Abstract
The effects of low-carbohydrate diets (≤45% of energy from carbohydrates) versus low-fat diets (≤30% of energy from fat) on metabolic risk factors were compared in a meta-analysis of randomized controlled trials. Twenty-three trials from multiple countries with a total of 2,788 participants met the predetermined eligibility criteria (from January 1, 1966 to June 20, 2011) and were included in the analyses. Data abstraction was conducted in duplicate by independent investigators. Both low-carbohydrate and low-fat diets lowered weight and improved metabolic risk factors. Compared with participants on low-fat diets, persons on low-carbohydrate diets experienced a slightly but statistically significantly lower reduction in total cholesterol (2.7 mg/dL; 95% confidence interval: 0.8, 4.6), and low density lipoprotein cholesterol (3.7 mg/dL; 95% confidence interval: 1.0, 6.4), but a greater increase in high density lipoprotein cholesterol (3.3 mg/dL; 95% confidence interval: 1.9, 4.7) and a greater decrease in triglycerides (−14.0 mg/dL; 95% confidence interval: −19.4, −8.7). Reductions in body weight, waist circumference and other metabolic risk factors were not significantly different between the 2 diets. These findings suggest that low-carbohydrate diets are at least as effective as low-fat diets at reducing weight and improving metabolic risk factors. Low-carbohydrate diets could be recommended to obese persons with abnormal metabolic risk factors for the purpose of weight loss. Studies demonstrating long-term effects of low-carbohydrate diets on cardiovascular events were warranted.
Keywords: carbohydrate-restricted diet, fat-restricted diet, meta-analysis, metabolic syndrome, obesity
There were an estimated 937 million overweight and 396 million obese people worldwide in 2005 (1). Moreover, it was estimated that 68.0% of American adults were either overweight or obese in 2009 (2). Overweight and obesity are important risk factors for diabetes, cardiovascular disease, cancer, and premature death. The high prevalence of obesity has become a serious public health challenge. The dietary recommendations for weight loss from the American Heart Association and the National Institutes of Health emphasize the importance of low-fat, high-carbohydrate diets (3, 4). However, low-carbohydrate diets have recently become very popular for weight loss (5–7). Because low-carbohydrate diets may include significant amounts of fat and cholesterol, which have been associated with elevated low density lipoprotein (LDL) cholesterol levels, there is concern about their adverse effects on metabolic risk factors.
Some previous studies (6, 8–12), but not others (13–15), showed significant changes in metabolic risk factors associated with low-carbohydrate diets. Many clinical trials of low-carbohydrate diets have small sample sizes and insufficient statistical power to detect small changes in metabolic risk factors that may have public health importance. A previous meta-analysis of clinical trials comparing low-carbohydrate and low-fat diets reported differences in metabolic risk factors between the 2 diets (16). However, that study included only 5 trials, with a total of 447 participants (16). Since then, several larger trials of longer duration have been published (6, 11, 13, 14, 17). Given this recent additional evidence, a meta-analysis of randomized controlled trials comparing the effects of low-carbohydrate diets with those of low-fat diets on metabolic risk factors is timely and important to public health.
In the present meta-analysis, we aimed to estimate the long-term (6 or more months) effect of low-carbohydrate diets compared with those of low-fat diets on body weight, waist circumference, and metabolic risk factors, including systolic and diastolic blood pressure, total cholesterol, LDL cholesterol, high density lipoprotein (HDL) cholesterol, ratio of total to HDL cholesterol, triglycerides, fasting blood glucose, and serum insulin. In addition, we explored the possible underlying reasons (i.e., study duration, diabetic status, age, gender, and levels of carbohydrate intake in diets) for the observed heterogeneity of effect sizes.
MATERIALS AND METHODS
Data sources and searches
We used the MEDLINE online database (from January 1, 1966 to June 20, 2011), EMBASE, Web of Science, and the Cochrane Database of Systematic Reviews to identify randomized controlled trials that compared the low-carbohydrate diet with the low-fat diet. The following key words or medical subject headings on MEDLINE were used: (“low-carbohydrate diet” or “low sugar diet” or “carbohydrate restriction” or “diet, carbohydrate-restricted”) AND (“body mass index” or “BMI” or “waist circumference” or “obesity” or “diabetes” or “blood glucose” or “hypertension” or “HDL” or “LDL” or “triglycerides” or “cholesterol” or “lipids” or “dyslipidemias” or “blood pressure” or “diabetes mellitus” or “heart diseases” or “cardiovascular diseases”). The search was restricted to include studies classified as randomized controlled trials, and no language restriction was applied. In addition, manual searches of the references from selected original research and review articles were conducted.
Study selection
To be included in this meta-analysis, the studies had to be randomized controlled trials conducted in adults that compared a low-carbohydrate diet (≤45% of energy from carbohydrates) with a low-fat diet (≤30% of energy from fat) (18, 19), had an intervention duration of 6 months or more, and reported metabolic risk factors as outcomes. Studies were excluded if treatment allocation was not random, the study included participants less than 18 years of age, there was no difference in carbohydrate or fat intakes between diets, there were differences other than macronutrient and energy intake between the 2 diets, metabolic risk factors were not reported by treatment status, the variance of outcomes could not be calculated, and/or the duration of intervention was less than 6 months (3). When the results of a study were published more than once, only the most recent or most complete article was included in the analysis. Two investigators independently reviewed all potentially relevant publications and made decisions on inclusion. Where these decisions conflicted, additional investigators (co-authors) were involved to discuss discrepancies until mutual agreement was reached.
Data extraction and quality assessment
Two investigators independently abstracted the data in duplicate using a standardized data collection form. The following data were collected: article title, primary author's name, year and source of publication, country of origin, study design (parallel, cross-over, or factorial trial), blinding (open, single, or double), dietary composition, loss to follow-up, intention-to-treat analysis, characteristics of the study population (sample size, age, sex, prior disease status, and baseline levels of metabolic risk factors), and the net changes in metabolic risk factors with measures of variance, overall and by prespecified subgroups. Disagreement was resolved by consensus with additional investigators.
Data synthesis and analysis
Mean net change was calculated by subtracting mean change (from baseline to end of trial) in the low-fat-diet group from mean change in the low-carbohydrate-diet group for each metabolic risk factor. Pooled mean net changes and their 95% confidence intervals in metabolic risk factors were calculated using DerSimonian and Laird random-effects models (20). The presence of heterogeneity was assessed with the Q test and the extent of heterogeneity was quantified with the I-squared index. To assess publication bias, we constructed a funnel plot for each outcome. Begg's rank correlation test was used to examine the asymmetry of the funnel plot, and Egger's weighted linear regression test was used to examine the association between the mean effect estimate and its variance. Where these tests or funnel plots indicated possible publication bias, we used the trim-and-fill method to determine whether publication bias could have accounted for the results we observed. Additionally, sensitivity analyses were conducted by excluding each study in turn, by removing studies with a completion rate less than 70%, by removing studies with fewer than 20 participants per group, and by removing studies among postsurgery patients or those with severe diseases, such as cancer, to evaluate their relative influence on the pooled estimates. Finally, subgroup analyses including diabetic versus nondiabetic samples, very-low-carbohydrate (≤60 g carbohydrates per day) versus moderate-carbohydrate (>60 g carbohydrates per day) diets, longer (≥12 months intervention) versus shorter (<12 months) study duration, predominantly male (at least 50% men) versus predominantly female (at least 50% women) samples, and older (mean age ≥50 years) versus younger (mean age <50) samples were conducted to examine differences in all study outcomes between the 2 diets. The Bonferroni and false discovery rate methods were used to correct for multiple comparisons (21). All analyses were conducted using Stata, version 10 (StataCorp LP, College Station, Texas).
