Abstract
The association between birth weight and long-term within-individual variability of blood pressure (BP) was examined in a longitudinal cohort of 1,454 adults (939 whites and 515 blacks; adulthood age = 19–50 years) enrolled in the Bogalusa Heart Study in Bogalusa, Louisiana, in 1973–2010. BP variability was depicted as standard deviation, coefficient of variation, and deviation from age-predicted values using 6–15 serial BP measurements from childhood to adulthood over an average of 25.7 years. Birth weight was significantly and negatively associated with adulthood BP levels, long-term BP levels, and rate of change. Importantly, low birth weight was significantly associated with increased BP variability in terms of standard deviation, coefficient of variation, and deviation. As evaluated using the regression coefficients, a 1-kg lower birth weight was associated with increases in systolic BP variability measures (−0.38 mm Hg, P = 0.04 for standard deviation; −0.004 mm Hg, P = 0.01 for coefficient of variation; and −0.16 mm Hg, P = 0.04 for deviation) after adjustment for race, age, sex, mean BP levels, and gestational age; similar trends in the associations were noted for diastolic BP variability measures. In conclusion, these findings suggest that birth weight affects not only BP levels but also the magnitude of within-individual BP fluctuations over time through fetal programming in BP regulation mechanisms.
Keywords: birth weight, black-white, blood pressure variability, childhood
Blood pressure (BP) is a highly variable physiologic trait. In addition to variability in levels among individuals at one time point, the total variation of BP in a population has another important component, variation within the same individual at different time points (within-individual variability). Studies have shown that increased 24-hour (1) and long-term (2–7) within-individual variability of BP is associated with the severity of end-organ damage and a higher rate of cardiovascular events, even after adjustment for BP levels. Importantly, data from the Bogalusa Heart Study showed that long-term BP variability during childhood was associated with adulthood hypertension, and blacks had greater long-term BP variability than did whites (8). Three large-scale clinical trials in Europe have demonstrated that the visit-to-visit BP variability is not random, but rather is significantly reproducible over a long period of follow-up (9). More recently, the importance of long-term BP variability in the risk of stroke was highlighted in multiple articles appearing in the March 13, 2010 issue of The Lancet (2–4), indicating that visit-to-visit variability of clinic systolic BP was more predictive of stroke and coronary events than was the variability measured by ambulatory BP monitoring.
Since the “fetal origins” hypothesis was proposed by Barker et al. (10), epidemiologic studies have documented the association between low birth weight and elevated BP levels and hypertension in adult life (11–15) and shown that the effect size of birth weight on BP increases with age (12–15). However, information is limited on the association between birth weight and BP variability measured over a long period of follow-up. The objective of the present study was to examine the influence of birth weight on BP variability from childhood to young adulthood in black and white subjects enrolled in the Bogalusa Heart Study.
MATERIALS AND METHODS
Study cohort
Between 1973 and 2010, 9 cross-sectional surveys of children 4–17 years of age and 10 cross-sectional surveys of adults 18–52 years of age who had been previously examined as children were conducted in Bogalusa, Louisiana. This panel design of repeated cross-sectional examinations has resulted in serial observations from childhood to adulthood every 2–3 years. By linking these 19 surveys, we have data on 12,164 individuals, with a total of 38,058 observations. Of these individuals, 1,454 adult subjects (939 whites and 515 blacks; 45.2% males; adulthood age range = 19.2–50.1 years; mean age = 35.2 years) who have had BP measurements taken 6–15 times from childhood to adulthood formed the study cohort for this report. The average follow-up period was 25.7 years. Birth weight information (birth weight and gestational age) for the study cohort was obtained from Louisiana state birth certificates.
All subjects in this study gave informed consent at each examination, and for participants who were under 18 years of age, consent of a parent/guardian was obtained. Study protocols were approved by the institutional review board of the Tulane University Health Sciences Center.
BP measurements
Identical protocols have been used by trained examiners across all surveys since 1973 (16). BP levels were measured between 8:00 am and 10:00 am on the right arm of subjects in a relaxed, sitting position by 2 trained observers (3 replicates each). The mean value of the 6 readings was used for analysis. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded using mercury sphygmomanometers. The fourth Korotkoff phase was used for DBP in children and adults because the fourth phase is more reliably measured in childhood and more predictive of hypertension in adults (17). BP values were set as missing for subjects who were taking medications for hypertension at the examinations, and the remaining values were used for variability analysis.
