Abstract
The authors conducted a genome-wide linkage scan and positional association analysis to identify the genetic determinants of salt sensitivity of blood pressure (BP) in a large family-based, dietary-feeding study. The dietary intervention was conducted among 1,906 participants in rural China (2003–2005). A 7-day low-sodium intervention was followed by a 7-day high-sodium intervention. Salt sensitivity was defined as BP responses to low- and high-sodium interventions. Signals of the logarithm of the odds to the base 10 (LOD ≥ 3) were detected at 33–42 centimorgans of chromosome 2 (2p24.3-2p24.1), with a maximum LOD score of 3.33 for diastolic blood pressure responses to high-sodium intervention. LOD scores were 2.35–2.91 for mean arterial pressure (MAP) and 0.80–1.49 for systolic blood pressure responses in this region, respectively. Correcting for multiple tests, single nucleotide polymorphism (SNP) rs11674786 (2.7 kilobases upstream of the family with sequence similarity 84, member A, gene (FAM84A)) in the linkage region was significantly associated with diastolic blood pressure (P = 0.0007) and MAP responses (P = 0.0007), and SNP rs16983422 (2.8 kilobases upstream of the visinin-like 1 gene (VSNL1)) was marginally associated with diastolic blood pressure (P = 0.005) and MAP responses (P = 0.005). An additive interaction between SNPs rs11674786 and rs16983422 was observed, with P = 7.00 × 10−5 and P = 7.23 × 10−5 for diastolic blood pressure and MAP responses, respectively. The authors concluded that genetic region 2p24.3-2p24.1 might harbor functional variants for the salt sensitivity of BP.
Keywords: allelic association, blood pressure, genetic linkage, salt sensitivity
Epidemiologic studies and randomized clinical trials have documented that dietary sodium reduction lowers blood pressure (BP) in both hypertensive and normotensive persons (1). In addition, experimental studies in human subjects and animal models have shown that high dietary sodium intake increases BP (2, 3). It is also well known that BP responses to dietary sodium intake vary among individuals (4–6). Age, gender, ethnicity, and environmental and genetic factors may all play a role in determining an individual's salt sensitivity of BP (4, 5). Salt sensitivity is inheritable, as suggested by different family studies (7–9), indicating that genetic factors contribute to salt sensitivity. The Genetic Epidemiology Network of Salt Sensitivity (GenSalt) Study (10), which we conducted in a Chinese population, has shown that heritabilities of BP responses were 0.24, 0.34, and 0.27 during low-sodium intervention and 0.27, 0.42, and 0.35 during high-sodium intervention for systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP), respectively. However, the genetic etiology underlying salt sensitivity is not yet clear (10).
Understanding the genetic determinants of BP response to sodium intake has important public health significance. The identification of genetic variants that contribute to salt sensitivity of BP could help identify people with a genetic predisposition to salt-sensitive hypertension for early intervention. It will also assist in the development of new antihypertensive medications targeting biologic pathways related to sodium metabolites. The objective of the current study is to identify genetic variants underlying salt sensitivity of BP in a homogeneous Chinese population. We conducted a genome-wide linkage scan to identify linkage regions that may harbor potential susceptibility genes for salt sensitivity. In the follow-up analysis, we examined the association of tag single nucleotide polymorphisms (SNPs) in the linkage regions with BP responses to sodium intervention.
MATERIALS AND METHODS
Study participants
The GenSalt Study was conducted in rural areas of north China, where people have habitually high dietary sodium intake, from October 2003 to July 2005. A community-based BP screening was conducted among persons aged 18–60 years in study villages to identify potential probands for the study. Those with average SBP between 130 and 160 mm Hg and/or DBP between 85 and 100 mm Hg had a second BP screening visit 1 day later to confirm their eligibility. The detailed eligibility criteria for the probands and siblings/offspring are presented elsewhere (11). Individuals who had stage-2 hypertension, secondary hypertension, use of antihypertensive medications, history of clinical cardiovascular disease, diabetes, or chronic kidney disease, pregnancy, or heavy alcohol use were excluded from the GenSalt Study for safety purposes. After probands and their siblings were confirmed for eligibility, they were invited to participate in the study and signed an informed consent form. Both 2-generation (proband, siblings, and their parents) and 3-generation (proband, siblings, their parents, and proband's spouse and offspring) families were recruited for the study. Only probands, siblings, offspring, and spouses of probands from 3-generation families participated in the dietary sodium intervention. Of the 1,906 eligible participants, 1,860 participants (97.6%) from 624 families completed low- and high-sodium intervention.
Institutional review boards at all of the participating institutions approved the GenSalt Study. Written, informed consents for the baseline observation and for the intervention program were obtained from each participant.
Low- and high-sodium intervention
The dietary sodium intervention included low-sodium and high-sodium feeding among probands and their siblings and offspring. In the first 3 days at the baseline observation, the study participants consumed their usual diet. The dietary feeding study started on day 4. The intervention participants received a 7-day low-sodium diet (3 g of sodium chloride or 51.3 mmol of sodium per day) followed by a 7-day high-sodium diet (18 g of sodium chloride or 307.8 mmol of sodium per day). During the period of sodium intervention, dietary potassium intake remained unchanged. Total energy intake was varied according to each participant's baseline energy intake. All study foods were cooked without salt, and prepackaged salt was added to the individual study participant's meal when it was served by the study staff. To ensure study participants' compliance to the intervention program, they were required to have their breakfast, lunch, and dinner at the study kitchen under supervision of the study staff during the entire study period. Food consumption of study participants was carefully recorded at each meal by study staff members. The study participants were instructed to avoid consuming any foods that were not provided by the study. In addition, 3 timed urinary specimens were collected at baseline and in each phase of intervention to monitor participants' compliance to dietary sodium intake. The results from 24-hour urinary excretion of sodium at the baseline examination and during each of the dietary intervention phases showed excellent compliance. For example, the mean 24-hour urinary excretions of sodium and potassium were 242.4 (standard deviation (SD), 66.7) mmol and 36.9 (SD, 9.6) mmol at baseline, 47.5 (SD, 16.0) mmol and 31.4 (SD, 7.7) mmol during the low-sodium intervention, and 244.3 (SD, 37.7) mmol and 35.7 (SD, 7.5) mmol during the high-sodium intervention, respectively. Baseline 24-hour urinary sodium excretions were not significantly different from those of the high-sodium intervention phase, suggesting that sodium intake during the high-sodium diet was similar to the habitual sodium intake of this population.