RESULTS
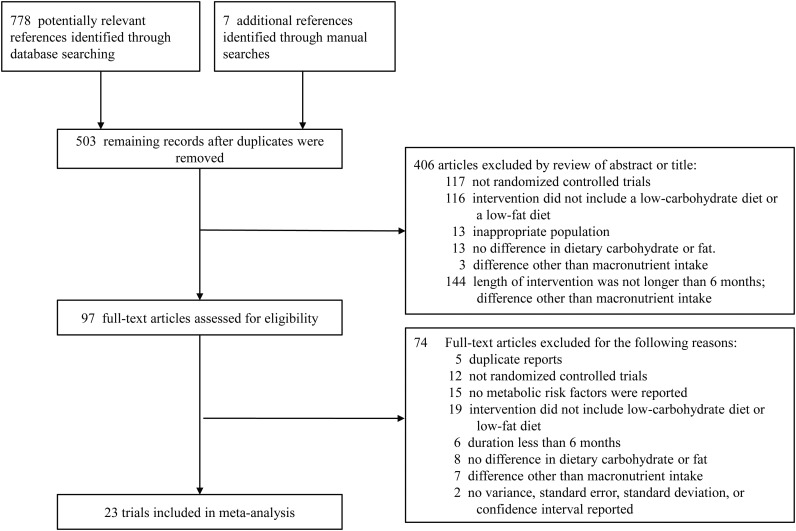
The flow of studies in our meta-analysis is depicted in Figure 1. From 785 potentially relevant references, 503 records remained after duplicates between databases were eliminated, and 406 articles were eliminated after reviewing titles and abstracts. A total of 97 full-text articles were reviewed for eligibility. Of those, 23 studies met all of the eligibility criteria and were included in the meta-analysis (5, 6, 8–14, 17, 22–34). These studies included data from 2,788 trial participants (1,392 on low-carbohydrate diets and 1,396 on low-fat diets).
Figure 1.
Flow diagram of systematic review (from January 1, 1966 to June 20, 2011).
The characteristics of these 23 randomized controlled trials are presented in Table 1. All trials were parallel except for 1 trial that had a factorial design (13). Trial participants were usually not blinded to their assignment because of the nature of the intervention—most interventions provided dietary instruction, leaving food buying and/or preparation to the participants. Study duration ranged from 6 to 24 months, and 16 studies had an intervention duration of 12 months or longer (6, 8, 9, 11–14, 17, 22, 23, 25, 26, 28–31). Most trials were conducted among obese or overweight patients without cardiovascular diseases or diabetes mellitus. However, 4 studies were conducted in patients with diabetes (23, 26, 28, 29), 1 was conducted in patients with prediabetes (31), and 1 was conducted in patients with coronary heart disease (23). The goal dietary nutritional composition varied across the studies, with carbohydrate consumption ranging from 4% to 45% (weighted mean, 23%) of energy intake in the low-carbohydrate group and fat ranging from 10% to 30% (weighted mean, 26%) of energy intake in the low-fat group. Self-reported mean energy intake weighted by trial sample sizes was similar for both diets at approximately 2,000 kcal.
Table 1.
Characteristics of 23 Randomized Controlled Clinical Trials From Multiple Countries Comparing Low-Carbohydrate Diets With Low-Fat Diets, 1966–2011
| First Author, Year (Reference No.) | Country | Design | Blinding | Inclusion Criteria | Intervention or Targeted Dietary Composition |
Mean Intervention Duration, months | Completion % |
||
|---|---|---|---|---|---|---|---|---|---|
| LCD | LFD | LCD | LFD | ||||||
| Brehm, 2003 (5) | United States | Parallel | Open | Female sex; BMIa 30–35; no DM or CVD; normal BP; NP | <20 g carbohydrates/day for 1–2 weeks, then increasing to 40–60 g/day only if self-testing of urinary ketones continued to indicate ketosis | 30% fat, 55% carbohydrate, 15% protein | 6 | 85 | 74 |
| Brinkworth, 2009 (22) | Australia | Parallel | Open | Abdominal obesity and ≥1 METS components; no DM or CVD; NP | 4% carbohydrate, 35% protein, 61% fat (20% saturated fat), <20 g carbohydrates/day during weeks 1–8 and then <40 g/day thereafter | 30% fat (8% or 10 g/day saturated fat), 46% carbohydrate, 24% protein | 12 | 58 | 59 |
| Dansinger, 2005 (8) | United States | Parallel | Open | Obesity and ≥1 METS; BMI 27–42; no CKD; NP | <20 g carbohydrates/day, increasing to 50 g/day | Vegetarian diet, 10% fat | 12 | 52 | 50 |
| Davis, 2009 (23) | United States | Parallel | Open | DM; overweight; no CKD; coronary heart disease | 20–25 g carbohydrates/day in weeks 1–2 depending on baseline weight, then increasing by 5 g carbohydrates/week | 25% fat | 12 | 86 | 88 |
| Due, 2008 (24) | Denmark | Parallel | Open | BMI 28–36; NP | 45% carbohydrate, 40% fat (20% MUFA), 15% protein | 60% carbohydrate, 25% fat (20% MUFA), 15% protein | 6 | 56 | 73 |
| Ebbeling, 2007 (25) | United States | Parallel | Open | BMI ≥30; no DM | 40% carbohydrate, 35% fat, 25% protein; low-glycemic-load foods and limited intake of high-glycemic-load foods | 20% fat, 55% carbohydrates, 25% protein; low-fat grains, vegetables, fruits, and legumes and limited intake of added fats sweets and high-fat snacks | 18 | 78 | 62 |
| Elhayany, 2009 (26) | Israel | Parallel | Open | DM; BMI 27–34 | 35% carbohydrate, 45% fat (23% MUFA), 20% protein; 4–6 meals/day, only low-glycemic-index carbohydrates | 30% fat (10% MUFA), 20% protein, 50% carbohydrate; 4–6 meals/day, mixed glycemic index carbohydrates | 12 | 72 | 71 |
