levels of risk factors than metabolically abnormal, nonoverweight/obese children and, to a much higher degree, metabolically abnormal, overweight/obese children (Table 4).
Table 4.
Childhood Characteristics of the Study Sample by Obesity Status in Childhood: The Bogalusa Heart Study, Bogalusa, Louisiana, 1973–2002
| Variable in Childhood | Healthy Obesity Status in Childhooda |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MHNO |
MANO |
MAO |
MHO |
||||||||
| Mean | 95% CI | P Valueb | Mean | 95% CI | P Valueb | Mean | 95% CI | P Valueb | Mean | 95% CI | |
| Age, years | 13.58 | 13.31, 13.85 | 0.62 | 13.88 | 13.67, 14.08 | 0.77 | 13.68 | 13.38, 13.98 | 0.83 | 13.78 | 13.15, 14.40 |
| Body mass indexc | 18.25 | 18.03, 18.47 | <0.0001 | 18.95 | 18.77, 19.13 | <0.0001 | 25.80 | 25.28, 26.32 | <0.0001 | 24.30 | 23.48, 25.12 |
| LDL cholesterol, mg/dL | 77.46 | 75.78, 79.14 | 0.69 | 92.84 | 90.72, 94.96 | <0.0001 | 101.90 | 98.11, 105.68 | <0.0001 | 78.82 | 75.27, 82.38 |
| HDL cholesterol, mg/dL | 63.94 | 62.70, 65.17 | 0.04 | 55.52 | 54.13, 56.91 | 0.09 | 51.25 | 48.93, 53.56 | 0.001 | 59.88 | 57.29, 62.48 |
| Triglycerides, mg/dL | 55.56 | 54.03, 57.08 | 0.07 | 74.46 | 72.05, 76.88 | 0.0003 | 90.68 | 85.73, 95.62 | <0.0001 | 59.24 | 55.10, 63.39 |
| Glucose, mg/dL | 83.10 | 82.55, 83.65 | 0.68 | 86.80 | 86.13, 87.47 | <0.0001 | 87.02 | 86.07, 87.97 | <0.0001 | 82.61 | 81.10, 84.13 |
| Systolic blood pressure, mm Hg | 102.86 | 102.04, 103.68 | 0.06 | 107.39 | 106.61, 108.18 | 0.13 | 110.59 | 109.53, 111.66 | <0.0001 | 105.40 | 103.53, 107.27 |
| Diastolic blood pressure, mm Hg | 63.24 | 62.54, 63.95 | 0.39 | 67.39 | 66.67, 68.12 | 0.009 | 68.18 | 67.31, 69.05 | 0.0001 | 64.34 | 63.00, 65.67 |
| Mean arterial pressure, mm Hg | 76.45 | 75.81, 77.09 | 0.16 | 80.73 | 80.04, 81.41 | 0.01 | 82.32 | 81.50, 83.14 | <0.0001 | 78.02 | 76.81, 79.24 |
Abbreviations: CI, confidence interval; HDL, high density lipoprotein; LDL, low density lipoprotein; MANO, metabolically abnormal nonoverweight/obesity; MAO, metabolically abnormal overweight/obesity; MHNO, metabolically healthy nonoverweight/obesity; MHO, metabolically healthy overweight/obesity.
a MHO was defined as body mass indexin the top quartile, while LDL cholesterol, triglycerides, mean arterial pressure, and plasma glucose were in the bottom 3 quartiles, and HDL cholesterol was in the top 3 quartiles.
b Compared with the MHO group, adjusted for race and sex (and age) in a general linear model.
c Body mass index: weight (kg)/height (m)2.
Overall, MHO children showed substantially increased BMI and waist circumference in their adulthood compared with nonoverweight/obese (either metabolically healthy or abnormal) children (Table 5). Despite this striking difference in obesity measures in their adulthood (amounting to ∼7 BMI units or ∼20 cm in waist circumference) between MHO children and metabolically abnormal, nonoverweight/obese children, MHO children had comparable or even favorable cardiometabolic risk factors as adults (Table 5). Moreover, MHO children and metabolically healthy, nonoverweight/obese children had comparable cardiometabolic risk profiles in adulthood, except that the former had higher blood pressure and, to a lesser degree, higher levels of insulin and glucose than the latter in adulthood (Table 5). MHO children showed a very favorable cardiometabolic profile in their adulthood, compared with metabolically abnormal, overweight/obese children (Table 5).
Table 5.