Phenotype measurement
A standard questionnaire was administered by a trained staff member at the baseline examination to collect information on family structure, demographic characteristics, personal and family medical history, and lifestyle risk factors. Three BP measurements were obtained at each clinic visit by trained and certified observers according to a common protocol adapted from procedures recommended by the American Heart Association (12). BP was measured with the participant in the sitting position after 5 minutes of rest. In addition, participants were advised to avoid alcohol, cigarette smoking, coffee/tea, and exercise for at least 30 minutes prior to their BP measurement. A random-zero sphygmomanometer was used, and 1 of 4 cuff sizes (pediatric, regular adult, large, or thigh) was chosen on the basis of the circumference of the participant's arm. BP was measured each morning of the 3-day baseline observation and on days 2, 5, 6, and 7 of each intervention period by the same BP technician using the same sphygmomanometer to avoid interobservation variation. All BP observers were blinded to the dietary intervention.
Marker genotyping and tag SNP selection
Lymphocytic DNA samples were obtained from GenSalt family members (probands, parents, spouses, siblings, and offspring) and used for genotyping microsatellite markers spaced at approximately 9-centimorgan (cM) intervals (407 markers of Weber Lab Screening Set 12; Marshfield Center, Marshfield, Wisconsin). Microsatellite genotyping used fluorescently labeled polymerase chain reaction primers for marker amplification followed by capillary electrophoresis on automated DNA sequencers (ABI 3730xl DNA Analyzer; Applied Biosystems, Carlsbad, California). Quality control samples included blind duplicates, no DNA controls, and Centre d'Etudes du Polymorphisme Humain (CEPH) DNA standards (mother, father, offspring with known genotypes). Genotypes were assigned by using GeneMapper software (Applied Biosystems). Graphical representation of relation (GRR) and affected sib-pair exclusion (ASPEX) mapping were used to check for potential misreported relations in the GenSalt family pedigrees (13, 14). MapMaker/Sibs and PedCheck were used to check for Mendelian inconsistencies within families for each marker (15, 16). After quality control, 359 markers were used for analysis.
SNPs located within identified linkage regions (LOD ≥ 3.0) were genotyped as part of the SNP Array 6.0 platform (Affymetrix, Inc., Cleveland, Ohio), which should provide excellent coverage of all common genetic variation. On the basis of human haplotype map data of Han Chinese in Beijing (17), these SNPs were analyzed to select tag SNPs by using Haploview (18) software and the following criteria: 1) minor allele frequency ≥0.01; 2) P value of a Hardy-Weinberg equilibrium test ≥0.01; and 3) pairwise correlation coefficient ≤0.1. The positions and corresponding genes of tag SNPs were identified by the National Center for Biotechnology Information map viewer (www.ncbi.nlm.nih.gov/mapview/) and Affymetrix annotation data.
Statistical analyses
BP levels at baseline and during intervention were calculated as the average of 9 measurements from 3 clinic visits during the 3-day baseline observation or on days 5, 6, and 7 of each intervention period. Mean arterial pressure (MAP) was calculated as DBP + (SBP − DBP)/3. The BP responses to low-sodium intervention were calculated as BP in the low-sodium intervention minus BP at baseline, and BP responses to high-sodium intervention were calculated as BP in the high-sodium intervention minus BP in the low-sodium intervention. BP responses were used to define salt sensitivity for linkage and association tests.
The BP responses were adjusted for the effects of age and BP examination room temperature separately within sex-generation-field center groups. In brief, each phenotype was regressed on the covariates in a stepwise manner, and only significant terms (P < 0.05) were retained. The residual variance was also examined (i.e., heteroscedasticity) by regressing the squared residual from the first regression on the same covariates (stepwise) and retaining significant terms. The final adjusted phenotype was computed as the residual from the first regression, divided by the square root of the predicted score from the second regression. A final standardization step was taken to ensure a mean of 0 and a standard deviation of 1. These adjusted and standardized scores were used as the analysis phenotypes.
Multipoint genome-wide linkage analyses of salt sensitivity BP were conducted by using SOLAR software (19). The resolution for a multipoint linkage scan is set as 1 cM. The linkage region is defined as 0.5 cM up- and downstream of the tested locus that has LOD ≥3.0. The association of every tag SNP in linkage regions (LOD ≥3.0) with salt-sensitivity BP was examined by using the lme4 package of R (version 2.10.1; http://www.r-project.org) and SAS (version 9.1; SAS Institute, Inc., Cary, North Carolina) statistical software using a mixed linear regression model. A sandwich estimator was used to account for the nonindependence of family members. This method assumes the same degree of dependency among family members. Age, gender, BP measurement, room temperature, and study site were adjusted in multivariable analyses. The Bonferroni method was used to adjust for multiple tests. For significant or marginally significant SNPs, additive effects were examined by comparing the statistical model including all SNPs with that without SNPs. Nonadditive interactions were examined by comparing the statistical model including an interaction term with that without the term. Hardy-Weinberg equilibrium was examined by Fisher's exact test (20).
