Abstract
To clarify the role of previous lung diseases (chronic bronchitis, emphysema, pneumonia, and tuberculosis) in the development of lung cancer, the authors conducted a pooled analysis of studies in the International Lung Cancer Consortium. Seventeen studies including 24,607 cases and 81,829 controls (noncases), mainly conducted in Europe and North America, were included (1984–2011). Using self-reported data on previous diagnoses of lung diseases, the authors derived study-specific effect estimates by means of logistic regression models or Cox proportional hazards models adjusted for age, sex, and cumulative tobacco smoking. Estimates were pooled using random-effects models. Analyses stratified by smoking status and histology were also conducted. A history of emphysema conferred a 2.44-fold increased risk of lung cancer (95% confidence interval (CI): 1.64, 3.62 (16 studies)). A history of chronic bronchitis conferred a relative risk of 1.47 (95% CI: 1.29, 1.68 (13 studies)). Tuberculosis (relative risk = 1.48, 95% CI: 1.17, 1.87 (16 studies)) and pneumonia (relative risk = 1.57, 95% CI: 1.22, 2.01 (12 studies)) were also associated with lung cancer risk. Among never smokers, elevated risks were observed for emphysema, pneumonia, and tuberculosis. These results suggest that previous lung diseases influence lung cancer risk independently of tobacco use and that these diseases are important for assessing individual risk.
Keywords: bronchitis, chronic, emphysema, lung diseases, lung neoplasms, meta-analysis, pneumonia, pulmonary disease, chronic obstructive, tuberculosis
Lung cancer continues to be the leading cause of cancer incidence and mortality worldwide, with an estimated 1,608,800 new cases and 1,378,400 deaths in 2008 (1). Disease survival remains dismal, with 5-year survival rates of approximately 15% among developed populations (2, 3). Although tobacco smoking continues to be the primary determinant of risk, further investigation is required concerning the additional risk factors for lung cancer, particularly among never smokers (4). One particular set of risk factors that may play an important role in lung cancer development is previous lung diseases. Recent evidence suggests that inflammatory processes may play a central role in carcinogenesis (5–8).
Previous lung diseases such as chronic obstructive pulmonary disease (COPD) (including emphysema and chronic bronchitis), pneumonia, and tuberculosis are major sources of inflammation in lung tissue (9, 10). The resulting inflammation has been suggested to increase the risk of lung cancer (11–13), and these diseases may act as catalysts in the development of lung neoplasms (14, 15). The associations between COPD (emphysema and/or chronic bronchitis), pneumonia, and tuberculosis and lung cancer have been investigated previously; however, a recent meta-analysis of the literature showed that most of the studies were small-scale initiatives—65% were identified as having fewer than 500 cases (16). In addition, the meta-analysis was not able to address the issues of standardized covariate adjustment, and data on never smokers and histologic type were limited. To address these limitations, we conducted a pooled analysis using primary data from 17 studies included in the International Lung Cancer Consortium to examine the risk of lung cancer associated with previous lung diseases.
MATERIALS AND METHODS
Data collection
Requirements for inclusion of studies in the International Lung Cancer Consortium and other details have been previously published (17) and are available on the Consortium's website (http://ilcco.iarc.fr). Investigators from 17 participating studies (out of 52 studies included in the Consortium) contributed data on previous lung diseases and agreed to participate in this pooled analysis (Table 1). There was 1 population-based cohort study and 16 case-control studies, of which 9 were population-based, 4 were hospital-based, and 3 had mixed controls (where both population and hospital-based controls were sampled). Eleven studies were conducted in North America, 5 in Europe, and 1 in China; the dates of the studies ranged from 1984 to 2011. The control groups in all of the case-control studies were, at a minimum, frequency-matched with cases on age and sex. Written informed consent was obtained from all study subjects, and ethics review boards at each study center approved the study protocols. The data submitted from all 17 studies were checked for missing values, inadmissible values, aberrant distributions, and inconsistencies. Queries were sent to the investigators to resolve all discrepancies and possible errors. Subjects with unknown age or sex were excluded from the analysis (n = 6). A total of 24,607 cases and 81,829 controls were available for the present investigation.
Table 1.