Adulthood Characteristics of the Study Sample by Obesity Status in Childhood: The Bogalusa Heart Study, Bogalusa, Louisiana, 1973–2002
| Variable in Adulthood | Healthy Obesity Status in Childhooda |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MHNO |
MANO |
MAO |
MHO |
||||||||
| Mean | 95% CI | P Valueb | Mean | 95% CI | P Valueb | Mean | 95% CI | P Valueb | Mean | 95% CI | |
| Age, years | 35.71 | 35.23, 36.19 | 0.77 | 36.39 | 36.02, 36.76 | 0.46 | 35.39 | 34.78, 36.00 | 0.47 | 35.88 | 34.57, 37.19 |
| Body mass indexc | 26.77 | 26.18, 27.37 | <0.0001 | 27.35 | 26.88, 27.82 | <0.0001 | 36.85 | 35.92, 37.79 | 0.006 | 34.53 | 32.30, 36.77 |
| Waist, cm | 87.99 | 86.47, 89.51 | <0.0001 | 88.97 | 87.75, 90.19 | <0.0001 | 109.88 | 107.57, 112.18 | 0.16 | 107.35 | 101.70, 113.00 |
| LDL cholesterol, mg/dL | 115.87 | 112.54, 119.20 | 0.95 | 126.61 | 123.52, 129.69 | 0.04 | 131.68 | 126.80, 136.55 | 0.004 | 115.30 | 108.11, 122.49 |
| HDL cholesterol, mg/dL | 49.99 | 48.39, 51.59 | 0.70 | 47.57 | 46.40, 48.73 | 0.24 | 43.66 | 42.27, 45.05 | 0.006 | 49.85 | 44.09, 55.60 |
| Triglycerides, mg/dL | 115.05 | 105.35, 124.76 | 0.79 | 134.04 | 124.90, 143.18 | 0.13 | 147.23 | 134.32, 160.13 | 0.004 | 111.98 | 90.58, 133.38 |
| Insulin, µU/mL | 11.09 | 10.24, 11.95 | 0.10 | 12.23 | 11.03, 13.43 | 0.21 | 17.71 | 16.15, 19.26 | 0.002 | 13.43 | 10.45, 16.42 |
| Glucose, mg/dL | 83.43 | 80.90, 85.96 | 0.21 | 85.55 | 83.87, 87.23 | 0.39 | 93.64 | 89.29, 97.98 | 0.15 | 88.93 | 79.74, 98.13 |
| Systolic blood pressure, mm Hg | 113.78 | 112.42, 115.14 | 0.01 | 116.16 | 115.05, 117.28 | 0.09 | 122.37 | 120.37, 124.37 | 0.04 | 119.97 | 115.35, 124.59 |
| Diastolic blood pressure, mm Hg | 76.68 | 75.68, 77.68 | 0.04 | 78.58 | 77.75, 79.40 | 0.26 | 82.42 | 80.99, 83.85 | 0.03 | 80.43 | 77.26, 83.60 |
Abbreviations: CI, confidence interval; HDL, high density lipoprotein; LDL, low density lipoprotein; MANO, metabolically abnormal nonoverweight/obesity; MAO, metabolically abnormal overweight/obesity; MHNO, metabolically healthy nonoverweight/obesity; MHO, metabolically healthy overweight/obesity.
a MHO was defined as body mass index in the top quartile, while LDL cholesterol, triglycerides, mean arterial pressure, and plasma glucose were in the bottom 3 quartiles, and HDL cholesterol was in the top 3 quartiles.
b Compared with the MHO group, adjusted for race and sex (and age) in a general linear model.
c Body mass index: weight (kg)/height (m)2.