RESULTS
The baseline characteristics of 3,142 participants, including 676 probands, 69 spouses, 1,236 parents, 956 siblings, and 205 offspring from 625 families, are presented in Table 1. The baseline SBP ranged from 107 mm Hg among offspring to 137 mm Hg among parents, the DBP ranged from 65 mm Hg among offspring to 80 mm Hg among probands, and the MAP ranged from 79 mm Hg among offspring to 96 mm Hg among probands. Among the 1,860 participating probands, spouses, siblings, and offspring who completed the entire low- and high-sodium interventions, the average SBP, DBP, and MAP decreased during low-sodium intervention (SBP: −2.3 to −8.0 mm Hg; DBP: −1.7 to −4.2 mm Hg; and MAP: −1.9 to −5.5 mm Hg) and increased during high-sodium intervention (SBP: 10.1 to 13.4 mm Hg; DBP: 8.7 to 9.5 mm Hg; and MAP: 8.3 to 10.4 mm Hg). The effects of low- and high-sodium intake on BP were also observed in whites and blacks (21) that showed increased BP (SBP: 6.7 mm Hg; DBP: 3.5 mm Hg) comparing high-sodium intake with low-sodium intake and decreased BP (SBP: 4.6 mm Hg; DBP: 2.4 mm Hg) comparing low-sodium intake with intermediate-sodium intake.
Table 1.
Characteristics and BP Responses to Sodium Intervention Among 3,142 Participants From 633 Families, GenSalt Study, China, 2003–2005
| Probands (n = 676) |
Spouses (n = 69) |
Parents (n = 1,236)a |
Siblings (n = 956) |
Offspring (n = 205) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | |
| Men | 60.4 | 33.3 | 48.6 | 51.1 | 55.6 | |||||
| Age, years | 41.0 (8.3) | 49.1 (6.7) | 67.6 (8.4) | 39.6 (7.7) | 23.5 (6.5) | |||||
| Body mass indexb | 24.2 (3.3) | 23.4 (3.7) | 22.8 (3.4) | 23.1 (2.8) | 21.5 (3.3) | |||||
| Baseline BP, mm Hg | ||||||||||
| SBP | 128.0 (11.4) | 112.6 (14.9) | 136.6 (23.9) | 111.6 (11.5) | 106.6 (10.3) | |||||
| DBP | 80.3 (9.0) | 72.6 (10.0) | 75.0 (11.7) | 71.0 (8.9) | 65.3 (9.0) | |||||
| MAP | 96.2 (8.5) | 85.9 (11.2) | 95.6 (14.3) | 84.5 (9.2) | 79.1 (8.7) | |||||
| BP during low-sodium intervention, mm Hg | ||||||||||
| SBP | 119.9 (11.3) | 106.2 (11.7) | NA | 107.2 (9.8) | 104.5 (9.7) | |||||
| DBP | 76.0 (9.1) | 69.5 (8.7) | NA | 69.1 (8.6) | 63.7 (8.7) | |||||
| MAP | 94.2 (9.3) | 81.7 (9.1) | NA | 81.8 (8.3) | 77.3 (8.2) | |||||
| BP response to low-sodium intervention, mm Hg | ||||||||||
| SBP | −8.0 (7.9) | −5.59 (7.7) | NA | −4.3 (6.0) | −2.3 (4.9) | |||||
| DBP | −4.2 (5.9) | −2.7 (5.0) | NA | −2.0 (5.1) | −1.7 (5.3) | |||||
| MAP | −5.5 (5.8) | −3.7 (5.4) | NA | −2.7 (4.8) | −1.9 (4.5) | |||||
| BP during high-sodium intervention, mm Hg | ||||||||||
| SBP | 125.6 (12.4) | 111.9 (13.4) | NA | 111.8 (11.1) | 106.9 (10.1) | |||||
| DBP | 78.5 (9.4) | 71.3 (9.5) | NA | 70.8 (9.1) | 64.5 (8.7) | |||||
| MAP | 94.2 (9.3) | 84.9 (10.4) | NA | 84.5 (9.2) | 78.7 (8.3) | |||||
| BP response to high-sodium intervention, mm Hg | ||||||||||
| SBP | 5.7 (6.4) | 5.8 (5.9) | NA | 4.7 (5.8) | 2.6 (4.3) | |||||
| DBP | 2.5 (5.8) | 2.0 (4.3) | NA | 1.7 (5.2) | 0.9 (5.0) | |||||
| MAP | 3.3 (4.4) | −2.9 (4.7) | NA | 2.7 (4.8) | 1.4 (4.0) | |||||
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; GenSalt, Genetic Epidemiology Network of Salt Sensitivity; MAP, mean arterial pressure; NA, not available; SBP, systolic blood pressure; SD, standard deviation.
a Characteristics for parents in the sample. Parents did not take part in the dietary intervention, and their BP responses to sodium intervention are not available.
b Body mass index: weight (kg)/height (m)2.
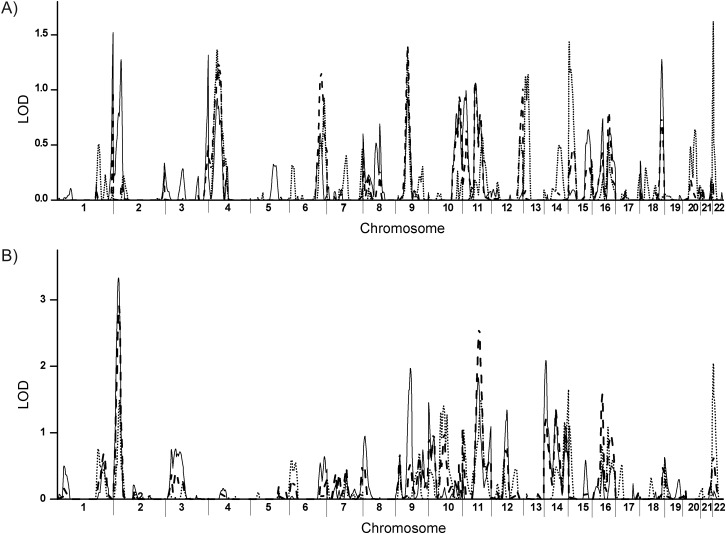
Genome-wide linkage results for BP responses to the low-sodium and high-sodium interventions are illustrated in Figure 1, A and B, respectively. We observed significant linkage (LOD score = 3.08–3.33) for DBP responses (ΔDBP) to the high-sodium intervention to chromosomal region 2p24.3-2p24.1. We also observed suggestive linkage (LOD score = 2.35–2.91) for MAP responses to this chromosomal region (ΔMAP). The linkage scan of the SBP response (ΔSBP) has a LOD score of 0.80–1.49. There is no significant linkage region identified for BP responses to the low-sodium intervention.