Statistical methods
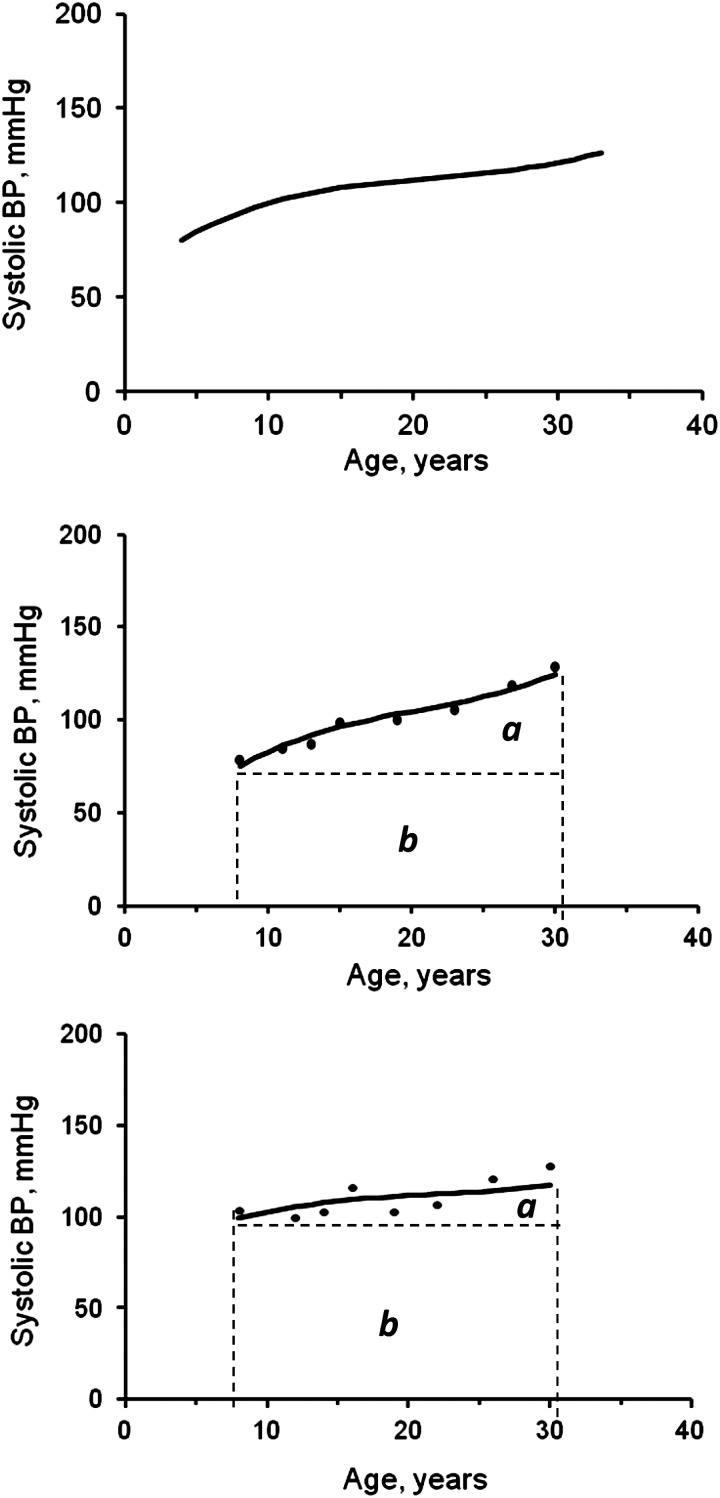
Long-term levels of BP and body mass index (BMI; weight (kg)/height (m)2) were measured as the area under the curve (AUC) calculated using the growth curve of multiple serial BP measurements from childhood to adulthood in a random-effects model using SAS, version 9.2 (SAS Institute Inc., Cary, North Carolina) proc MIXED. As shown in Figure 1, the AUC of SBP was calculated as the integral of the growth curve parameters during the follow-up period for each individual (18, 19). The panel at the top shows the overall growth curve of the entire cohort; the panel in the middle shows the curve and AUC of individual 1; and the panel at the bottom shows the curve and AUC of individual 2. Because individuals had different follow-up periods, the individual's AUC value was divided by the number of follow-up years for further analyses. The AUC measures have advantages over other longitudinal analysis models in that they measure both long-term levels and trends. Total AUC (a + b), where a is incremental AUC and b is baseline AUC, can be considered a measure of long-term levels; incremental AUC represents a combination of linear and nonlinear longitudinal trends.
Figure 1.
The area under the curve (AUC) of systolic blood pressure (BP) and 2 examples (individuals 1 and 2), Bogalusa Heart Study, Bogalusa, Louisiana, 1973–2010. Fixed parameters of the overall growth curve (top panel): SBP = 113 + (0.89 × age) – (0.54 × age2) + (0.08 × age3); random effects incorporated for individual 1 (middle panel): SBP = 100 + (1.7 × age) – (0.63 × age2) + (0.12 × age3); and random effects incorporated for individual 2 (bottom panel): SBP =110 + (0.61 × age) – (0.41 × age2) + (0.06 × age3). a, incremental AUC; b, baseline AUC.