| Foster, 2003 (9) | United States | Parallel | Open | No DM; obesity | 20 g carbohydrates/day, gradually increasing until a stable and desired weight is achieved | 25% fat, 60% carbohydrate, 15% protein; limited energy intake of 1200–1500 kcal/day for women and 1500–1800 kcal/day for men | 12 | 61 | 57 |
| Foster, 2010 (17) | United States | Parallel | Open | BMI 30–40; no DM; normal BP | 20 g carbohydrates/day (low-glycemic index-vegetables) during weeks 1–12, increasing by 5 g/week through consumption of a limited amount of fruits, more vegetables, and eventually small quantities of whole grains and dairy products, until a stable and desired weight was achieved | 30% fat, 55% carbohydrate, 15% protein; energy intake limited to 1200–1500 kcal/day for women and 1500–1800 kcal/day for men | 24 | 58 | 68 |
| Frisch, 2009 (27) | Germany | Parallel | Open | BMI >27; no CVD or type 1 DM | 40% carbohydrate, 35% fat, 25% protein | 30% fat, 55% carbohydrates, 15% protein | 6 | 95 | 89 |
| Gardner, 2007 (6) | United States | Parallel | Single | Female sex; BMI 27–40; normal BP; no DM CVD; NP | ≤20 g carbohydrates/day for the first 2–3 months and ≤50 g carbohydrates/day for the subsequent phase | 10% fat | 12 | 88 | 78 |
| Hockaday, 1978 (28) | United Kingdom | Parallel | Open | DM; no myocardial infarction or stroke | 40% carbohydrates, 40% fat, 20% protein | 25% fat, 54% carbohydrates, 21% protein | 12 | ||
| Iqbal, 2010 (29) | United States | Parallel | Open | DM; obesity; no CKD | 30 g/day carbohydrates | 30% fat with a deficit of 500 kcal/day | 24 | 40 | 54 |
| Klemsdal, 2010 (14) | Norway | Parallel | Open | No DM or CVD; ≥1 METS components and BMI 28–40 for men and 28–35 for women | 30%–35% carbohydrates, 35%–40% fat, 25%–30% protein | 30% fat, 55–60% carbohydrate, 15% protein, | 12 | 78 | 84 |
| Lim, 2009 (30) | Australia | Parallel | Open | BMI 28–40; ≥1 CVD risk factors; No CVD or DM | 4% carbohydrate, 60% fat (20% saturated fat), 35% protein | 10% fat (3% saturated fat), 70% carbohydrate, 20% protein | 15 | 63 | 64 |
| McAuley, 2006 (31) | New Zealand | Parallel | Open | Female sex; prediabetes; NP | ≤20 g carbohydrates/day during weeks 1–2, increasing to 50 g/day by 8 weeks and continuing thereafter | 30% fat, 55% carbohydrate, 15% protein | 12 | 77 | 75 |
| Moran, 2006 (32) | United Kingdom | Parallel | Open | Female sex; overweight with polycystic ovary syndrome; no DM; NP | 120 g carbohydrates/day | 50 g fat/day | 6 | 78 | 57 |
| Sacks, 2009 (13) | United States | Factorial | Double | No DM or CVD; BMI >25 | 35% carbohydrate, 40% fat, 25% protein | 30% fat, 55% carbohydrate, 25% protein | 24 | 83 | 78 |
| Shai, 2008 (11) | Israel | Parallel | Open | DM or CVD or BMI >27; NP | 20 g carbohydrates/day for the 2-month induction phase and with a gradual increase to ≤120 g/day to maintain the weight loss | 30% fat (10% saturated fat) with 1500 kcal/day for women and 1800 kcal/day for men, and 300 mg cholesterol/day | 24 | 78 | 90 |
| Stern, 2004 (12) | United States | Parallel | Open | BMI ≥35; no CKD | <30 g carbohydrates/day | <30% fat with a deficit of 500 kcal/day | 12 | 69 | 63 |
| Swenson, 2007 (33) | United States | Parallel | Single | Gastric bypass surgery; obesity | South Beach diet | 16% fat, 72%–74% carbohydrate, 10–12% protein | 6 | ||
| Thomson, 2010 (34) | United States | Parallel | Open | Female sex; stage 1–2 breast cancer; BMI 25–35; No DM, CKD, or CVD | 35% carbohydrates, 35%–40% fat, 25%–30% protein | 25% fat, 55%–60% carbohydrates, 15%–20% protein | 6 | 91 | 68 |
| Yancy, 2004 (10) | United States | Parallel | Open | BMI 30–60; dyslipidemia; NP | <20 g carbohydrates/day during weeks 1–10, increasing by 5 g/week until body weight was maintained | <30% fat (<10% saturated fat) | 6 | 76 | 57 |
Abbreviation: BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease; CVD, cardiovascular diseases; DM, diabetes mellitus; LCD, low-carbohydrate diets; LFD, low-fat diets; METS, metabolic syndrome; MUFA, monounsaturated fatty acid; NP, no pregnancy.
a Weight (kg)/height (m)2.
Table 2 shows baseline characteristics of trial participants in the included studies. The mean age ranged from 27 to 60 years. Approximately 40% of participants were male. Baseline levels of body weight and metabolic risk factors were similar between the 2 diets in each study but varied among studies.
Table 2.