Figure 1.
Genome-wide linkages scan of BP responses to low-sodium (A) and high-sodium (B) interventions, GenSalt Study, China, 2003–2005. Linkage logarithm of the odds (LOD) scores for the blood pressure (BP) responses are distinguished by different line styles: solid line, ΔDBP (absolute diastolic blood pressure (DBP) change); dashed line, ΔMAP (absolute mean arterial pressure (MAP) change); and dotted line, ΔSBP (absolute systolic blood pressure (SBP) change). GenSalt, Genetic Epidemiology Network of Salt Sensitivity.
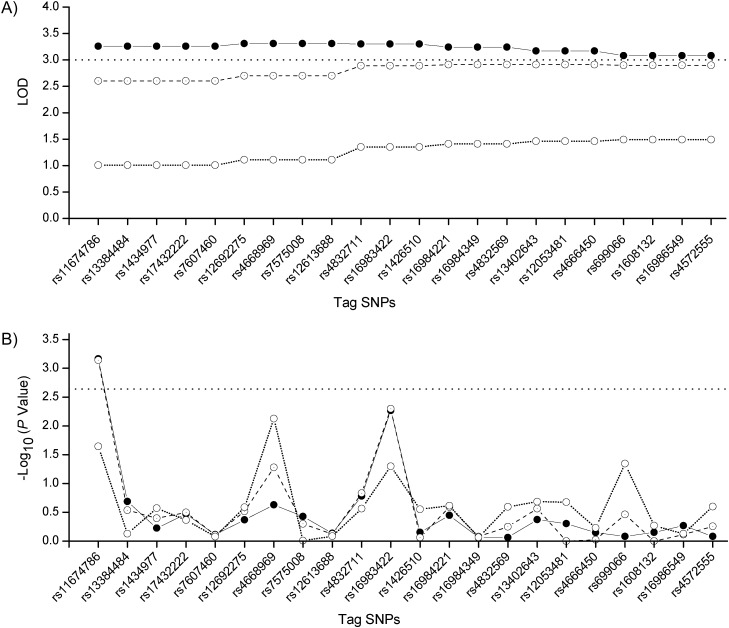
Twenty-two tag SNPs in the significant linkage region were selected for testing the association with BP responses to the high-sodium intervention. Table 2 shows the characteristics of selected tag SNPs including alleles, minor allele frequency, physical position in base pairs, and the P value of a Hardy-Weinberg equilibrium Fisher's exact test. The linkage disequilibrium, measured as r2 ≤ 0.16, is presented in Web Figure 1, which appears on the Journal's Web site (http://aje.oxfordjournals.org/) along with Web Figure 2 and Web Table 1. For each tag SNP, its LOD score in the linkage region and −log10 (P value) of association were shown in Figure 2, A and B, respectively. SNP rs11674786, downstream from the family with sequence similarity 84, member A, gene (FAM84A), has significant linkage to DBP responses (LOD = 3.26) and suggestive linkage to MAP responses (LOD = 2.60) during high-sodium intervention. The corresponding P values are 6.9 × 10−4 and 7.2 × 10−4 for associations with DBP and MAP responses, respectively, and are both significant after Bonferroni correction. SNP rs16983422, upstream from the visinin-like 1 gene (VSNL1), has significant linkage to DBP responses (LOD = 3.30) and suggestive linkage to MAP responses (LOD = 2.89) during high-sodium intervention. The corresponding P values of 0.005 and 0.005 are marginally significant for associations with DBP and MAP responses, respectively, when compared with the Bonferroni-corrected threshold of significance (P = 0.05/22).
Table 2.
Description of Tag SNPs Within Linkage Regions, GenSalt Study, China, 2003–2005
| SNP | Minor Allele/Major Allele | MAF | Position, base pairs | HWE P Value | Region | Gene Symbola |
|---|---|---|---|---|---|---|
| rs11674786 | G/T | 0.107 | 14417953 | 1 | Upstream | FAM84A |
| rs13384484 | A/G | 0.001 | 14610298 | 1 | Upstream | FAM84A |
| rs1434977 | A/C | 0.349 | 14652091 | 0.876953511 | Upstream | FAM84A |
| rs17432222 | A/T | 0.391 | 14873031 | 0.695026883 | Downstream | FAM84A, NAG |
| rs7607460 | A/T | 0.476 | 15139806 | 0.743512478 | Downstream | FAM84A , NAG |
| rs12692275 | T/C | 0.246 | 15634232 | 0.068854963 | Upstream | NAG, DDX1 |
| rs4668969 | G/A | 0.234 | 15803805 | 0.603205915 | Downstream | DDX1 |
| rs7575008 | A/G | 0.263 | 15881442 | 0.506276473 | Downstream | DDX1 |
| rs12613688 | A/G | 0.423 | 15893199 | 1 | Downstream | DDX1 |
| rs4832711 | C/T | 0.247 | 17218488 | 0.230969457 | Upstream | VSNL1 |
| rs16983422 | G/A | 0.145 | 17308144 | 0.924923419 | Upstream | VSNL1 |
| rs1426510 | A/T | 0.205 | 17646037 | 0.77517763 | Intron | VSNL1 |
| rs16984221 | G/A | 0.212 | 18091241 | 0.944454778 | Downstream | RDH14, NT5C1B |
| rs16984349 | G/A | 0.259 | 18142831 | 0.012544094 | Downstream | RDH14, NT5C1B |
| rs4832569 | C/A | 0.454 | 18315827 | 0.17281924 | Downstream | RDH14, NT5C1B |
| rs13402643 | T/G | 0.381 | 18519802 | 0.804634338 | Downstream | RDH14, NT5C1B |
| rs12053481 | A/C | 0.476 | 18695827 | 0.175628829 | Upstream | RDH14, NT5C1B |
| rs4666450 | C/G | 0.221 | 19149985 | 0.734119022 | Downstream | OSR1 |
| rs699066 | C/A | 0.276 | 19354652 | 0.682966192 | Downstream | OSR1 |
| rs1608132 | T/C | 0.235 | 19501366 | 0.136379003 | Upstream | OSR1 |
| rs16986476 | A/C | 0.228 | 19522847 | 0.14446073 | Upstream | OSR1 |
| rs4572555 | G/A | 0.352 | 19778919 | 0.328785215 | Upstream | OSR1 |
Abbreviations: GenSalt, Genetic Epidemiology Network of Salt Sensitivity; HWE, Hardy-Weinberg equilibrium; MAF, minor allele frequency; SNP, single nucleotide polymorphism.