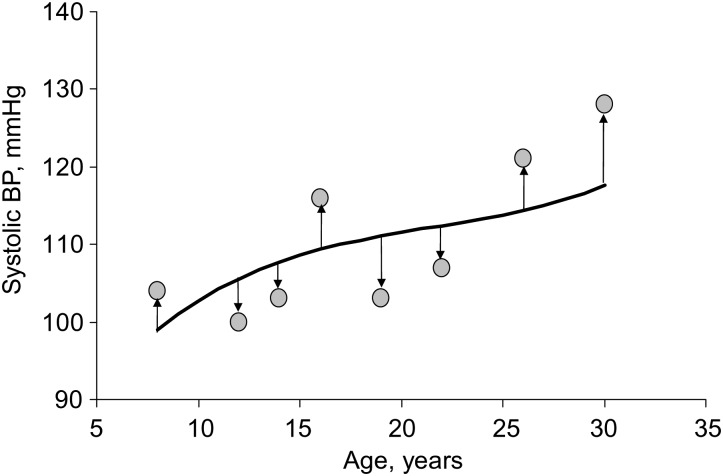
Long-term variability was measured as standard deviation, coefficient of variation (CV), and deviation from age-predicted values (DEV). Standard deviation of multiple serial measurements of BP from childhood to adulthood was calculated for each individual using the conventional standard deviation definition. Standard deviation was the most commonly used index of long-term BP variability in previous studies (2–4, 8, 9). CV was calculated as standard deviation divided by mean, a measure of mean-adjusted standard deviation. The curve as shown in Figure 2 represents a nonlinear trend of increase in BP with age of individual 2. The cubic curve was constructed using multiple BP measurements in a random-effects model, so the data points are supposed to be located below and above the curve. The DEV was then calculated as the mean of distances between the observed data values and the curve (sum of absolute values of deviations divided by the number of values). This measure is similar to the random variability measure of BP used in the Honolulu-Asia Ageing Study and Framingham Heart Study and is shown to be an important predictor of stroke (5) and coronary heart disease (6, 7).
Figure 2.
Deviation from age-predicted values of systolic blood pressure (BP) of individual 2, Bogalusa Heart Study, Bogalusa, Louisiana, 1973–2010.
Analyses of covariance were performed to test differences in study variables between blacks and whites and between men and women. The associations of birth weight with childhood BP (first measurement) and adulthood BP (last measurement), long-term BP levels (total AUC) and trends (incremental AUC), and long-term BP variability measures (standard deviation, CV, and DEV) were examined using separate multivariable linear regression models adjusted for appropriate covariates. For the long-term BP trend (incremental AUC) analyses, the baseline value of BP was included in the model to control for the regression-to-the-mean bias. For BP variability (standard deviation and DEV) analyses, the long-term BP levels (total AUC) were included in the model for adjustment because the BP variability measures are highly correlated with their mean levels (2, 3, 6).
RESULTS
Table 1 shows mean levels (plus/minus standard deviation) of study variables by race and sex. The mean levels of study variables were compared between race and sex groups and were adjusted for age except age itself; gestational age and average age were used for birth weight and long-term measures (AUC and variability), respectively. Blacks had significantly lower birth weights than did whites. Adulthood BP showed significant differences between race and sex groups. The AUC values of SBP and DBP were significantly higher in blacks and men than in whites and women, respectively, except for race difference in DBP for men. Incremental AUC of SBP was significantly higher in blacks and men than in whites and women, respectively; however, incremental AUC of DBP had a significant difference for sex only. All 3 variability measures (standard deviation, CV, and DEV) of both SBP and DBP showed significant sex differences (men > women) except for DEV of DBP. The standard deviation, CV, and DEV of SBP showed significant race differences (blacks > whites) for both men and women, but these variability measures of DBP showed significant race differences (blacks > whites) in women only. Furthermore, significantly positive correlations of the long-term levels (total AUC) with variability for SBP (r = 0.327 with standard deviation, P < 0.001; r = 0.096 with DEV, P < 0.001) were noted in this cohort.
Table 1.
Mean Levels of Study Variables by Race and Sex, Bogalusa Heart Study, Bogalusa, Louisiana, 1973–2010
| Characteristic | Whites, mean (SD) |
Blacks, mean (SD) |
Racial Differencea |
|||
|---|---|---|---|---|---|---|
| Men (n = 440) | Women (n = 499) | Men (n = 217) | Women (n = 298) | Men | Women | |
| Gestational age, weeks | 39.7 (1.7) | 39.7 (1.8) | 39.3 (2.7) | 39.5 (2.0) | 0.064 | 0.262 |
| Birth weight, kg | 3.47 (0.54) | 3.32 (0.53)** | 3.08 (0.61) | 3.04 (0.50) | <0.001 | <0.001 |
| Childhood (first examination) | ||||||
| Age, years | 9.8 (3.1) | 9.7 (3.2) | 9.4 (2.8) | 9.2 (2.8) | 0.130 | 0.038 |
| SBP, mm Hg | 99.9 (9.8) | 99.0 (9.7) | 98.6 (10.8) | 97.3 (9.9)* | 0.966 | 0.391 |