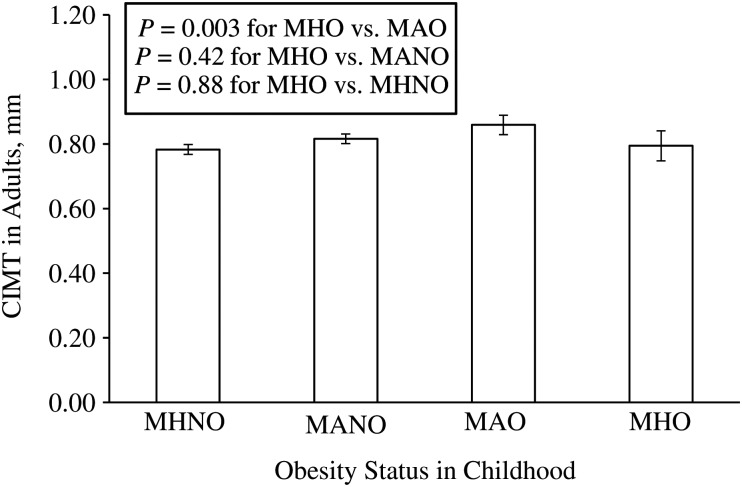
MHO children showed lower CIMT in adulthood compared with metabolically abnormal, overweight/obese children (0.794 (95% confidence interval (CI): 0.748, 0.841) mm vs. 0.859 (95% CI: 0.829, 0.889) mm) (P = 0.003) (Figure 1). Notably, CIMT in adults was comparable between MHO children and metabolically healthy, nonoverweight/obese children (0.794 (95% CI: 0.748, 0.841) mm vs. 0.783 (95% CI: 0.768, 0.798) mm) (P = 0.88) and between MHO children and metabolically abnormal, nonoverweight/obese children (0.794 (95% CI: 0.748, 0.841) mm vs. 0.816 (95% CI: 0.801, 0.831) mm) (P = 0.42) (Figure 1), despite the striking difference in obesity measures in childhood and in adulthood (Tables 4 and 5).
Figure 1.
Carotid intima-media thickness (CIMT) in adults by childhood obesity status: The Bogalusa Heart Study, Bogalusa, Louisiana, 1973–2002. MANO, metabolically abnormal nonoverweight/obesity; MAO, metabolically abnormal overweight/obesity; MHNO, metabolically healthy nonoverweight/obesity; MHO, metabolically healthy overweight/obesity. A general linear model was used, with P values adjusted for adult age, race, and sex.
DISCUSSION
In this community-based, black-white cohort, we showed that MHO children had considerably favorable cardiometabolic profiles as adults compared with metabolically abnormal, overweight/obese children. Further, their adulthood cardiometabolic profiles were, overall, comparable to those of metabolically healthy, nonoverweight/obese children despite substantially increased obesity in adulthood. Such observations are in agreement with previous findings that there exist substantial heterogeneities in the metabolic consequences of obesity. Importantly, our findings indicate that MHO in adults has its origin in the childhood years.
Our results support the argument by Gerald Reaven that “all obese individuals are not created equal” (10, p. 105), with observations that MHO children showed a comparable cardiometabolic profile compared with their metabolically healthy, nonoverweight/obese counterparts, and that metabolically abnormal, overweight/obese children had the most adverse cardiometabolic profile in adulthood. Our observations clearly show that such “inequality” in cardiometabolic consequences starts in childhood. Although only 13% of MHO children retained MHO status as adults, MHO children were 2.7–9.3 times more likely to be MHO adults compared with children in other categories. Importantly, MHO children did not show increased CIMT, a surrogate marker of atherosclerosis, in adulthood compared with nonoverweight/obese children, suggesting that MHO children may not have increased risk of cardiovascular disease in their adulthood. It should be noted, however, that MHO children seemed to have intermediate levels of blood pressure and insulin and glucose in adulthood, compared with metabolically healthy, nonoverweight/obese children and metabolically abnormal, overweight/obese children. This indicates that MHO children may be at intermediate risk of developing hypertension and type 2 diabetes as adults. It should also be noted that metabolically healthy, nonoverweight/obese children had the best risk profile in adults. Interestingly, 62.0% of MHO children were nonoverweight/obese in adulthood, indicating that the majority of MHO children are not destined to have increased weight in adulthood.
There is compelling evidence that obesity in childhood is a predictor of cardiovascular risk in adulthood (23, 24), and it is strongly recommended to adopt weight-loss interventions for overweight/obese children (25). Our observations, however, raise an important question as to whether MHO children would benefit from weight-loss interventions, from either increased physical activity or a healthy diet or both. It has been reported that obese adults respond differently to dietary intervention or physical activity intervention for weight loss, and MHO individuals may not benefit, or even suffer, metabolically from these interventions (26–28). Whether this is true for MHO children is not known and cannot be addressed in this study, because we did not have enough information on physical activity and dietary intake in children. Studies addressing this question should have important public health implications in promoting health in obese children. It has to be noted, however, that there is no doubt that interventions should target those metabolically abnormal, overweight/obese children and that these children may benefit the most from such interventions.