a DDX1, DEAD (Asp-Glu-Ala-Asp) box helicase 1 gene; FAM84A, family with sequence similarity 84, member A, gene; NAG, neuroblastoma amplified sequence gene; NT5C1B, 5’-nucleotidase, cytosolic IB gene; OSR1, odd-skipped related 1 gene; RDH14, retinol dehydrogenase 14 gene; VSNL1, visinin-like 1 gene.
Figure 2.
Logarithm of the odds (LOD) scores of tag SNPs in the linkage region (LOD ≥3.0) for diastolic blood pressure (DBP, solid line), mean arterial pressure (MAP, dashed line), and systolic blood pressure (SBP, dotted line) responses (A) and –log10 (P value) of tag SNPs associated with DBP, MAP, and SBP responses (B), GenSalt Study, China, 2003–2005. GenSalt, Genetic Epidemiology Network of Salt Sensitivity; SNP, single nucleotide polymorphism.
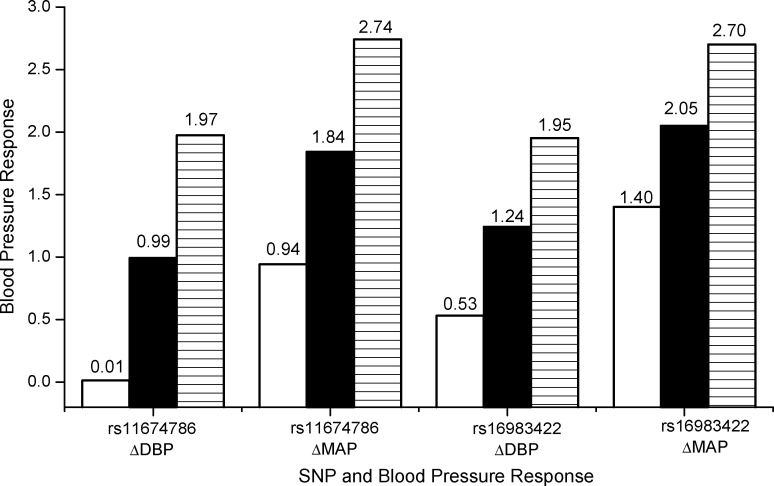
The effect sizes of SNP rs11674786 and rs16983422 on BP responses are shown in Figure 3. For SNP rs11674786, genotypes GG, GT, and TT have increased DBP of 0.01, 0.99, and 1.97 mm Hg and MAP of 0.94, 1.84, and 2.74 mm Hg during high-sodium intervention. For SNP rs16983422, genotypes GG, GA, and AA have increased DBP of 0.53, 1.24, and 1.95 mm Hg and MAP of 1.40, 2.05, and 2.70 mm Hg during high-sodium intervention. The numbers of allele T at SNP rs11674786 and allele A at SNP rs16983422 are linearly associated with increased DBP and MAP responses at high-sodium intervention.
Figure 3.
The effect sizes of SNP rs11674786 (GG: white; GT: black; TT: line) and rs16983422 (GG: white; GA: black; AA: line) on diastolic blood pressure (DBP) responses (ΔDBP) and mean arterial pressure (MAP) responses (ΔMAP) during the high-sodium intervention, GenSalt Study, China, 2003–2005. P = 6.9 × 10−4 and 7.3 × 10−4 for rs11674786 associated with DBP and MAP responses; P = 0.005 and 0.005 for rs16983422 associated with DBP and MAP responses. GenSalt, Genetic Epidemiology Network of Salt Sensitivity; SNP, single nucleotide polymorphism.
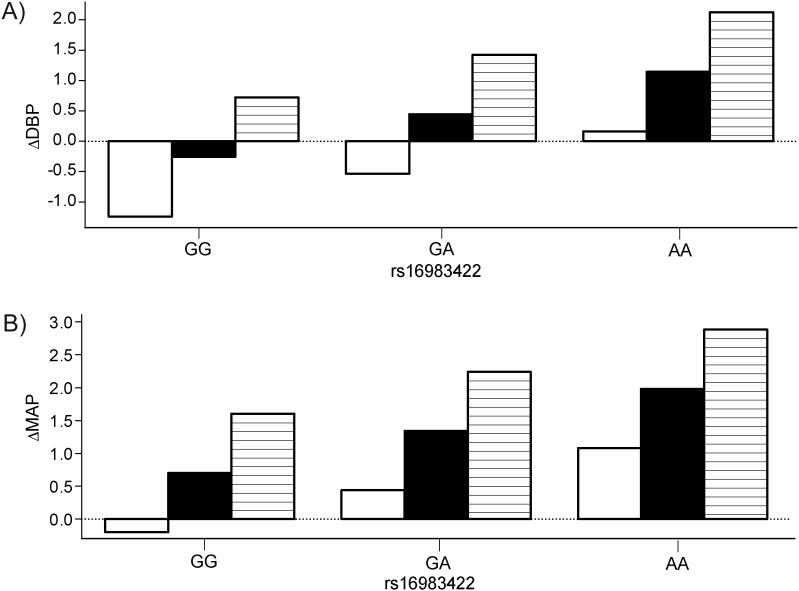
Additive interactions between rs11674786 and rs16983422 on BP responses to dietary sodium interventions are shown in Figure 4. Genotypes GG, GT, and TT of rs11674786 have DBP responses of −1.24, −0.26, and 0.72 mm Hg under GG; −0.54, 0.44, and 1.42 mm Hg under GA; and 0.16, 1.14, and 2.12 mm Hg under AA of rs16983422. Genotypes GG, GT, and TT of rs11674786 have MAP responses of −0.20, 0.70, and 1.60 mm Hg under GG; 0.44, 1.34, and 2.24 mm Hg under GA; and 1.08, 1.98, and 2.88 mm Hg under AA of rs16983422. The effect of rs11674786 increased as the number of allele A's at rs16983422 increased. The additive interaction between rs11674786 and rs16983422 on BP responses was significant for both DBP (P = 7.00 × 10−5) and MAP (P = 7.23 × 10−5) responses (Figure 4), and nonadditive interaction effects were not significant for DBP (P = 0.58) or MAP (P = 0.72) responses during high-sodium intervention.