| DBP, mm Hg | 61.3 (8.0) | 61.5 (8.6) | 61.6 (7.9) | 60.3 (8.0)* | 0.099 | 0.995 |
| Adulthood (last examination) | ||||||
| Age, years | 36.5 (8.5) | 36.8 (8.2) | 31.8 (9.5) | 33.3 (8.7) | <0.001 | <0.001 |
| SBP, mm Hg | 117.8 (11.5) | 110.8 (12.0)** | 120.9 (15.5) | 115.3 (14.6)** | <0.001 | <0.001 |
| DBP, mm Hg | 80.0 (9.5) | 75.0 (8.7)** | 79.0 (12.9) | 75.7 (10.7)** | 0.093 | 0.003 |
| AUC measures, mm Hg | ||||||
| Total AUC of SBP | 112.1 (6.9) | 106.9 (6.3)** | 112.7 (8.1) | 109.0 (6.5)** | 0.002 | <0.001 |
| Total AUC of DBP | 71.3 (5.7) | 69.4 (4.7)** | 70.1 (6.3) | 69.7 (5.0)* | 0.365 | <0.001 |
| Incremental AUC of SBP | 12.0 (5.2) | 7.8 (5.0)** | 14.7 (6.5) | 11.6 (5.6)** | <0.001 | <0.001 |
| Incremental AUC of DBP | 10.7 (3.7) | 8.4 (3.6)** | 9.9 (4.5) | 9.3 (3.8)* | 0.387 | 0.056 |
| Variability, mm Hg | ||||||
| SD of SBP | 9.1 (3.4) | 7.7 (3.0)** | 11.1 (4.4) | 9.4 (4.2)** | <0.001 | <0.001 |
| SD of DBP | 9.0 (3.2) | 7.5 (2.7)** | 9.4 (4.0) | 8.5 (3.2)** | 0.060 | <0.001 |
| DEV of SBP | 5.0 (1.6) | 4.6 (1.4)** | 5.9 (1.7) | 5.3 (1.7)** | <0.001 | <0.001 |
| DEV of DBP | 4.5 (1.4) | 4.5 (1.4) | 4.8 (1.5) | 4.9 (1.5) | 0.194 | 0.007 |
| CV of SBP | 0.08 (0.03) | 0.07 (0.03)** | 0.10 (0.04) | 0.09 (0.04)** | <0.001 | <0.001 |
| CV of DBP | 0.13 (0.05) | 0.11 (0.04)** | 0.14 (0.06) | 0.12 (0.05)** | 0.190 | 0.005 |
Abbreviations: AUC, area under the curve; CV, coefficient of variation; DBP, diastolic blood pressure; DEV, deviation from age-predicted values; SBP, systolic blood pressure; SD, standard deviation.
* P < 0.05; **P < 0.01 for sex differences within racial groups.
a The mean levels were compared between race and sex groups, adjusted for age except age itself using analysis of covariance; gestational age and average age were used for birth weight and long-term measures (AUC and variability), respectively.
Table 2 shows associations of birth weight with BP levels, trend, and variability using separate linear regression models by race. In whites, low birth weight was associated with elevated adulthood SBP and DBP and total AUC of both SBP and DBP after adjustment for age, sex, and gestational age; with greater incremental AUC of BP after adjustment for age, sex, gestational age, and baseline values of BP; with increased standard deviation and DEV of BP except DEV of SBP after adjustment for age, sex, gestational age, and total AUC of BP; and with increased CV of SBP and DBP after adjustment for age, sex, and gestational age. With respect to age, the ages at the first and last examinations were included in the models with childhood and adulthood BP levels, respectively; the average age was included in the models with AUC and BP variability. In blacks, birth weight was associated with DEV of SBP only. Of note, all of these association parameters did not differ significantly between blacks and whites as tested using race-birth weight interaction models.
Table 2.
Regression Coefficients of Blood Pressure Levels, Trends, and Variability on Birth Weight by Race, Adjusted for Age, Sex, and Gestational Age, Bogalusa Heart Study, Bogalusa, Louisiana, 1973–2010
| Dependent Variable, mm Hg | Whites |
Blacks |
Race Difference |
||
|---|---|---|---|---|---|
| β | P Value | β | P Value | P Value | |
| Childhood SBP | −0.66 | 0.251 | 0.22 | 0.791 | 0.542 |
| Childhood DBP | 0.27 | 0.578 | −0.21 | 0.749 | 0.354 |
| Adulthood SBP | −2.65 | <0.001 | −0.24 | 0.854 | 0.121 |
| Adulthood DBP | −1.65 | 0.003 | −0.34 | 0.710 | 0.331 |
| Total AUC of SBP | −1.44 | <0.001 | −0.31 | 0.616 | 0.145 |
| Total AUC of DBP | −0.67 | 0.027 | −0.12 | 0.784 | 0.493 |
| Incremental AUC of SBPa | −0.86 | 0.002 | −0.69 | 0.152 | 0.884 |
| Incremental AUC of DBPa | −0.49 | 0.022 | −0.36 | 0.285 | 0.621 |
| SD of SBPb | −0.40 | 0.040 | −0.42 | 0.231 | 0.908 |
| SD of DBPb | −0.60 | 0.002 | −0.31 | 0.314 | 0.394 |
| DEV of SBPb | −0.06 | 0.554 | −0.34 | 0.027 | 0.143 |
| DEV of DBPb | −0.19 | 0.031 | −0.20 | 0.129 | 0.684 |
| CV of SBPc | −0.004 | 0.031 | −0.004 | 0.173 | 0.898 |
| CV of DBPc | −0.009 | 0.003 | −0.004 | 0.327 | 0.336 |
Abbreviations: AUC, area under the curve; CV, coefficient of variation; DBP, diastolic blood pressure; DEV, deviation from age-predicted values; SBP, systolic blood pressure; SD, standard deviation.
a Baseline BP values were included in the model.
b Total AUC was included in the model.
c Total AUC was not included in the model.