Characteristics of Participating Studies in a Pooled Analysis of Previous Lung Diseases and Lung Cancer Risk, International Lung Cancer Consortium, 1984–2011
| Continent and Study/Center | Principal Investigator | Control Source | Study Period | Location | No. of Cases | No. of Controls | Total No. |
|---|---|---|---|---|---|---|---|
| North America | |||||||
| Family Health Study (WSU/KCI-1) (22) | A. G. Schwartz | Population | 1984–2004 | Detroit, Michigan, US | 1,006 | 1,184 | 2,190 |
| Study of women's lung cancer epidemiology (WSU/KCI-2) (30) | A. G. Schwartz | Population | 2001–2007 | Detroit, Michigan, US | 576 | 575 | 1,151 |
| University of California, Los Angeles (21) | Z. F. Zhang | Population | 1999–2004 | Los Angeles, California, US | 611 | 1,040 | 1,651 |
| New England Lung Cancer Study (25) | E. Duell | Population | 2005–2008 | New Hampshire, US | 277 | 251 | 528 |
| Samuel Lunenfeld Research Institute (18) | J. McLaughlin | Mixed | 1997–2002 | Toronto, Ontario, Canada | 445 | 947 | 1,392 |
| Mayo Clinic (27) | P. Yang | Mixed | 1997–2006 | Rochester, Minnesota, US | 5,700 | 2,269 | 7,969 |
| New York Multicenter Study (26) | J. Muscat | Hospital | 1969–1999 | New York State, US | 5,130 | 4,942 | 10,072 |
| Moffitt Cancer Study (24) | P. Lazarus | Hospital | 1999–2003 | Florida, US | 497 | 898 | 1,395 |
| University of California, San Francisco (29) | J. Wiencke | Population | 1999–2002 | San Francisco, California, US | 428 | 900 | 1,328 |
| Memorial Sloan-Kettering Cancer Center (33) | I. Orlow | Hospital | 2003–2005 | New York City, US | 102 | 101 | 203 |
| Hawaii (28) | L. Le Marchand | Population | 1992–1997 | Hawaii, US | 635 | 588 | 1,223 |
| Europe | |||||||
| Liverpool Lung Project (35) | J. K. Field | Population | 1998–2006 | Liverpool, United Kingdom | 475 | 954 | 1,429 |
| CREST Biorepository (19) | M. Neri | Mixed | 1996–ongoing | Genova, Italy | 413 | 555 | 968 |
| Helmholtz Center Munich (39, 40, 69, 70) | E. Wichmann | Population | 2000–2004 | Germany | 4,735 | 8,178 | 12,913 |
| Central Europe (23) | P. Boffetta | Hospital | 1998–2002 | Central/Eastern Europe | 2,633 | 2,702 | 5,335 |
| Danish Diet, Cancer, and Health Studya (20) | A. Tjønneland | Population-based cohort | 1993–2009 | Copenhagen, Denmark | 822 | 55,623 | 56,445 |
| Asia | |||||||
| NCI-China (34) | Q. Lan | Population | 1985–1990 | Xuan Wei, People's Republic of China | 122 | 122 | 244 |
| Total | 24,607 | 81,829 | 106,436 |
Abbreviations: CREST, Cancer of the Respiratory Tract; KCI, Karmanos Cancer Institute; NCI, National Cancer Institute; US, United States; WSU, Wayne State University.
a Population-based cohort included in counts as cases and controls.
Previous lung diseases were based on self-reported status of being previously diagnosed with chronic bronchitis, emphysema, pneumonia, or tuberculosis by a physician. Two of the studies asked open-ended questions about previous lung diseases, where responses were recorded using free text (18) or were coded using International Classification of Diseases, Ninth Revision, codes (19). Dichotomous variables were created for each of the previous lung diseases. Several studies also recorded the date of diagnosis of the disease (18, 20–28). Detailed descriptions of the 17 study populations within this analysis have been published elsewhere (18–34). Four of the studies had previously reported effect estimates for prior lung diseases (18, 25, 30, 35) and were included in the previous meta-analysis (16), whereas the other 13 studies (88% of the pooled study population) represented new data and were not included in the previous meta-analysis (Table 1).