Figure 4.
Additive interaction between SNP rs11674786 (GG: white; GT: black; TT: line) and rs16983422 on diastolic blood pressure (DBP) responses (ΔDBP), P = 7.00 × 10−5, and mean arterial pressure (MAP) responses (ΔMAP), P = 7.23 × 10−5, during the high-sodium intervention, GenSalt Study, China, 2003–2005. GenSalt, Genetic Epidemiology Network of Salt Sensitivity; SNP, single nucleotide polymorphism.
DISCUSSION
In this study, we identified a linkage region on chromosome 2 for salt sensitivity of BP and observed susceptibility genetic variants significantly associated with BP responses to high-sodium intervention in the linkage region. Genome-wide linkage scans in a rat model (22) and candidate linkage scans in humans (23, 24) have been reported in the past decades related to BP responses to sodium intake. However, this study is the first to conduct genome-wide linkage scans in humans and to identify genomic regions by using a family-based, dietary-feeding study. We also conducted follow-up association analyses of tag SNPs to identify susceptibility genetic variants in the linkage region.
Our study was performed in a rural Han Chinese population with similar lifestyles and other environmental risk factors, including diet and physical activity (11). Thus, confounding due to these exposures should be minimized. Participation in the dietary interventions was high (97.6%). To ensure study participants' compliance to the low-sodium and high-sodium interventions, consumption of food with prepackaged salt was supervised and recorded at each meal with dietary potassium intake unchanged. The controlled food consumption will minimize the confounding effects of dietary intakes during the sodium intervention study. Furthermore, bias due to population stratification is unlikely, because the study is conducted in a homogenous Chinese Han population. Compliance with dietary low- and high-sodium intervention assessed by urinary 24-hour excretion was excellent in our study. Stringent quality control during the intervention, data collection, genotyping, and statistical analyses all served to reduce type I error and improve power.
Our genome-wide linkage scan observed a significant linkage region at 33–42 cM on chromosome 2 (2p24.3-2p24.1) for DBP responses to the high-sodium intervention. This region also observed suggestive linkage for MAP responses to high-sodium intervention (LOD > 2.0). Genome-wide linkage scans for salt sensitivity have not been conducted in human subjects previously. Our findings, however, are consistent with genomic regions identified in linkage scans for regular BP (25–32), which are close to or overlap with linkage regions in our study. This suggests that genetic determinants underlying salt sensitivity of BP and regular BP might share the same genes.
In the follow-up association study of linkage region, we found that SNP rs11674786, upstream from a gene encoding FAM84A, was strongly associated with DBP and MAP responses to the high-sodium intervention. The function of FAM84A has not been widely studied yet. Previous research indicated that expression of FAM84A was associated with morphology of cells, and up-regulation of FAM84A may be involved with progression of colon cancer (33). Our study suggests that regulation of the region upstream from FAM84A may be related to salt sensitivity of BP. The second SNP rs16983422, upstream from VSNL1 (Online Mendelian Inheritance in Man (OMIM) identifier (ID) 600817), has a marginally significant association with DBP and MAP responses to a high-sodium intervention. VSNL1 is highly expressed in the brain and associates with membranes in a calcium-dependent manner by regulating the activity of adenylyl cyclase (EntrezGene ID 7447). The family of neuronal calcium sensor proteins can modulate a variety of pathophysiologic processes including cancer (34), hypertension (35), and insulin secretion (36). Implication of VSNL1 in these processes could be attributable to its regulation of the natriuretic peptide system (37). Our study added novel information that VSNL1 may be involved in regulation of salt sensitivity of BP. However, rs11674786 is 2.7 kilobases upstream of FAM84A, and rs16983422 is 2.8 kilobases upstream of VSNL1. These distances may also possibly suggest that causative variants are out of gene regions, and that these significant intergenic SNPs lie in regulatory sequences. The function of these regions and potential regulations on FAM84A and VSNL1 are worth future investigations.
Our study identified strongly significant association of rs11674786 and marginally significant association of rs16983422 with BP responses during the high-sodium intervention. The joint test of both SNPs had a much more significant P value than separate tests of single SNPs, indicating strong additive effects between them. These findings suggest that the salt sensitivity of BP could involve multiple variants, and that each variant contributes additively to BP responses. In addition, these findings suggest that a strong linkage signal in a chromosomal region can be the result of multiple variants, each with modest effect.
We conducted association tests in the significant linkage region for BP responses to dietary intervention, which has the advantages of identifying genetic variants with the evidence of both cosegregation and linkage disequilibrium with causal genetic variants. For SNPs rs11674786 and rs16983422, which were associated with DBP and MAP responses to high-sodium intervention (P ≤ 0.005), the family-based association test (38, 39) jointly detects the presence of both linkage and association as evidenced by the similar significant results (not shown). After adjustment for either rs11674786 or rs16983422 as a covariate in the linkage test, the linkage peak at 33–42 cM of chromosome 2 (2p24.3-2p24.1) presented decreased signals for both (Web Figure 2). The results indicate that the linkage signals were partially attributed to SNPs rs11674786 and rs16983422.