As there was no heterogeneity in the association parameters between races, data for black and white individuals were combined. Table 3 presents the regression coefficients in 2 models, one without (model 1) and one with (model 2) BMI included, both of which were adjusted for the same covariates in Table 2 plus race. In model 1, adulthood BP, AUC values, and variability measures showed significant associations with birth weight except total AUC of DBP. In model 2 (which included childhood BMI, adulthood BMI, and total or incremental AUC values of BMI), the adjustment for BMI strengthened the associations for childhood BP, adulthood BP, and total AUC values, whereas it did not substantially affect the regression coefficients for incremental AUC and variability measures.
Table 3.
Regression Coefficients of Blood Pressure Levels, Trends, and Variability on Birth Weight, Adjusted for Race, Age, Sex, and Gestational Age, Bogalusa Heart Study, Bogalusa, Louisiana, 1973–2010
| Dependent Variable, mm Hg | Model I Without BMI Included |
Model II With BMI Included |
||
|---|---|---|---|---|
| β | P Value | β | P Value | |
| Childhood SBP | −0.35 | 0.460 | −1.36 | 0.002 |
| Childhood DBP | 0.08 | 0.830 | −0.45 | 0.232 |
| Adulthood SBP | −1.82 | 0.007 | −2.34 | <0.001 |
| Adulthood DBP | −1.20 | 0.013 | −1.56 | <0.001 |
| Total AUC of SBP | −1.04 | 0.003 | −1.45 | <0.001 |
| Total AUC of DBP | −0.47 | 0.059 | −0.68 | 0.004 |
| Incremental AUC of SBPa | −0.80 | 0.001 | −0.82 | <0.001 |
| Incremental AUC of DBPa | −0.44 | 0.019 | −0.46 | 0.004 |
| SD of SBPb | −0.38 | 0.038 | −0.33 | 0.069 |
| SD of DBPb | −0.48 | 0.004 | −0.54 | 0.001 |
| DEV of SBPb | −0.16 | 0.045 | −0.13 | 0.121 |
| DEV of DBPb | −0.19 | 0.008 | −0.20 | 0.006 |
| CV of SBPc | −0.004 | 0.010 | −0.004 | 0.009 |
| CV of DBPc | −0.007 | 0.004 | −0.008 | 0.002 |
Abbreviations: AUC, area under the curve; BMI, body mass; CV, coefficient of variation; DBP, diastolic blood pressure; DEV, deviation from age-predicted values; SBP, systolic blood pressure; SD, standard deviation.
a Baseline BP values were included in the model.
b Total AUC was included in the model.
c Total AUC was not included in the model.
DISCUSSION
Labile hypertension, which is transient elevation of BP induced by emotion, cold, pain, exercise, or other stimuli, has long been regarded as evidence of a possible prehypertensive state followed by fixed hypertension (20–22). In addition to environmental factors, BP changes over time are also influenced by neurohormonal regulation. The sympathetic nervous system modulates BP variation by affecting cardiac output and peripheral vascular resistance (23, 24). Despite the vast accumulated information on the influencing factors of BP variability, the role of low birth weight in the magnitude of BP variability is not clear. In a previous study of ambulatory 24-hour BP monitoring in children and adolescents, birth weight was found to be inversely correlated with SBP variability measured as standard deviation, independent of the increases in ambulatory BP mean levels (25). In the present study, lower birth weight was associated with increased long-term BP variability from childhood to adulthood measured as both standard deviation and fluctuations around the age-predicted values. In our previous study in the same population (26), we noted that the association between birth weight and age-related trend of BP was dependent on the combination of adrenergic receptor β2 and β3 genotypes, suggesting that the adrenergic receptor gene variants play a role, in an interactive manner, in BP regulation of adults who have a low birth weight. Furthermore, low birth weight was reported to be associated with elevated sympathetic nervous system activity in adulthood as measured by heart rate, pre-ejection period, and respiratory sinus arrhythmia (27, 28). The findings from the present and previous studies suggest that increased sympathetic nervous system activity established in utero may be one of the mechanisms linking birth weight with BP levels, trajectories, and fluctuations during the life course.
The within-individual BP variability over time has been mainly studied in terms of 24 hours of ambulatory monitoring. Visit-to-visit BP variability has also been extensively investigated using serial measurements over a long follow-up period (2–9). It is well known that the magnitude of both long-term (6) and short-term (29, 30) BP variability is highly correlated with their mean levels. We also found significantly positive correlations between the long-term levels (total AUC) and variability measures of SBP in this study cohort. The observed significant correlations indicated the need for adjustment of the mean BP levels in BP variability analysis. There are 2 commonly used approaches for this purpose. One is adjustment of the variability index for mean levels before the association analysis like CV, calculated as standard deviation divided mean (31). The other approach is including the mean BP levels in the model (8, 9, 25, 26). In the present study, we used both approaches. After adjustment for the long-term BP levels (total AUC), low birth weight was still significantly associated with higher BP variability measures.