Statistical methods
The frequency distributions of demographic variables and putative risk factors for lung cancer, including age, sex, ethnicity, and smoking, were examined among cases and controls combined. The ethnicity of the subjects was categorized according to the National Institutes of Health definition as non-Hispanic white, black or African-American, Hispanic or Latino, Asian, Native Hawaiian or Pacific Islander, American Indian, or other. Former smokers were defined as smokers who had quit smoking at least 2 years before the interview or diagnosis. Never smokers were defined as persons who had smoked fewer than 100 cigarettes over their lifetime. Cumulative tobacco smoking was calculated as the product of smoking duration and intensity throughout the life course, standardized across studies and expressed as pack-years.
For those studies that recorded the date of lung disease diagnosis, indicator variables for whether the diagnosis had been made 5 years or 10 years before the date of cancer diagnosis or control interview were created. For case-control studies, we estimated odds ratios and their associated 95% confidence intervals for the relation of each previous lung disease with lung cancer, using unconditional logistic regression, adjusting for age, sex, cumulative tobacco smoking (in pack-years), and country (when the study participants were from multiple countries). For the cohort study (20), we used Cox regression (with time since study entry as the time scale) to estimate hazard ratios, adjusted for age, sex, and pack-years, and their associated 95% confidence intervals for each previous lung disease. Follow-up time at risk was calculated as the time between study entry and lung cancer diagnosis (for cases) or the last known date of query (for noncases) from the cancer registry. Although we estimated hazard ratios or odds ratios across study sites, we refer to all effect estimates henceforth as relative risks for consistency.
When information on cumulative tobacco smoking was missing (<1%), it was imputed using the median of the study-specific control population for the smoking group (never/former/current) of the individual. We estimated pooled effects across studies, employing random-effects models to account for variability between study populations. Studies in which the odds ratio could not be estimated because of small numbers in one or more of the 4 categories in the 2 × 2 table of case-control status and history of previous lung diseases were omitted. We conducted an analysis stratified by smoking status to investigate the potential modifying effects of smoking or differential etiology across smoking groups. We also compared effect estimates across histologic subtypes to search for differential effects. We adjusted estimates for other lung diseases; however, since not all studies collected data for all diseases, this limited the sample in which we could conduct such an analysis. Subgroup analyses for large cell carcinoma are omitted from the results because there were very small numbers of cases in most studies and risk measures could not be estimated across studies unless data were pooled as a single study. We estimated population attributable fractions for each of the previous lung diseases based on the pooled adjusted effect estimates and the proportion of exposed persons among the cases (36).
Heterogeneity was evaluated for each of the summary estimates based on a test of the Cochrane Q statistic as well as the I2 statistic (37). Where there was evidence of heterogeneity across studies, we evaluated the source of heterogeneity by means of meta-regression using control type, prevalence of ever smoking among controls, median year of the study period, and continents as predictors. If the heterogeneity could not be accounted for by the different study characteristics, we conducted an influence analysis to evaluate the source of heterogeneity from single studies using Galbraith plots (38) and Q statistics through an iterative process. Statistical analyses were conducted using SAS, version 9.1 (SAS Institute Inc., Cary, North Carolina), and STATA, version 10 (StataCorp LP, College Station, Texas).
RESULTS
The demographic distribution of the pooled data set for previous lung diseases is displayed in Table 2. The majority of cases were Caucasian, male, and over the age of 60 years. As expected, there was a much higher proportion of never smokers among the controls. Adenocarcinoma and squamous cell carcinoma were the most commonly characterized histologic subtypes among cases in the pooled population. The prevalences of the 4 lung diseases examined among cases and controls across studies/centers, smoking groups, and histology groups are shown in Table 3.
Table 2.