Baseline Characteristics of Study Participants in 23 Randomized Controlled Trials From Multiple Countries Comparing Low-Carbohydrate Diets With Low-Fat Dietsa, 1966–2011
| First Author, Year (Reference No.) | Diet | No. | Age, years (mean (SD)) | Men, % | Weight, kg (mean (SD)) | Metabolic Risk Factors, mean (SD) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WC, cm | TC, mg/dL | LDL, mg/dL | HDL, mg/dL | TG, mg/dL | SBP, mmHg | DBP, mmHg | Glucose, mg/dL | Insulin, μIU/mL | ||||||
| Brehm, 2003 (5) | LFD | 20 | 43 (9) | 0 | 92 (6) | 185 (29) | 114 (30) | 49 (11) | 109 (45) | 115 (12) | 75 (9) | 91 (10) | 24 (11) | |
| LCD | 22 | 44 (7) | 0 | 91 (8) | 206 (30) | 125 (24) | 52 (13) | 149 (60) | 116 (14) | 79 (12) | 99 (12) | 17 (8) | ||
| Brinkworth, 2009 (22) | LFD | 36 | 50 (7) | 41 | 96 (15) | 107 (3) | 212 (4) | 131 (4) | 53 (2) | 159 (12) | 135 (12) | 77 (11) | 101 (2) | 10 (4) |
| LCD | 33 | 51 (8) | 32 | 95 (16) | 106 (3) | 209 (8) | 124 (4) | 56 (2) | 148 (12) | 132 (13) | 72 (10) | 103 (2) | 8 (3) | |
| Dansinger, 2005 (8) | LFD | 40 | 49 (12) | 43 | 103 (15) | 111 (13) | 214 (34) | 136 (37) | 45 (2) | 174 (130) | 133 (17) | 76 (9) | 121 (55) | 30 (18) |
| LCD | 40 | 47 (12) | 53 | 100 (14) | 109 (11) | 214 (31) | 136 (31) | 48 (16) | 152 (98) | 129 (17) | 77 (9) | 127 (62) | 22 (16) | |
| Davis, 2009 (23) | LFD | 50 | 53 (7) | 26 | 101 (19) | 166 (33) | 93 (28) | 46 (11) | 124 (59) | 130 (17) | 77 (10) | |||
| LCD | 55 | 54 (6) | 18 | 94 (18) | 170 (32) | 97 (27) | 50 (9) | 124 (74) | 125 (18) | 73 (9) | ||||
| Due, 2008 (24) | LFD | 43 | 29 (5) | 41 | 97 (14) | 103 (9) | 175 (39) | 107 (32) | 48 (13) | 102 (59) | 87 (6) | 6 (2) | ||
| LCD | 39 | 27 (5) | 43 | 95 (13) | 104 (9) | 171 (31) | 106 (31) | 47 (12) | 90 (42) | 90 (9) | 6 (3) | |||
| Ebbeling, 2007 (25) | LFD | 37 | 27 (4) | 22 | 103 (15) | 126 (34) | 54 (13) | 126 (81) | 108 (11) | 62 (9) | 88 (10) | 10 (7) | ||
| LCD | 36 | 28 (4) | 19 | 104 (17) | 102 (35) | 57 (20) | 112 (96) | 105 (12) | 63 (8) | 86 (8) | 11 (6) | |||
| Elhayany, 2009 (26) | LFD | 63 | 57 (6) | 56 | 86 (11) | 111 (9) | 212 (31) | 124 (31) | 43 (8) | 266 (62) | 182 (32) | 12 (7) | ||
| LCD | 61 | 56 (7) | 51 | 87 (14) | 113 (10) | 209 (35) | 120 (31) | 43 (8) | 283 (71) | 189 (36) | 14 (6) | |||
| Foster, 2003 (9) | LFD | 30 | 44 (7) | 26 | 98 (16) | 192 (33) | 120 (30) | 49 (13) | 123 (83) | 123 (14) | 75 (9) | |||
| LCD | 33 | 44 (9) | 36 | 99 (20) | 189 (30) | 130 (30) | 47 (11) | 131 (114) | 121 (11) | 78 (11) | ||||
| Foster, 2010 (17) | LFD | 154 | 45 (10) | 32 | 104 (14) | 124 (9) | 45 (12) | 124 (74) | 125 (16) | 76 (10) | ||||
| LCD | 153 | 46 (9) | 33 | 103 (16) | 120 (9) | 46 (14) | 113 (55) | 124 (14) | 74 (9) | |||||
| Frisch, 2009 (27) | LFD | 100 | 47 (10) | 24 | 99 (17) | 108 (13) | 214 (43) | 138 (35) | 56 (14) | 123 (58) | 128 (14) | 86 (8) | 101 (15) | |
| LCD | 100 | 47 (11) | 38 | 100 (16) | 110 (11) | 212 (36) | 137 (31) | 58 (14) | 116 (50) | 126 (13) | 86 (8) | 102 (20) | ||
| Gardner, 2007 (6) | LFD | 76 | 42 (6) | 0 | 86 (10) | 111 (27) | 50 (11) | 118 (62) | 116 (10) | 75 (8) | 93 (13) | 10 (5) | ||
| LCD | 77 | 42 (5) | 0 | 86 (13) | 109 (29) | 53 (14) | 125 (78) | 118 (11) | 75 (8) | 92 (9) | 10 (7) | |||
| Hockaday, 1978 (28) | LFD | 39 | 50 (24–65)b | 51 | 82 (56–114)b | 239 (48) | 141 (66) | 225 (81) | 11 (7) | |||||
| LCD | 54 | 53 (22–65)b | 59 | 76 (51–99)b | 251 (60) | 150 (78) | 195 (77) | 11 (7) | ||||||
| Iqbal, 2010 (29) | LFD | 72 | 60 (10) | 95 | 116 (17) | 181 (42) | 108 (37) | 41 (13) | 167 (96) | 140 (20) | 80 (12) | 145 (51) | ||
| LCD | 53 | 60 (9) | 84 | 118 (21) | 180 (46) | 110 (39) | 41 (13) | 155 (108) | 139 (20) | 79 (10) | 158 (62) | |||
| Klemsdal, 2010 (14) | LFD | 102 | 50 (8) | 38 | 100 (15) | 110 (10) | 232 (40) | 148 (39) | 50 (14) | 169 (100) | 129 (16) | 92 (10) | 101 (12) | 18 (11) |
| LCD | 100 | 50 (5) | 46 | 100 (16) | 111 (11) | 224 (38) | 145 (36) | 49 (14) | 171 (107) | 130 (13) | 91 (9) | 101 (17) | 16 (8) | |
| Lim, 2009 (30) | LFD | 28 | 49 (11) | 20 | 89 (3) | 220 (46) | 104 (73) | 54 (15) | 142 (53) | 129 (12) | 76 (10) | 96 (11) | 8 (4) | |
| LCD | 27 | 48 (8) | 20 | 88 (2) | 228 (39) | 120 (66) | 50 (12) | 159 (89) | 130 (15) | 77 (13) | 97 (11) | 11 (6) | ||
| McAuley, 2006 (31) | LFD | 24 | 45 (8) | 0 | 98 (16) | 109 (13) | 232 (35) | 151 (31) | 45 (9) | 166 (50) | 126 (12) | 81 (11) | 90 (11) | |
| LCD | 24 | 45 (7) | 0 | 97 (10) | 110 (10) | 224 (43) | 147 (35) | 44 (11) | 166 (73) | 131 (14) | 84 (10) | 92 (11) | ||
| Moran, 2006 (32) | LFD | 16 | 33 (5) | 0 | 96 (22) | 94 (7) | 11 (5) | |||||||
| LCD | 18 | 32 (6) | 0 | 96 (18) | 94 (7) | 15 (9) | ||||||||
| Sacks, 2009 (13) | LFD | 202 | 50 (10) | 33 | 92 (13) | 102 (12) | 203 (36) | 126 (32) | 49 (13) | 144 (79) | 120 (13) | 75 (9) | 92 (17) | 12 (8) |
| LCD | 201 | 51 (9) | 36 | 94 (16) | 104 (13) | 204 (35) | 126 (31) | 51 (16) | 141 (85) | 120 (15) | 76 (10) | 92 (13) | 12 (8) | |
| Shai, 2008 (11) | LFD | 104 | 51 (7) | 86 | 91 (12) | 105 (9) | 117 (36) | 39 (10) | 157 (62) | 130 (13) | 79 (9) | 87 (26) | 13 (7) | |
| LCD | 109 | 52 (7) | 91 | 92 (14) | 106 (9) | 117 (35) | 38 (9) | 182 (117) | 131 (15) | 79 (9) | 93 (29) | 14 (10) | ||
| Stern, 2004 (12) | LFD | 64 | 54 (9) | 85 | 129 (20) | 121 (28) | 41 (9) | 162 (78) | 139 (16) | 82 (9) | ||||
| LCD | 62 | 53 (9) | 80 | 132 (23) | 112 (32) | 41 (10) | 201 (204) | 133 (16) | 77 (11) | |||||
| Swenson, 2007 (33) | LFD | 13 | 40 (8) | 15 | 167 (71) | 140 (25) | ||||||||
| LCD | 19 | 42 (10) | 5 | 198 (85) | 145 (16) | |||||||||
| Thomson, 2010 (34) | LFD | 19 | 56 (9) | 0 | 83 (11) | 98 (10) | 201 (40) | 119 (34) | 54 (18) | 138 (54) | 136 (21) | 78 (10) | 100 (12) | 16 (9) |
| LCD | 21 | 56 (9) | 0 | 85 (14) | 102 (11) | 195 (26) | 112 (23) | 60 (15) | 115 (45) | 127 (14) | 81 (11) | 98 (15) | 17 (17) | |
| Yancy, 2004 (10) | LFD | 60 | 46 (9) | 22 | 97 (19) | 240 (35) | 147 (31) | 54 (15) | 191 (106) | 133 (16) | 82 (8) | |||
| LCD | 59 | 44 (10) | 25 | 98 (15) | 245 (35) | 159 (27) | 55 (15) | 158 (106) | 134 (16) | 82 (9) | ||||
| Total | LFD | 1396 | 48 | 39 | 98 | 108 | 208 | 125 | 48 | 149 | 127 | 79 | 109 | 13 |
| LCD | 1392 | 48 | 40 | 99 | 108 | 209 | 124 | 49 | 149 | 126 | 78 | 111 | 13 | |
Abbreviations: DBP, diastolic blood pressure; HDL, high density lipoprotein cholesterol; LCD, low-carbohydrate diet; LDL, low density lipoprotein cholesterol; LFD, low-fat diet; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol; TG, triglycerides; WC, waist circumference.