In addition to BP variability measures, low birth weight was significantly associated with elevated adulthood BP levels (the last measurement) and a greater rate of change (incremental AUC) in the current study. However, birth weight was not associated with childhood BP levels (the first measurement) when BMI was not included in the model (model 1 in Table 2). A huge number of studies have shown extensive and almost consistent evidence for an association between low birth weight and elevated BP or hypertension in adults. The results in children are very conflicting. In a prospective study, birth weight was not predictive of BP levels in 1,417 Australian children followed to the age of 8 years (32). However, in a different study, birth weight was found to be significantly associated with both SBP and DBP in 1,860 children at 3 years of age (33). Importantly, epidemiologic studies and systematic reviews have provided evidence that the effect of birth weight on BP increases with age in adults (14, 15) and even from childhood (12, 13, 33). The age-amplification hypothesis suggests that low birth weight might have a synergistic influence on BP with a large number of environmental factors that are accumulative during lifetime. On the other hand, one of the concerns in this regard is that the age-related trend of the association may be largely resulted from the adjustment for current body size (12, 34–37).
A substantive challenge to the birth weight-BP association has been made. The major criticism is overestimation of the birth weight-BP association by the statistical adjustment for current body size in testing this hypothesis (12, 14, 35–37). In a meta-analysis of birth weight-BP association studies (35), the regression coefficients were adjusted for current body weight in almost all of the studies, and removal of this adjustment reduced the regression coefficients from −1.5 mm Hg/kg to −0.4 mm Hg/kg. In our previous analysis of 6,251 children and adults (12), the adjustment for current BMI inflated the regression coefficients more in children than in adults. In the present study, we examined the birth weight-BP relation in 2 regression analysis models, with and without BMI adjustment. The adjustment for BMI substantially strengthened the birth weight-BP associations for childhood and adulthood BP and long-term levels measured as total AUC, particularly for the childhood BP, whereas it did not affect the effect size of birth weight on variability measures and long-term trends measured as incremental AUC. For this reason, we discussed our results of the regression models without BMI included, even though it did not affect the birth weight-BP variability association parameters.
It is well known that blacks have higher BP levels and prevalence of hypertension than do whites (38). The racial difference in BP was observed even in children in the Bogalusa population (39). In the present study, blacks also had significantly higher long-term BP variability from childhood to adulthood in terms of standard deviation, CV, and DEV for both SBP and DBP (P ≤ 0.001–0.004 for race difference). In our previous study (8), serial childhood BP measurements also showed significantly greater variability in blacks than in whites. Of particular interest, our previous studies of orthostatic changes, cold pressure responses, and effect of hand grip isometric changes indicated a greater response of BP in blacks, especially in black men (40, 41). In addition, studies from the Bogalusa population showed that status of sympathetic activity, renin-angiotensin system, and electrolytes balance had more influence for black subjects (42, 43). All of the above mechanisms may be responsible for the significant racial difference in the long-term BP variability observed in this study. With respect to the associations of birth weight with various BP measures, the strength of the associations did not differ significantly between blacks and whites, although black subjects had lower values of the association parameters, especially for adulthood BP and total AUC, as shown in Table 2. The findings on racial contrasts from the present study need to be confirmed and replicated in a larger sample from other population studies.
In summary, we examined various aspects of BP, including childhood BP, adulthood BP, increasing trends, and fluctuations, in relation to birth weight in a population that included both blacks and whites. The most striking finding was that low birth weight was significantly associated not only with BP levels but also with its long-term variability from childhood to adulthood. The long-term variability (fluctuations) in BP represents an overall indicator of response to the intrinsic physiologic and metabolic changes and the cumulative burden of lifestyles, nutrition, and other environmental stimuli. Results from the present study suggest a joint influence of birth weight and environment factors on BP regulation during the lifetime. Investigation of BP variability as a new phenotype is a promising research area and may yield important information about the etiology and pathogenesis of hypertension and related disorders, and thus its prevention.
ACKNOWLEDGMENTS
Author affiliation: Tulane Center for Cardiovascular Health, Department of Epidemiology, Tulane University, New Orleans, Louisiana (Wei Chen, Sathanur R. Srinivasan, Lu Yao, Shengxu Li, Pronabesh Dasmahapatra, Camilo Fernandez, Jihua Xu, Gerald S. Berenson).
This study was supported by grants ES-021724 from National Institute of Environmental Health Science, HD-061437 and HD-062783 from the National Institute of Child Health and Human Development, and AG-16592 from the National Institute on Aging.
Conflict of interest: none declared.