Determinants of the MHO phenotype are not very clear. Studies have shown that insulin resistance, visceral fat, and inflammation are strongly associated with metabolic consequences of obesity (9, 13, 15, 29). Other factors, such as fitness and physical activity, may also play a part (30). Although in our study waist circumference in adulthood of MHO children was increased (P < 0.001) compared with nonoverweight/obese children and was comparable to that of metabolically abnormal, overweight/obese children, waist circumference per se cannot differentiate visceral fat from subcutaneous fat in the abdomen. Previously in the Bogalusa Heart Study, subjects were showed that a high waist/height ratio is associated with adverse cardiometabolic risk among normal weight children and adults (31, 32). Whether or not this ratio can be used among obese children and adults remains to be addressed. Other factors, such as genetic factors, have been proposed to be determinants of the MHO phenotype (33). In this regard, Kilpelainen et al. (34) have recently reported that variants near the insulin receptor substrate 1 gene (IRS1) are associated with reduced adiposity and an impaired metabolic profile.
Some limitations of our study need to be addressed. First, we arbitrarily defined the MHO phenotype by quartiles, because there is no consensus on the definition of MHO, especially in childhood. Second, grouping children based on extreme values of multiple variables might have subjected our results to be influenced by the effect of “regression to the mean” (35–37). However, we expect such an effect might have been mitigated by using the average values of at least 2 measurements to define the MHO phenotype in children. Finally, we recognize that the sample size of our study was relatively small, and the findings should be interpreted with caution. Future studies with large sample sizes are needed to confirm our findings.
In conclusion, MHO children have favorable cardiometabolic profiles and CIMT in adulthood compared with metabolically abnormal, overweight/obese children. Further, their cardiometabolic profiles and CIMT are comparable to those of nonoverweight/obese children. Such observations provide important evidence that the MHO phenotype starts in childhood and continues into adulthood. Whether MHO children should be targeted with weight-loss interventions needs to be addressed in future studies.
ACKNOWLEDGMENTS
Author affiliation: Tulane Center for Cardiovascular Health, Department of Epidemiology, Tulane University, New Orleans, Louisiana (Shengxu Li, Wei Chen, Sathanur R. Srinivasan, Jihua Xu, Gerald S. Berenson).
This study was supported by grants HD-061437 and HD-062783 from the National Institute of Child Health and Human Development, 0855082E from the American Heart Association, and AG-16592 from the National Institute on Aging.
Conflict of interest: none declared.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 3.Chen CM. Overview of obesity in Mainland China. Obes Rev. 2008;9(1):14–21. doi: 10.1111/j.1467-789X.2007.00433.x. [DOI] [PubMed] [Google Scholar]
- 4.de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92(5):1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 5.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 6.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 7.Caterson ID, Hubbard V, Bray GA, et al. Prevention Conference VII: obesity, a worldwide epidemic related to heart disease and stroke: Group III: worldwide comorbidities of obesity. Circulation. 2004;110(18):e476–e483. doi: 10.1161/01.CIR.0000140114.83145.59. [DOI] [PubMed] [Google Scholar]
- 8.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin T, Abbasi F, Lamendola C, et al. Heterogeneity in the prevalence of risk factors for cardiovascular disease and type 2 diabetes mellitus in obese individuals: effect of differences in insulin sensitivity. Arch Intern Med. 2007;167(7):642–648. doi: 10.1001/archinte.167.7.642. [DOI] [PubMed] [Google Scholar]
- 10.Reaven G. All obese individuals are not created equal: insulin resistance is the major determinant of cardiovascular disease in overweight/obese individuals. Diab Vasc Dis Res. 2005;2(3):105–112. doi: 10.3132/dvdr.2005.017. [DOI] [PubMed] [Google Scholar]
- 11.Ferrannini E, Natali A, Bell P, et al. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR) J Clin Invest. 1997;100(5):1166–1173. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168(15):1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 13.Brochu M, Tchernof A, Dionne IJ, et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86(3):1020–1025. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 14.Iacobellis G, Ribaudo MC, Zappaterreno A, et al. Prevalence of uncomplicated obesity in an Italian obese population. Obes Res. 2005;13(6):1116–1122. doi: 10.1038/oby.2005.130. [DOI] [PubMed] [Google Scholar]