a Baseline data were from all participants in the study.
b Expressed as mean (range).
Pooled mean net changes and 95% confidence intervals for metabolic risk factors are presented in Web Figure 1 (available at http://aje.oxfordjournals.org/). The weighted mean changes in outcomes were −6.1 versus −5.0 kg for body weight, −6.2 versus −6.0 cm for waist circumference, −4.6 versus −10.1 mg/dL for total cholesterol, −2.1 versus −6.0 mg/dL for LDL cholesterol, 4.5 versus 1.6 mg/dL for HDL cholesterol, −0.7 versus −0.5 for ratio of total to HDL cholesterol, −30.4 versus −17.1 mg/dL for triglycerides, −3.5 versus −3.0 mm Hg for systolic blood pressure, and −10.4 versus −10.1 mg/dL for fasting blood glucose for low-carbohydrate versus low-fat diets, respectively. Pooled mean net changes and 95% confidence intervals representing differences between the diets in body weight (−1.0 kg, 95% confidence interval (CI): −2.2, 0.2) and waist circumference (−0.1 cm, 95% CI: −0.6, 0.4) reductions were not statistically significant. Compared with participants on low-fat diets, those on low-carbohydrate diets experienced slightly but statistically significantly less reduction in total cholesterol (pooled mean net change, 2.7 mg/dL, 95% CI: 0.8, 4.6) and LDL cholesterol (pooled mean net change, 3.7 mg/dL, 95% CI: 1.0, 6.4) but a greater increase in HDL cholesterol (pooled mean net change, 3.3 mg/dL, 95% CI: 1.9, 4.7) and a greater decrease in triglycerides (pooled mean net change, −14.0 mg/dL, 95% CI: −19.4, −8.7). These differences remained statistically significant after correction for multiple comparisons. Pooled mean net changes in systolic blood pressure (−1.0 mm Hg, 95% CI: −3.5, 1.5), ratio of total to HDL cholesterol (−0.1, 95% CI: −0.3, 0.1), and fasting blood glucose (−0.3 mg/dL, 95% CI: −1.9, 1.3) were not significantly different between the 2 diets. The pooled mean net changes in diastolic blood pressures and serum insulin were also not significant (data not shown).
There was no significant heterogeneity in the net changes in total cholesterol (I2 = 0.2%, P = 0.45), triglycerides (I2 = 55.6%, P = 0.07), waist circumference (I2 = 12.5%, P = 0.33), fasting blood glucose (I2 = 41.2%, P = 0.06), or serum insulin (I2 = 7.8%, P = 0.29) among these trials. However, statistically significant heterogeneity was detected for body weight (I2 = 85.7%, P < 0.001), systolic blood pressure (I2 = 91.7%, P < 0.001), diastolic blood pressure (I2 = 40.8%, P = 0.04), LDL cholesterol (I2 = 50.0%, P = 0.01), HDL cholesterol (I2 = 78.6%, P < 0.001), and ratio of total to HDL cholesterol (I2 = 75.0%, P = 0.003) among trials.
We examined the potential for publication bias by plotting sample sizes against mean net changes in each metabolic risk factor (Web Figure 2). Possible publication bias was detected for triglycerides (P = 0.02) using Begg's rank correlation and for body weight (P = 0.03), total cholesterol (P = 0.03), LDL cholesterol (P = 0.001), HDL cholesterol (P < 0.001), ratio of total to HDL cholesterol (P = 0.03), and insulin (P = 0.03) using Egger's linear regression tests. We used the trim-and-fill method to estimate the potential effect of publication bias on our results. When corrected for the effects of possible publication bias, pooled net change estimates for total cholesterol, LDL cholesterol, and HDL cholesterol became nonsignificant, but the pooled mean net change in body weight was significant at −3.2 kg (95% CI: −4.5 to −2.0), favoring low-carbohydrate diets.
In sensitivity analyses, the exclusion of any one study from the analysis did not significantly alter the net changes in metabolic risk factors. In addition, after removing studies with fewer than 20 participants per group or studies among patients with breast cancer, polycystic ovarian syndrome, or coronary heart disease or who had undergone gastric bypass surgery, body weight reduction was significantly greater on low-carbohydrate diets, with pooled mean net changes in body weight of −1.3 kg (95% CI: −2.5 to −0.1, n = 19 studies) and −1.4 kg (95% CI: −2.6 to −0.2, n = 18 studies), respectively (Table 3).
Table 3.