REFERENCES
- 1.Mancia G, Parati G. The role of blood pressure variability in end-organ damage. J Hypertens Suppl. 2003;21(6):S17–S23. doi: 10.1097/00004872-200307006-00004. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375(9718):938–948. doi: 10.1016/S0140-6736(10)60309-1. [DOI] [PubMed] [Google Scholar]
- 3.Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375(9718):895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 4.Webb AJ, Fischer U, Mehta Z, et al. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375(9718):906–915. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
- 5.Havlik RJ, Foley DJ, Sayer B, et al. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: the Honolulu-Asia Aging study. Stroke. 2002;33(1):26–30. doi: 10.1161/hs0102.101890. [DOI] [PubMed] [Google Scholar]
- 6.Grove JS, Reed DM, Yano K, et al. Variability in systolic blood pressure—a risk factor for coronary heart disease? Am J Epidemiol. 1997;145(9):771–776. doi: 10.1093/oxfordjournals.aje.a009169. [DOI] [PubMed] [Google Scholar]
- 7.Hathaway DK, D'Agostino RB. A technique for summarizing longitudinal data. Stat Med. 1993;12(23):2169–2178. doi: 10.1002/sim.4780122303. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Srinivasan SR, Ruan L, et al. Adult hypertension is associated with blood pressure variability beginning in childhood in blacks and whites: the Bogalusa Heart Study. Am J Hypertens. 2010;24(1):77–82. doi: 10.1038/ajh.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard SC, Rothwell PM. Reproducibility of measures of visit-to-visit variability in blood pressure after transient ischaemic attack or minor stroke. Cerebrovasc Dis. 2009;28(4):331–340. doi: 10.1159/000229551. [DOI] [PubMed] [Google Scholar]
- 10.Barker DJP, Osmond C, Golding J, et al. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298(6673):564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leon DA, Johansson M, Rasmussen F. Gestational age and growth rate of fetal mass are inversely associated with systolic blood pressure in young adults: an epidemiologic study of 165,136 Swedish men aged 18 years. Am J Epidemiol. 2000;152(7):597–604. doi: 10.1093/aje/152.7.597. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Srinivasan SR, Berenson GS. Amplification of the association between birth weight and blood pressure with age: the Bogalusa Heart Study. J Hypertens. 2010;28(10):2046–2052. doi: 10.1097/HJH.0b013e32833cd31f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Law CM, de Swiet M, Osmond C, et al. Initiation of hypertension in utero and its amplification throughout life. BMJ. 1993;306(6869):24–27. doi: 10.1136/bmj.306.6869.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamborg M, Byberg L, Rasmussen F, et al. Birth weight and systolic blood pressure in adolescence and adulthood: meta-regression analysis of sex- and age-specific results from 20 Nordic studies. Am J Epidemiol. 2007;166(6):634–645. doi: 10.1093/aje/kwm042. [DOI] [PubMed] [Google Scholar]
- 15.Davies AA, Smith GD, May MT, et al. Association between birth weight and blood pressure is robust, amplifies with age, and may be underestimated. Hypertension. 2006;48(3):431–436. doi: 10.1161/01.HYP.0000236551.00191.61. [DOI] [PubMed] [Google Scholar]
- 16.Berenson GS. Cardiovascular Risk Factors in Children—The Early Natural History of Atherosclerosis and Essential Hypertension. Oxford, UK: Oxford University Press; 1980. [Google Scholar]
- 17.Elkasabany AM, Urbina EM, Daniels SR, et al. Prediction of adult blood pressure by K4 and K5 diastolic blood pressure in children: the Bogalusa Heart Study. J Pediatr. 1998;132(4):687–692. doi: 10.1016/s0022-3476(98)70361-0. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Li S, Srinivasan SR, et al. Autosomal genome scan for loci linked to blood pressure levels and trends since childhood: the Bogalusa Heart Study. Hypertension. 2005;45(5):954–959. doi: 10.1161/01.HYP.0000161881.02361.11. [DOI] [PubMed] [Google Scholar]
- 19.Cook NR, Rosner BA, Chen W, et al. Using area under the curve to predict adult blood pressure from childhood measures in the Bogalusa Heart Study. Stat Med. 2004;23(22):3421–3435. doi: 10.1002/sim.1921. [DOI] [PubMed] [Google Scholar]
- 20.Levy RI, Stroud WD, White PD. Transient hypertension: its significance in terms of later development of sustained hypertension cardiovascular-renal diseases. JAMA. 1944;126(3):829–833. [Google Scholar]
- 21.Levy RI, White PD, Stroud WD. Transient hypertension: the relative prognostic importance of various systolic diastolic levels. JAMA. 1945;128(5):1059–1061. [Google Scholar]
- 22.Hines EA., Jr. Range of normal blood pressure and development of hypertension: a follow-up study of 1,522 patients. JAMA. 1940;115(2):271–273. [Google Scholar]
- 23.Julius S, Esler M. Autonomic nervous cardiovascular regulation in borderline hypertension. Am J Cardiol. 1975;36(5):685–696. doi: 10.1016/0002-9149(75)90170-8. [DOI] [PubMed] [Google Scholar]
- 24.Grassi G, Bombelli M, Seravalle G, et al. Diurnal blood pressure variation and sympathetic activity. Hypertens Res. 2010;33(5):381–385. doi: 10.1038/hr.2010.26. [DOI] [PubMed] [Google Scholar]