This study would have important clinical and public health implications. All participants lived in the rural area of northern China with a conventional high-salt diet. The dissection of the underlying risk genes for BP responses to dietary salt intake will contribute to the understanding of cardiovascular disease development. The findings could contribute to identifying individuals at high risk for developing hypertension and cardiovascular diseases and will enable the discovery of a new preventive strategy for people living in areas of conventional high dietary-salt intake.
However, this study has 2 potential limitations. First, the linkage scan may not be able to detect chromosomal regions that incubate variants with only modest effect (40). Therefore, the follow-up association analysis will fail to identify variants in these missed genomic regions. Additionally, we did not investigate rare variants for salt sensitivity of BP. Rare variants are considered to be more enriched in the functional region and exhibit stronger effect sizes (39). The linkage region at chromosome 2 contains 50 genes on either the forward (+) or backward (−) strand (Web Table 1). The functions for most of these genes are not known yet. The linkage and the follow-up association studies suggest potential effects from functional rare variants of one or several genes in this region. In the next step, we expect to perform deep resequencing of these candidate genes to identify functional rare variants that influence the salt sensitivity of BP.
In summary, the current study identified chromosomal region 2p24.3-2p24.1 linkage to BP responses to a high-sodium intervention. Furthermore, variants near FAM84A and VSNL1 in the linkage region are associated with BP responses to sodium intervention. The findings are supported by evidence from both linkage and association analyses. Although this study was performed on a Chinese population, the findings can be used to instruct the study of gene functions on different populations. This study provides new evidence of genetic factors that might be partially responsible for BP responses to dietary sodium intake. Replication studies of candidate genes from independent samples of different populations should be conducted to further validate the findings. In addition, experimental studies are warranted to explore the effect of functional variants in FAM84A, VSNL1, and other genes in this genetic region on the salt sensitivity of BP.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Tulane University, New Orleans, Louisiana (Hao Mei, Jing Chen, Tanika N. Kelly, Jiang He); Cardiovascular Institute and Fuwai Hospital, Beijing, China (Dongfeng Gu, Shufeng Chen, Jian-feng Huang); Health Science Center, University of Texas School of Public Health, Houston, Texas (James E. Hixson, Lawrence C. Shimmin); Division of Biostatistics, Washington University, St. Louis, Missouri (Treva K. Rice, Karen Schwander, Dabeeru C. Rao); National Laboratory of Medical Molecular Biology, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China (De-Pei Liu); and Division of Epidemiology and Clinical Applications, National Heart, Lung, and Blood Institute, Bethesda, Maryland (Cashell E. Jaquish).
The Genetic Epidemiology Network of Salt Sensitivity is supported by research grants (U01HL072507, R01HL087263, and R01HL090682) from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland.
Conflict of interest: none declared.
REFERENCES
- 1.Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: clinical and public health advisory from the National High Blood Pressure Education Program. JAMA. 2002;288(15):1882–1888. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 2.Jones DW. Dietary sodium and blood pressure. Hypertension. 2004;43(5):932–935. doi: 10.1161/01.HYP.0000126610.89002.c6. [DOI] [PubMed] [Google Scholar]
- 3.Adrogue HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med. 2007;356(19):1966–1978. doi: 10.1056/NEJMra064486. [DOI] [PubMed] [Google Scholar]
- 4.Kawasaki T, Delea CS, Bartter FC, et al. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med. 1978;64(2):193–198. doi: 10.1016/0002-9343(78)90045-1. [DOI] [PubMed] [Google Scholar]
- 5.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27(3 pt 2):481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 6.Franco V, Oparil S. Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Am Coll Nutr. 2006;25(3 suppl):247S–255S. doi: 10.1080/07315724.2006.10719574. [DOI] [PubMed] [Google Scholar]
- 7.Beeks E, Kessels AG, Kroon AA, et al. Genetic predisposition to salt-sensitivity: a systematic review. J Hypertens. 2004;22(7):1243–1249. doi: 10.1097/01.hjh.0000125443.28861.0d. [DOI] [PubMed] [Google Scholar]
- 8.Giner V, Poch E, Bragulat E, et al. Renin-angiotensin system genetic polymorphisms and salt sensitivity in essential hypertension. Hypertension. 2000;35(1 pt 2):512–517. doi: 10.1161/01.hyp.35.1.512. [DOI] [PubMed] [Google Scholar]
- 9.Poch E, Gonzalez D, Giner V, et al. Molecular basis of salt sensitivity in human hypertension. Evaluation of renin-angiotensin-aldosterone system gene polymorphisms. Hypertension. 2001;38(5):1204–1209. doi: 10.1161/hy1101.099479. [DOI] [PubMed] [Google Scholar]
- 10.Gu D, Rice T, Wang S, et al. Heritability of blood pressure responses to dietary sodium and potassium intake in a Chinese population. Hypertension. 2007;50(1):116–122. doi: 10.1161/HYPERTENSIONAHA.107.088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The GenSalt Collaborative Research Group. GenSalt: rationale, design, methods and baseline characteristics of study participants. J Hum Hypertens. 2007;21(8):639–646. doi: 10.1038/sj.jhh.1002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88(5 pt 1):2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 13.Abecasis GR, Cherny SS, Cookson WO, et al. GRR: graphical representation of relationship errors. Bioinformatics. 2001;17(8):742–743. doi: 10.1093/bioinformatics/17.8.742. [DOI] [PubMed] [Google Scholar]