Main Results and Results From Sensitivity Analyses, 1966–2011
| Variable | All Trials |
Trials With ≥70% Completion Rate |
Trials ≥With 20 Participants per Group |
Trials With Healthy Participantsa |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Trials | Net Change | 95% CI | No. of Trials | Net Change | 95% CI | No. of Trials | Net Change | 95% CI | No. of Trials | Net Change | 95% CI | |
| Weight, kg | 22 | −1.0 | −2.2, 0.2 | 12 | −1.3 | −2.8, 0.2 | 19 | −1.3 | −2.5, −0.1 | 18 | −1.4 | −2.6, −0.2 |
| WC, cm | 10 | −0.1 | −0.6, 0.4 | 8 | −0.3 | −1.0, 0.5 | 8 | −0.2 | −0.7, 0.3 | 8 | −0.2 | −0.7, 0.3 |
| TC, mg/dL | 15 | 2.7 | 0.8, 4.6 | 9 | 3.5 | 0.1, 6.9 | 14 | 3.3 | 1.0, 5.5 | 13 | 2.7 | 0.6, 4.9 |
| LDL, mg/dL | 19 | 3.7 | 1.0, 6.4 | 11 | 2.8 | 0.3, 5.3 | 18 | 3.7 | 1.0, 6.4 | 17 | 3.6 | 0.7, 6.4 |
| HDL, mg/dL | 19 | 3.3 | 1.9, 4.7 | 11 | 3.0 | 1.9, 4.1 | 18 | 3.4 | 1.9, 4.8 | 17 | 3.3 | 1.9, 4.8 |
| TC:HDL ratio | 5 | −0.1 | −0.3, 0.1 | 1 | −0.5 | −0.8, −0.1 | 5 | −0.1 | −0.3, 0.1 | 5 | −0.1 | −0.3, 0.1 |
| TG, mg/dL | 20 | −14.0 | −19.4, −8.7 | 12 | −11.2 | −16.9, −5.5 | 19 | −13.4 | −18.7, −8.1 | 18 | −13.6 | −19.2, −8.0 |
| SBP, mmHg | 18 | −1.0 | −3.5, 1.5 | 10 | −0.1 | −2.5, 2.3 | 17 | −1.4 | −3.9, 1.2 | 17 | −1.6 | −4.2, 1.0 |
| DBP, mmHg | 18 | −0.7 | −1.6, 0.2 | 10 | −1.1 | −2.4, 0.2 | 17 | −0.8 | −1.7, 0.2 | 17 | −0.8 | −1.7, 0.2 |
| Glucose, mg/dL | 14 | −0.3 | −1.9, 1.3 | 10 | −0.6 | −2.7, 1.4 | 13 | −0.4 | −2.1, 1.4 | 13 | −0.4 | −2.1, 1.4 |
| Insulin, IU/mL | 12 | −0.1 | −0.8, 0.6 | 8 | −0.1 | −1.0, 0.8 | 10 | −0.1 | −0.9, 0.7 | 10 | −0.3 | −0.9, 0.7 |
Abbreviations: CI, confidence interval; DBP, diastolic blood pressure; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; WC, waist circumference.
a Trials with participants who had breast cancer, polycystic ovary syndrome, or coronary heart disease or who had undergone gastric bypass surgery were excluded.
Subgroup analyses by gender, diabetic status, level of carbohydrate restriction, and study duration did not identify statistically significant differences in the majority of metabolic risk factor reductions between low-carbohydrate and low-fat diets (Web Tables 1–5). However, there was a significantly greater reduction in body weight among participants who were on low-carbohydrate diets for more than 1 year (−0.9 kg, 95% CI: −1.6, −0.3) and those with a high level of carbohydrate restriction (−2.0 kg, 95% CI: −3.4 to −0.6) when compared with persons on low-fat diets. In addition, in the younger age group, low-carbohydrate diets resulted in significantly greater reductions in systolic blood pressure (−2.7 mm Hg, 95% CI: −4.5 to −0.9), diastolic blood pressure (−1.5 mm Hg, −2.7 to −0.2), and serum insulin (−0.9 μIU/mL, −1.8 to −0.1) as compared with results in the older age groups. However, these findings were not statistically significant after adjustment for multiple testing.
DISCUSSION
In the present meta-analysis of randomized controlled trials comparing low-carbohydrate diets with low-fat diets, we found that both diets were equally effective at reducing body weight and waist circumference. Both diets reduced participants' blood pressures, total to HDL cholesterol ratios, and total cholesterol, LDL cholesterol, triglycerides, blood glucose, and serum insulin levels and raised HDL cholesterol; however, participants on low-carbohydrate diets had greater increases in HDL cholesterol and greater decreases in triglycerides but experienced less reduction in total and LDL cholesterol compared with persons on low-fat diets. These findings have important clinical and public health implications. Over the past several decades, low-fat diets have been recommended to the public for weight loss primarily because of their beneficial effects on metabolic risk factors (4). Our study suggests that low-carbohydrate diets might provide an alternative approach for weight reduction without worsening metabolic risk factors.
Our results for blood pressure and lipids are consistent with those of a meta-analysis of randomized trials of low-carbohydrate dietary interventions conducted by Nordmann et al. in 2006 (16). That study found that low-carbohydrate diets produced significantly greater weight loss after 6 months than did low-fat diets, although the differences were not statistically significant at 1 year. In contrast, the present analysis showed that both diets were equally effective in reducing weight. The meta-analysis conducted by Nordmann et al. included only 5 trials with data on 447 participants, whereas the present study included 23 trials with data on 2,788 participants (16). Moreover, our study used a broader definition of low-carbohydrate diets (a maximum 45% of energy intake from carbohydrates) than did the previous study (≤60 g/day) and examined a much wider variety of metabolic outcomes.
There is a substantial body of evidence indicating that low-carbohydrate diets are effective for weight loss. With the exception of a study of severely obese patients, body weight and waist circumference were reduced among all of the low-carbohydrate dietary intervention studies, with mean reductions ranging from 1.5 to 14.3 kg and from 2.2 to 9.3 cm, respectively. Our findings suggest that low-carbohydrate diets are at least as effective as low-fat diets for weight loss, regardless of gender, age, length of intervention, diabetes status, and level of carbohydrate restriction.
Both diets also improved lipid profiles. Low-carbohydrate diets resulted in reductions in total cholesterol (−4.6 mg/dL), LDL cholesterol (−2.1 mg/dL), the ratio of total to HDL cholesterol (−0.7), and triglycerides (−30.4 mg/dL) and an increase in HDL cholesterol (4.5 mg/dL) from baseline to at least 6 months of follow-up. Although compared with low-fat diets, low-carbohydrate diets resulted in less reductions in total and LDL cholesterol but greater reductions in triglycerides and increases in HDL cholesterol, the reduction in ratio of total to HDL cholesterol, which has been identified as a predictor of coronary heart disease (35), was not significantly different between the 2 diets. It is tempting to suggest that the differential effect of the 2 diets on lipid fractions may translate to a differential effect on cardiovascular risk; however, to clearly demonstrate such a difference, randomized trials with clinical event outcomes may be necessary.