- 25.Lurbe E, Torro I, Rodríguez C, et al. Birth weight influences blood pressure values and variability in children and adolescents. Hypertension. 2001;38(3):389–393. doi: 10.1161/01.hyp.38.3.389. [DOI] [PubMed] [Google Scholar]
- 26.Chen W, Srinivasan SR, Hallman DM, et al. The relationship between birth weight and longitudinal changes of blood pressure is modulated by beta-adrenergic receptor genes: the Bogalusa Heart Study. J Biomed Biotechnol. 2010;2010:543514. doi: 10.1155/2010/543514. ( doi:10.1155/2010/543514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips DI, Barker DJ. Association between low birth weight and high resting pulse in adult life: is the sympathetic nervous system involved in programming the insulin resistance syndrome? Diabet Med. 1997;14(8):673–677. doi: 10.1002/(SICI)1096-9136(199708)14:8<673::AID-DIA458>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 28.IJzerman RG, Stehouwer CD, de Geus EJ, et al. Low birth weight is associated with increased sympathetic activity: dependence on genetic factors. Circulation. 2003;108(5):566–571. doi: 10.1161/01.CIR.0000081778.35370.1B. [DOI] [PubMed] [Google Scholar]
- 29.Watson RD, Stallard TJ, Flinn RM, et al. Factors determining direct arterial pressure and its variability in hypertensive man. Hypertension. 1980;2(3):333–341. doi: 10.1161/01.hyp.2.3.333. [DOI] [PubMed] [Google Scholar]
- 30.Berenson GS, Dalfres E, Jr, Savage D, et al. Ambulatory blood pressure measurements in children and young adults selected by high and low casual blood pressure levels and parental history of hypertension: the Bogalusa Heart Study. Am J Med Sci. 1993;305(6):374–382. doi: 10.1097/00000441-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Hata Y, Muratani H, Kimura Y, et al. Office blood pressure variability as a predictor of acute myocardial infarction in elderly patients receiving antihypertensive therapy. J Hum Hypertens. 2002;16(2):141–146. doi: 10.1038/sj.jhh.1001301. [DOI] [PubMed] [Google Scholar]
- 32.Burke V, Beilin LJ, Blake KV, et al. Indicators of fetal growth do not independently predict blood pressure in 8-year-old Australians: a prospective cohort study. Hypertension. 2004;43(2):208–213. doi: 10.1161/01.HYP.0000113296.77924.28. [DOI] [PubMed] [Google Scholar]
- 33.Whincup PH, Bredow M, Payne F, et al. Size at birth and blood pressure at 3 years of age. The Avon Longitudinal Study of Pregnancy and Childhood (ALSPAC) Am J Epidemiol. 1999;149(8):730–739. doi: 10.1093/oxfordjournals.aje.a009882. [DOI] [PubMed] [Google Scholar]
- 34.Hardy R, Sovio U, King VJ, et al. Birth weight and blood pressure in five European birth cohort studies: an investigation of confounding factors. Eur J Public Health. 2006;16(1):21–30. doi: 10.1093/eurpub/cki171. [DOI] [PubMed] [Google Scholar]
- 35.Huxley R, Neil A, Collins R. Unravelling the fetal origins hypothesis: is there really an inverse association between birth weight and subsequent blood pressure? Lancet. 2002;360(9334):659–665. doi: 10.1016/S0140-6736(02)09834-3. [DOI] [PubMed] [Google Scholar]
- 36.Hardy R, Kuh D, Langenberg C, et al. Birthweight, childhood social class change in adult blood pressure in the 1946 British birth cohort. Lancet. 2003;362(9391):1178–1183. doi: 10.1016/S0140-6736(03)14539-4. [DOI] [PubMed] [Google Scholar]
- 37.Tu YK, West R, Ellison GT, et al. Why evidence for the fetal origins of adult disease might be a statistical artifact: the “reversal paradox” for the relation between birth weight and blood pressure in later life. Am J Epidemiol. 2005;161(1):27–32. doi: 10.1093/aje/kwi002. [DOI] [PubMed] [Google Scholar]
- 38.Cornoni-Huntley J, LaCroix AZ, Havlik RJ. Race, sex differentials in the impact of hypertension in the United States. The National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Intern Med. 1989;149(4):780–788. [PubMed] [Google Scholar]
- 39.Voors AW, Berenson GS, Dalferes ER, Jr, et al. Racial differences in blood pressure control. Science. 1979;204(4397):1091–1094. doi: 10.1126/science.451554. [DOI] [PubMed] [Google Scholar]
- 40.Voors AW, Webber LS, Berenson GS. Racial contrasts in cardiovascular response tests for children from a total community. Hypertension. 1980;2(5):686–694. doi: 10.1161/01.hyp.2.5.686. [DOI] [PubMed] [Google Scholar]
- 41.Parker FC, Croft JB, Cresanta JL, et al. The association between cardiovascular response tasks and future blood pressure levels in children: Bogalusa Heart Study. Am Heart J. 1987;113(5):1174–1179. doi: 10.1016/0002-8703(87)90931-8. [DOI] [PubMed] [Google Scholar]
- 42.Victor RG, Leimbach WN, Jr, Seals DR, et al. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension. 1987;9(5):429–436. doi: 10.1161/01.hyp.9.5.429. [DOI] [PubMed] [Google Scholar]
- 43.Berenson GS, Voors AW, Webber LS, et al. Racial differences of parameters associated with blood pressure levels in children-the Bogalusa Heart Study. Metabolism. 1979;28(12):1218–1228. doi: 10.1016/0026-0495(79)90134-3. [DOI] [PubMed] [Google Scholar]