Demographic Characteristics of Participants in a Pooled Analysis of Previous Lung Diseases and Lung Cancer Risk, International Lung Cancer Consortium, 1984–2011
| Cases (n = 24,607) |
Controls (Noncases) (n = 81,829) |
|||||
|---|---|---|---|---|---|---|
| No. | % | Mean (SD) | No. | % | Mean (SD) | |
| Age at diagnosisa, years | 61.1 (10.9) | 56.4 (8.1) | ||||
| Age group, years | ||||||
| <50 | 4,434 | 18.0 | 12,135 | 14.8 | ||
| 51–60 | 6,713 | 27.3 | 46,522 | 56.9 | ||
| 61–70 | 8,392 | 34.1 | 19,156 | 23.4 | ||
| >70 | 5,068 | 20.6 | 4,016 | 4.9 | ||
| Sex | ||||||
| Male | 15,394 | 62.6 | 41,964 | 51.3 | ||
| Female | 9,213 | 37.4 | 39,865 | 48.7 | ||
| Ethnicity | ||||||
| White/Caucasian | 21,030 | 85.5 | 74,890 | 91.5 | ||
| Black/African-American | 1,379 | 5.6 | 1,698 | 2.1 | ||
| Asian | 561 | 2.3 | 574 | 0.7 | ||
| Hispanic/Latino | 313 | 1.3 | 629 | 0.8 | ||
| Other/unknown | 1,324 | 5.4 | 4,038 | 4.9 | ||
| Smoking status | ||||||
| Never smoker | 2,719 | 11.0 | 29,884 | 36.5 | ||
| Ever smoker | 21,888 | 88.9 | 51,945 | 63.5 | ||
| Former smoker | 13,113 | 53.3 | 27,022 | 52.0 | ||
| Current smoker | 8,775 | 35.7 | 24,923 | 48.0 | ||
| Pack-years of smokingb | 44.1 (28.0) | 28 (16.8) | ||||
| <15 | 5,191 | 21.1 | 41,719 | 51.0 | ||
| 15–<30 | 4,383 | 17.8 | 13,477 | 16.5 | ||
| 30–45 | 6,179 | 25.1 | 21,168 | 25.9 | ||
| >45 | 8,854 | 36.0 | 5,465 | 6.7 | ||
| Histologic typec | ||||||
| Adenocarcinoma | 6,684 | 27.1 | ||||
| Squamous cell carcinoma | 4,685 | 19.0 | ||||
| Small cell lung cancer | 1,810 | 7.4 | ||||
| Large cell lung cancer | 824 | 3.3 | ||||
Abbreviation: SD, standard deviation.
a Age at baseline in the cohort study.
b Among ever smokers only.
c The remaining cases had either mixed or other histologic types.
Table 3.
Prevalence of Previous Lung Disease Among Lung Cancer Cases and Controls, by Study/Center, Smoking Status, and Histologic Type, International Lung Cancer Consortium, 1984–2011
| Emphysema |
Chronic Bronchitis |
Tuberculosis |
Pneumonia |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cases |
No. of Controls |
No. of Cases |
No. of Controls |
No. of Cases |
No. of Controls |
No. of Cases |
No. of Controls |
|||||||||
| Exp. | Unexp. | Exp. | Unexp. | Exp. | Unexp. | Exp. | Unexp. | Exp. | Unexp. | Exp. | Unexp. | Exp. | Unexp. | Exp. | Unexp. | |
| Study/center | ||||||||||||||||
| UCLA (21) | 71 | 540 | 7 | 1,033 | 71 | 540 | 59 | 981 | 28 | 583 | 25 | 1,015 | 213 | 398 | 197 | 843 |
| Helmholtz Center Munich (39, 40, 69, 70) | 125 | 4,564 | 88 | 4,528 | 929 | 3,759 | 665 | 6,263 | 192 | 4,505 | 204 | 4,399 | 1,098 | 3,557 | 781 | 3,808 |
| Central Europe (23) | 75 | 2,552 | 47 | 2,652 | 207 | 2,420 | 150 | 2,550 | 900 | 1,727 | 689 | 2,009 | ||||
| NCI-China (34) | 10 | 111 | 2 | 119 | 38 | 84 | 34 | 87 | 12 | 110 | 1 | 119 | ||||
| Family Health Study (WSU/KCI-1) (22) | 30 | 967 | 18 | 1,164 | 29 | 388 | 28 | 441 | 24 | 975 | 12 | 1,171 | 174 | 819 | 171 | 1,011 |