Dietary intake may affect multiple body systems. Although to our knowledge, there have been no clinical trials examining the association between low-carbohydrate diets and clinical outcomes such as depression, some studies have suggested that low-carbohydrate diets may result in mood changes. However, weight loss has also been associated with improved mood, whereas obesity has often been associated with depression (36, 37). Similarly, low-carbohydrate diets, which are high in protein, may increase calcium excretion in urine; however, this increase has not been associated with low bone density in prospective cohort studies (38–41) and may be offset by increased calcium absorption in the intestines (42). Low-carbohydrate diets are often high in fat, and high-fat diets have been associated with increased risks of certain types of cancer in some observational studies (43, 44). Thus, moderating the amount and types of fat substituted for carbohydrates is prudent not only to improve cardiovascular and metabolic risk factors but also to avoid increasing risk for other chronic diseases. Given the difficulty in disentangling dietary components, weight status, and other confounding factors that can vary over time in observational studies, these remaining questions may require large clinical trials of many years' duration.
Protein and fat sources may mediate the association between low-carbohydrate diets and long-term cardiovascular and metabolic risk factors, cancer, and mortality (45). A prospective cohort study of 82,802 US nurses reported that a low-carbohydrate dietary pattern that incorporated high intakes of vegetable protein and unsaturated fat was associated with a lower risk of coronary heart disease over 19 years of follow-up (46). The investigators also found this pattern was associated with a lower risk of cardiovascular and all-cause mortality among both men and women (47). In contrast, a low-carbohydrate diet emphasizing animal sources of fat and protein was associated with a higher risk of type 2 diabetes mellitus and all-cause mortality (47). Unfortunately, few studies in the present meta-analysis reported data on sources of dietary protein and fat. In the future, randomized controlled clinical trials that examine and compare metabolic and cardiovascular effects of different low-carbohydrate dietary patterns are warranted.
The present study has several limitations. First, losses to follow-up were substantial, especially in some trials (8, 22, 29). Almost half of the studies included in our meta-analysis had completion rates less than 70%. However, the sensitivity analysis suggested a nonsignificant influence of studies with a low completion rate on the overall study results. Second, moderate to high heterogeneity existed for some metabolic risk factors. Thus, we used random-effect models, which allow for between-study heterogeneity. Third, publication bias may be responsible for the significant differences in reductions of total and LDL cholesterol and increases in HDL cholesterol between diets. Statistical testing indicated significant publication bias for lipid outcomes, and when using the trim-and-fill method, which accounted for potential publication bias, pooled mean net changes in total, LDL, and HDL cholesterol were no longer statistically significant. However, the results of the trim-and-fill analysis should be interpreted cautiously because publication bias against low-carbohydrate diets may be different from what is typically encountered in research in which publication bias leaves out predominantly the negative studies.
There are also several strengths in the present study. We conducted this meta-analysis following a stringent protocol. Two investigators independently reviewed articles and abstracted the data using a standard abstraction form. The studies that we used were all randomized controlled trials, which are subject to fewer biases than observational studies and are the gold standard for evaluating the effects of an intervention. This meta-analysis had a sample size of 2,788, which provided the power to detect statistically significant mean differences, assess publication bias, and conduct sensitivity and subgroup analyses. Moreover, we used both false discovery rate and Bonferroni correction to adjusted P values (21). The results with regard to lipids remained significant when they were corrected using either method. Finally, we only included trials that were at least 6 months in duration to evaluate long-term changes in metabolic risk factors.
Because metabolic risk factors are important determinants of cardiovascular disease morbidity and mortality, recommending a diet in clinical practice that can improve these factors is vital to curbing the epidemics of obesity and cardiovascular diseases in the general population. Low-carbohydrate diets had beneficial effects on weight loss and metabolic risk factors, and these effects were comparable to those seen on low-fat diets. Although the present study did not examine long-term clinical effects on cardiovascular diseases, these findings suggest that low-carbohydrate diets can be recommended to obese persons with metabolic risk factors for the purpose of weight loss. Dietary recommendations for weight loss should be revisited to consider additional evidence of the benefits of low-carbohydrate diets.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, Louisiana (Tian Hu, Katherine T. Mills, Lu Yao, Kathryn Demanelis, Mohamed Eloustaz, Tanika N. Kelly, Jiang He, Lydia A. Bazzano); Department of Medicine, School of Medicine, Tulane University, New Orleans, Louisiana (Mohamed Eloustaz, Jiang He, Lydia A. Bazzano); and Department of Medicine, School of Medicine, Duke University, Durham, North Carolina (William S. Yancy, Jr.).
Dr. Bazzano was supported by grant K08 HL091108 from the National Institutes of Health/National Heart, Lung, and Blood Institute.
Conflict of interest: none declared.
REFERENCES
- 1.Kelly T, Yang W, Chen CS, et al. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32(9):1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.National Heart, Lung, and Blood Institute Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Bethesda, MD: National Heart, Lung, and Blood Institutes in cooperation with The National Institute of Diabetes and Digestive and Kidney Diseases; 1998. Clinical Guidelines of the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. [Google Scholar]
- 4.Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 5.Brehm BJ, Seeley RJ, Daniels SR, et al. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88(4):1617–1623. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- 6.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A to Z weight loss study: a randomized trial. J Am Med Assoc. 2007;297(9):969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 7.Atkins RC. Dr. Atkins' New Diet Revolution. 1st. New York, NY: M. Evans & Company; 2002. [Google Scholar]
- 8.Dansinger ML, Gleason JA, Griffith JL, et al. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293(1):43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 9.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348(21):2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 10.Yancy WS, Jr, Olsen MK, Guyton JR, et al. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140(10):769–777. doi: 10.7326/0003-4819-140-10-200405180-00006. [DOI] [PubMed] [Google Scholar]
- 11.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. New Engl J Med. 2008;359(3):229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 12.Stern L, Iqbal N, Seshadri P, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med. 2004;140(10):778–785. doi: 10.7326/0003-4819-140-10-200405180-00007. [DOI] [PubMed] [Google Scholar]