- 15.Karelis AD, Faraj M, Bastard JP, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90(7):4145–4150. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- 16.Velho S, Paccaud F, Waeber G, et al. Metabolically healthy obesity: different prevalences using different criteria. Eur J Clin Nutr. 2010;64(10):1043–1051. doi: 10.1038/ejcn.2010.114. [DOI] [PubMed] [Google Scholar]
- 17.Lipid Research Clinics Program. Lipid and lipoprotein analysis. Manual of Laboratory Operations, I. Washington, DC: Department of Health, Education, and Welfare; 1974. pp. 75–628. National Institutes of Health. [Google Scholar]
- 18.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19(5):476–482. [PubMed] [Google Scholar]
- 19.Srinivasan SR, Berenson GS. Serum lipoproteins in children and methods for study. In: Lewis LA, editor. CRC Handbook of Electrophoresis. Vol III. Lipoprotein Methodology and Human Studies. Boca Raton, FL: CRC Press; 1983. pp. 185–204. [Google Scholar]
- 20.Bond MG, Barnes RW, Riley WA. High-resolution B-mode ultrasound reading methods in the Atherosclerosis Risk in Communities (ARIC) cohort. The ARIC Study Group. J Neuroimaging. 1991;1(4):168–172. [PubMed] [Google Scholar]
- 21.Tang R, Hennig M, Thomasson B, et al. Baseline reproducibility of B-mode ultrasonic measurement of carotid artery intima-media thickness: the European Lacidipine Study on Atherosclerosis (ELSA) J Hypertens. 2000;18(2):197–201. doi: 10.1097/00004872-200018020-00010. [DOI] [PubMed] [Google Scholar]
- 22.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290(17):2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 24.Raitakari OT, Juonala M, Kahonen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290(17):2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 25.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360(9331):473–482. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 26.Karelis AD. Metabolically healthy but obese individuals. Lancet. 2008;372(9646):1281–1283. doi: 10.1016/S0140-6736(08)61531-7. [DOI] [PubMed] [Google Scholar]
- 27.Shin MJ, Hyun YJ, Kim OY, et al. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes. 2006;30(10):1529–1534. doi: 10.1038/sj.ijo.0803304. [DOI] [PubMed] [Google Scholar]
- 28.Arsenault BJ, Cote M, Cartier A, et al. Effect of exercise training on cardiometabolic risk markers among sedentary, but metabolically healthy overweight or obese post-menopausal women with elevated blood pressure. Atherosclerosis. 2009;207(2):530–533. doi: 10.1016/j.atherosclerosis.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kloting N, Fasshauer M, Dietrich A, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299(3):E506–E515. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 30.McAuley PA, Blair SN. Obesity paradoxes. J Sports Sci. 2011;29(8):773–782. doi: 10.1080/02640414.2011.553965. [DOI] [PubMed] [Google Scholar]
- 31.Mokha JS, Srinivasan SR, Dasmahapatra P, et al. Utility of waist-to-height ratio in assessing the status of central obesity and related cardiometabolic risk profile among normal weight and overweight/obese children: the Bogalusa Heart Study. BMC Pediatr. 2010;10:73. doi: 10.1186/1471-2431-10-73. (doi:10.1186/1471-2431-10-73) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srinivasan SR, Wang R, Chen W, et al. Utility of waist-to-height ratio in detecting central obesity and related adverse cardiovascular risk profile among normal weight younger adults (from the Bogalusa Heart Study) Am J Cardiol. 2009;104(5):721–724. doi: 10.1016/j.amjcard.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 33.Pataky Z, Bobbioni-Harsch E, Golay A. Open questions about metabolically normal obesity. Int J Obes (Lond) 2010;34(2):S18–S23. doi: 10.1038/ijo.2010.235. [DOI] [PubMed] [Google Scholar]
- 34.Kilpelainen TO, Zillikens MC, Stancakova A, et al. Genetic variation near IRS1 associates with reduced adiposity and an impaired metabolic profile. Nat Genet. 2011;43(8):753–760. doi: 10.1038/ng.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bland JM, Altman DG. Statistic notes: regression towards the mean. BMJ. 1994;308(6942):1499. doi: 10.1136/bmj.308.6942.1499. (doi:10.1136/bmj.308.6942.1499). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson WD, George VT. Effect of regression to the mean in the presence of within-subject variability. Stat Med. 1991;10(8):1295–1302. doi: 10.1002/sim.4780100812. [DOI] [PubMed] [Google Scholar]
- 37.Davis CE. The effect of regression to the mean in epidemiologic and clinical studies. Am J Epidemiol. 1976;104(5):493–498. doi: 10.1093/oxfordjournals.aje.a112321. [DOI] [PubMed] [Google Scholar]