| Study of women's lung cancer epidemiology (WSU/KCI-2) (30) | 87 | 488 | 12 | 560 | 123 | 450 | 65 | 507 | 19 | 551 | 16 | 555 | 207 | 365 | 187 | 384 |
| Hawaii (28) | 105 | 525 | 20 | 568 | 36 | 593 | 15 | 573 | 26 | 605 | 28 | 560 | ||||
| Samuel Lunenfeld Research Institute (18) | 31 | 270 | 8 | 436 | 21 | 424 | 49 | 898 | 6 | 439 | 5 | 942 | 11 | 434 | 35 | 912 |
| Liverpool Lung Project (35) | 6 | 317 | 31 | 875 | 9 | 314 | 40 | 865 | 41 | 282 | 138 | 768 | ||||
| Mayo Clinic (27) | 1,167 | 4,533 | 36 | 2,233 | 63 | 5,637 | 18 | 2,251 | 897 | 4,803 | 123 | 2,146 | ||||
| New England Lung Cancer Study (25) | 46 | 228 | 10 | 241 | 41 | 235 | 13 | 238 | 3 | 272 | 2 | 249 | 123 | 152 | 73 | 178 |
| Moffitt Cancer Study (24) | 67 | 428 | 29 | 861 | 55 | 440 | 36 | 853 | ||||||||
| New York Multicenter Study (26) | 299 | 4,831 | 82 | 4,860 | 527 | 4,603 | 201 | 4,741 | 50 | 5,080 | 29 | 4,913 | ||||
| CREST Biorepository (19) | 10 | 403 | 4 | 551 | 77 | 336 | 14 | 541 | 7 | 406 | 3 | 552 | 21 | 392 | 14 | 541 |
| UCSF (29) | 77 | 349 | 45 | 853 | 20 | 407 | 19 | 881 | 168 | 258 | 167 | 733 | ||||
| MSKCC (33) | 6 | 90 | 4 | 96 | 4 | 90 | 1 | 97 | ||||||||
| Danish Diet, Cancer, and Health Study (20) | 15 | 807 | 271 | 55,352 | 45 | 777 | 1022 | 54,601 | 6 | 816 | 108 | 55,515 | 242 | 580 | 4,154 | 51,469 |
| Smoking status | ||||||||||||||||
| Never smoker | 44 | 2,514 | 94 | 28,007 | 90 | 1,516 | 459 | 26,312 | 70 | 2,588 | 208 | 27,883 | 343 | 1,848 | 1,852 | 24,018 |
| Ever smoker | 2,177 | 19,399 | 616 | 48,879 | 1,908 | 11,203 | 1,746 | 44,508 | 606 | 20,622 | 453 | 48,751 | 3,752 | 11,919 | 4,877 | 40,784 |
| Former smoker | 1,644 | 11,312 | 335 | 25,576 | 1,203 | 5,988 | 821 | 22,655 | 334 | 12,426 | 298 | 25,403 | 2,240 | 8,262 | 2,292 | 21,141 |
| Current smoker | 533 | 8,087 | 281 | 23,303 | 705 | 5,215 | 925 | 21,853 | 272 | 8,196 | 155 | 23,348 | 1,512 | 3,657 | 2,585 | 19,643 |
| Histologic type | ||||||||||||||||
| Adenocarcinoma | 705 | 5,551 | 277 | 2,792 | 159 | 6,287 | 950 | 3,933 | ||||||||
| Squamous cell carcinoma | 588 | 3,847 | 226 | 1,689 | 127 | 4,270 | 839 | 2,340 | ||||||||
| Small cell lung cancer | 175 | 1,607 | 62 | 618 | 50 | 1,700 | 301 | 1,098 | ||||||||
| Total | 2,221 | 21,913 | 710 | 76,886 | 1,998 | 12,719 | 2,205 | 70,820 | 676 | 23,210 | 661 | 76,634 | 4,095 | 13,767 | 6,729 | 64,802 |
| With removal(s)a | 769 | 9,028 | 498 | 65,641 | 453 | 18,044 | 440 | 71,561 | 2,602 | 7,432 | 2,033 | 9,061 | ||||
Abbreviations: CREST, Cancer of the Respiratory Tract; Exp., exposed; KCI, Karmanos Cancer Institute; MSKCC, Memorial Sloan-Kettering Cancer Center; UCLA, University of California, Los Angeles; UCSF, University of California, San Francisco; Unexp., unexposed; WSU, Wayne State University.
a Removal of one or more particular studies for each previous disease as specified in the figure legends.