- 14.Hinds D, Risch M. Fairfax, VA: Geeknet; 1996. The ASPEX package: affected sib-pair exclusion mapping. (http://aspex.sourceforge.net/). (Accessed December 12, 2011) [Google Scholar]
- 15.O'Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet. 1998;63(1):259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pratt SC, Daly MJ, Kruglyak L. Exact multipoint quantitative-trait linkage analysis in pedigrees by variance components. Am J Hum Genet. 2000;66(3):1153–1157. doi: 10.1086/302830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frazer KA, Ballinger DG, Cox DR, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449(7164):851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett JC, Fry B, Maller J, et al. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 19.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emigh TH. A comparison of tests for Hardy-Weinberg equilibrium. Biometrics. 1980;36(4):627–642. [PubMed] [Google Scholar]
- 21.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 22.Yagil C, Sapojnikov M, Kreutz R, et al. Salt susceptibility maps to chromosomes 1 and 17 with sex specificity in the Sabra rat model of hypertension. Hypertension. 1998;31(1):119–124. doi: 10.1161/01.hyp.31.1.119. [DOI] [PubMed] [Google Scholar]
- 23.Cusi D, Barlassina C, Azzani T, et al. Polymorphisms of alpha-adducin and salt sensitivity in patients with essential hypertension. Lancet. 1997;349(9062):1353–1357. doi: 10.1016/S0140-6736(97)01029-5. [DOI] [PubMed] [Google Scholar]
- 24.Svetkey LP, Chen YT, McKeown SP, et al. Preliminary evidence of linkage of salt sensitivity in black Americans at the beta 2-adrenergic receptor locus. Hypertension. 1997;29(4):918–922. doi: 10.1161/01.hyp.29.4.918. [DOI] [PubMed] [Google Scholar]
- 25.Angius A, Petretto E, Maestrale GB, et al. A new essential hypertension susceptibility locus on chromosome 2p24-p25, detected by genome-wide search. Am J Hum Genet. 2002;71(4):893–905. doi: 10.1086/342929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krushkal J, Ferrell R, Mockrin SC, et al. Genome-wide linkage analyses of systolic blood pressure using highly discordant siblings. Circulation. 1999;99(11):1407–1410. doi: 10.1161/01.cir.99.11.1407. [DOI] [PubMed] [Google Scholar]
- 27.Rao DC, Province MA, Leppert MF, et al. A genome-wide affected sibpair linkage analysis of hypertension: the HyperGEN Network. Am J Hypertens. 2003;16(2):148–150. doi: 10.1016/s0895-7061(02)03247-8. [DOI] [PubMed] [Google Scholar]
- 28.Rice T, Rankinen T, Chagnon YC, et al. Genome-wide linkage scan of resting blood pressure: HERITAGE Family Study. Health, Risk Factors, Exercise Training, and Genetics. Hypertension. 2002;39(6):1037–1043. doi: 10.1161/01.hyp.0000018911.46067.6e. [DOI] [PubMed] [Google Scholar]
- 29.Cooper RS, Luke A, Zhu X, et al. Genome scan among Nigerians linking blood pressure to chromosomes 2, 3, and 19. Hypertension. 2002;40(5):629–633. doi: 10.1161/01.hyp.0000035708.02789.39. [DOI] [PubMed] [Google Scholar]
- 30.Atwood LD, Samollow PB, Hixson JE, et al. Genome-wide linkage analysis of blood pressure in Mexican Americans. Genet Epidemiol. 2001;20(3):373–382. doi: 10.1002/gepi.7. [DOI] [PubMed] [Google Scholar]
- 31.Padmanabhan S, Wallace C, Munroe PB, et al. Chromosome 2p shows significant linkage to antihypertensive response in the British Genetics of Hypertension Study. Hypertension. 2006;47(3):603–608. doi: 10.1161/01.HYP.0000197947.62601.9d. [DOI] [PubMed] [Google Scholar]
- 32.Shi G, Gu CC, Kraja AT, et al. Genetic effect on blood pressure is modulated by age: the Hypertension Genetic Epidemiology Network Study. Hypertension. 2009;53(1):35–41. doi: 10.1161/HYPERTENSIONAHA.108.120071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi T, Masaki T, Sugiyama M, et al. A gene encoding a family with sequence similarity 84, member A (FAM84A) enhanced migration of human colon cancer cells. Int J Oncol. 2006;29(2):341–347. [PubMed] [Google Scholar]
- 34.Mahloogi H, Gonzalez-Guerrico AM, Lopez De Cicco R, et al. Overexpression of the calcium sensor visinin-like protein-1 leads to a cAMP-mediated decrease of in vivo and in vitro growth and invasiveness of squamous cell carcinoma cells. Cancer Res. 2003;63(16):4997–5004. [PubMed] [Google Scholar]
- 35.Kamide K, Kokubo Y, Yang J, et al. Hypertension susceptibility genes on chromosome 2p24-p25 in a general Japanese population. J Hypertens. 2005;23(5):955–960. doi: 10.1097/01.hjh.0000166835.70935.3c. [DOI] [PubMed] [Google Scholar]
- 36.Dai FF, Zhang Y, Kang Y, et al. The neuronal Ca2+ sensor protein visinin-like protein-1 is expressed in pancreatic islets and regulates insulin secretion. J Biol Chem. 2006;281(31):21942–21953. doi: 10.1074/jbc.M512924200. [DOI] [PubMed] [Google Scholar]
- 37.Buttgereit J, Qadri F, Monti J, et al. Visinin-like protein 1 regulates natriuretic peptide receptor B in the heart. Regul Pept. 2010;161(1-3):51–57. doi: 10.1016/j.regpep.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 38.Laird NM, Horvath S, Xu X. Implementing a unified approach to family-based tests of association. Genet Epidemiol. 2000;19(suppl 1):S36–S42. doi: 10.1002/1098-2272(2000)19:1+<::AID-GEPI6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 39.Lange C, Laird NM. On a general class of conditional tests for family-based association studies in genetics: the asymptotic distribution, the conditional power, and optimality considerations. Genet Epidemiol. 2002;23(2):165–180. doi: 10.1002/gepi.209. [DOI] [PubMed] [Google Scholar]
- 40.Risch NJ. Searching for genetic determinants in the new millennium. Nature. 2000;405(6788):847–856. doi: 10.1038/35015718. [DOI] [PubMed] [Google Scholar]