- 13.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klemsdal TO, Holme I, Nerland H, et al. Effects of a low glycemic load diet versus a low-fat diet in subjects with and without the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2010;20(3):195–201. doi: 10.1016/j.numecd.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Dyson PA, Beatty S, Matthews DR. A low-carbohydrate diet is more effective in reducing body weight than healthy eating in both diabetic and non-diabetic subjects. Diabet Med. 2007;24(12):1430–1435. doi: 10.1111/j.1464-5491.2007.02290.x. [DOI] [PubMed] [Google Scholar]
- 16.Nordmann AJ, Nordmann A, Briel M, et al. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors—a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166(3):285–293. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 17.Foster GD, Wyatt HR, Hill JO, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153(3):147–157. doi: 10.1059/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Heart Association. AHA Dietary Guidelines Revision 2000: A Statement for Healthcare Professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102(18):2284–2299. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Medicine of the National Academies. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) Washington, DC: Institute of Medicine of the National Academies; 2002. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003;19(3):368–375. doi: 10.1093/bioinformatics/btf877. [DOI] [PubMed] [Google Scholar]
- 22.Brinkworth GD, Noakes M, Buckley JD, et al. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am J Clin Nutr. 2009;90(1):23–32. doi: 10.3945/ajcn.2008.27326. [DOI] [PubMed] [Google Scholar]
- 23.Davis NJ, Tomuta N, Schechter C, et al. Comparative study of the effects of a 1-year dietary intervention of a low-carbohydrate diet versus a low-fat diet on weight and glycemic control in type 2 diabetes. Diabetes Care. 2009;32(7):1147–1152. doi: 10.2337/dc08-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Due A, Larsen TM, Mu H, et al. Comparison of 3 ad libitum diets for weight-loss maintenance, risk of cardiovascular disease, and diabetes: a 6-mo randomized, controlled trial. Am J Clin Nutr. 2008;88(5):1232–1241. doi: 10.3945/ajcn.2007.25695. [DOI] [PubMed] [Google Scholar]
- 25.Ebbeling CB, Leidig MM, Feldman HA, et al. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297(19):2092–2102. doi: 10.1001/jama.297.19.2092. [DOI] [PubMed] [Google Scholar]
- 26.Elhayany A, Lustman A, Abel R, et al. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: a 1-year prospective randomized intervention study. Diabetes Obes Metab. 2010;12(3):204–209. doi: 10.1111/j.1463-1326.2009.01151.x. [DOI] [PubMed] [Google Scholar]
- 27.Frisch S, Zittermann A, Berthold HK, et al. A randomized controlled trial on the efficacy of carbohydrate-reduced or fat-reduced diets in patients attending a telemedically guided weight loss program. Cardiovasc Diabetol. 2009;8(1):36. doi: 10.1186/1475-2840-8-36. (doi:10.1186/1475-2840-8-36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hockaday TD, Hockaday JM, Mann JI, et al. Prospective comparison of modified fat-high-carbohydrate with standard low-carbohydrate dietary advice in the treatment of diabetes: one year follow-up study. Br J Nutr. 1978;39(2):357–362. doi: 10.1079/bjn19780045. [DOI] [PubMed] [Google Scholar]
- 29.Iqbal N, Vetter ML, Moore RH, et al. Effects of a low-intensity intervention that prescribed a low-carbohydrate vs. a low-fat diet in obese, diabetic participants. Obesity (Silver Spring) 2010;18(9):1733–1738. doi: 10.1038/oby.2009.460. [DOI] [PubMed] [Google Scholar]
- 30.Lim SS, Noakes M, Keogh JB, et al. Long-term effects of a low carbohydrate, low fat or high unsaturated fat diet compared to a no-intervention control. Nutr Metab Cardiovasc Dis. 2010;20(8):599–607. doi: 10.1016/j.numecd.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 31.McAuley KA, Smith KJ, Taylor RW, et al. Long-term effects of popular dietary approaches on weight loss and features of insulin resistance. Int J Obes (Lond) 2006;30(2):342–349. doi: 10.1038/sj.ijo.0803075. [DOI] [PubMed] [Google Scholar]
- 32.Moran LJ, Noakes M, Clifton PM, et al. Short-term meal replacements followed by dietary macronutrient restriction enhance weight loss in polycystic ovary syndrome. Am J Clin Nutr. 2006;84(1):77–87. doi: 10.1093/ajcn/84.1.77. [DOI] [PubMed] [Google Scholar]
- 33.Swenson BR, Saalwachter Schulman A, Edwards MJ, et al. The effect of a low-carbohydrate, high-protein diet on post laparoscopic gastric bypass weight loss: a prospective randomized trial. J Surg Res. 2007;142(2):308–313. doi: 10.1016/j.jss.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 34.Thomson CA, Stopeck AT, Bea JW, et al. Changes in body weight and metabolic indexes in overweight breast cancer survivors enrolled in a randomized trial of low-fat vs. reduced carbohydrate diets. Nutr Cancer. 2010;62(8):1142–1152. doi: 10.1080/01635581.2010.513803. [DOI] [PubMed] [Google Scholar]
- 35.Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276(11):875–881. [PubMed] [Google Scholar]
- 36.Brinkworth GD, Buckley JD, Noakes M, et al. Long-term effects of a very low-carbohydrate diet and a low-fat diet on mood and cognitive function. Arch Intern Med. 2009;169(20):1873–1880. doi: 10.1001/archinternmed.2009.329. [DOI] [PubMed] [Google Scholar]
- 37.Geliebter A, Maher MM, Gerace L, et al. Effects of strength or aerobic training on body composition, resting metabolic rate, and peak oxygen consumption in obese dieting subjects. Am J Clin Nutr. 1997;66(3):557–563. doi: 10.1093/ajcn/66.3.557. [DOI] [PubMed] [Google Scholar]
- 38.Hannan MT, Tucker KL, Dawson-Hughes B, et al. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15(12):2504–2512. doi: 10.1359/jbmr.2000.15.12.2504. [DOI] [PubMed] [Google Scholar]
- 39.Munger RG, Cerhan JR, Chiu BC. Prospective study of dietary protein intake and risk of hip fracture in postmenopausal women. Am J Clin Nutr. 1999;69(1):147–152. doi: 10.1093/ajcn/69.1.147. [DOI] [PubMed] [Google Scholar]
- 40.Promislow JH, Goodman-Gruen D, Slymen DJ, et al. Protein consumption and bone mineral density in the elderly : the Rancho Bernardo Study. Am J Epidemiol. 2002;155(7):636–644. doi: 10.1093/aje/155.7.636. [DOI] [PubMed] [Google Scholar]
- 41.Kerstetter JE, Allen LH. Dietary protein increases urinary calcium. J Nutr. 1990;120(1):134–136. doi: 10.1093/jn/120.1.134. [DOI] [PubMed] [Google Scholar]
- 42.Kerstetter JE, O'Brien KO, Insogna KL. Dietary protein, calcium metabolism, and skeletal homeostasis revisited. Am J Clin Nutr. 2003;78(3 suppl):584S–592S. doi: 10.1093/ajcn/78.3.584S. [DOI] [PubMed] [Google Scholar]
- 43.Sieri S, Krogh V, Ferrari P, et al. Dietary fat and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;88(5):1304–1312. doi: 10.3945/ajcn.2008.26090. [DOI] [PubMed] [Google Scholar]
- 44.Freedman ND, Cross AJ, McGlynn KA, et al. Association of meat and fat intake with liver disease and hepatocellular carcinoma in the NIH-AARP cohort. J Natl Cancer Inst. 2010;102(17):1354–1365. doi: 10.1093/jnci/djq301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meinhold CL, Dodd KW, Jiao L, et al. Available carbohydrates, glycemic load, and pancreatic cancer: is there a link? Am J Epidemiol. 2010;171(11):1174–1182. doi: 10.1093/aje/kwq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halton TL, Willett WC, Liu S, et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355(19):1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 47.Fung TT, van Dam RM, Hankinson SE, et al. Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Ann Intern Med. 2010;153(5):289–298. doi: 10.1059/0003-4819-153-5-201009070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]