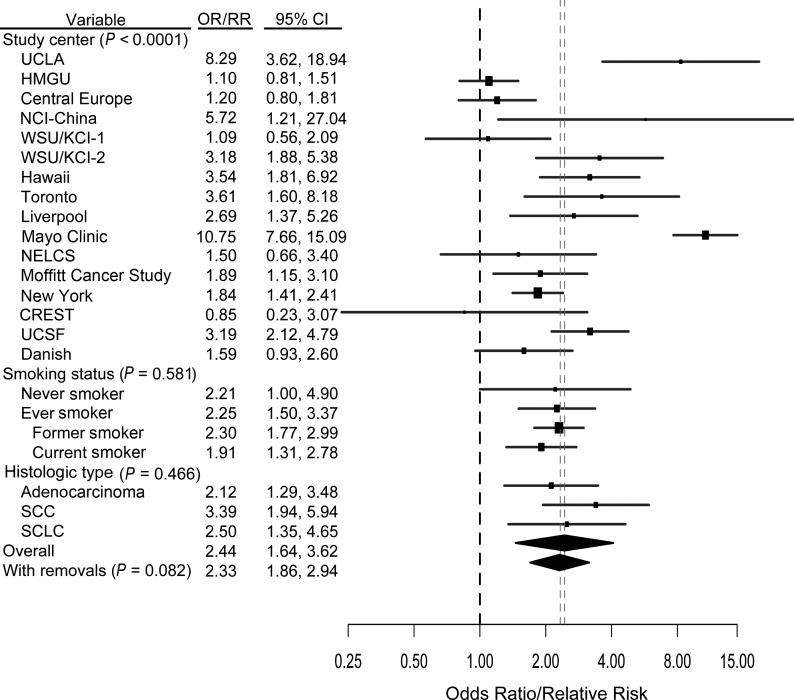
Overall, all of the 4 previous lung diseases examined were associated with increased incidence of lung cancer when adjusted estimates were examined individually. Specifically, a previous diagnosis of emphysema was associated with increased risk overall, based on 16 studies (relative risk (RR) = 2.44, 95% confidence interval (CI): 1.64, 3.62; I2 = 89.37%), and when stratified according to never (RR = 2.21, 95% CI: 1.00, 4.90; I2 = 88.52%) or ever (RR = 2.25, 95% CI: 1.50, 3.37; I2 = 44.28) smoking. The study-specific estimates, as well as estimates for subgroups of smoking status and histology, are shown in Figure 1. There was evidence of heterogeneity across studies that was not clearly explained by a single source (i.e., control type, proportion of ever smokers, time period, continent—all contributed (P < 0.001)). When we removed the outlying studies (21–23, 27, 39) as indicated by the Galbraith plot (see Web Figure 1 (http://aje.oxfordjournals.org/)), we observed marginal attenuation in the pooled effect estimate (RR = 2.33, 95% CI: 1.86, 2.94; I2 = 40.52%). After adjustment for other previous lung diseases, the relative risk associated with emphysema was 2.05 (95% CI: 1.33, 3.15; I2 = 89.95%) (data not shown).
Figure 1.
Results from a pooled analysis of emphysema as a risk factor for the development of lung cancer, International Lung Cancer Consortium, 1984–2011. The graph shows a forest plot of the association between emphysema and lung cancer risk by study center, smoking status, and histologic type. Models adjusted for age, sex, and pack-years of smoking. P values are from a test for heterogeneity across studies or across subgroups. “With removals” represents removal of the Mayo, Central Europe, HMGU, WSU/KCI-2, and UCLA studies. See Table 1 for published references. (CI, confidence interval; CREST, CREST (Cancer of the Respiratory Tract) Biorepository; Danish, Danish Diet, Cancer, and Health Study; HMGU, Helmholtz Center Munich; KCI, Karmanos Cancer Institute; Liverpool, Liverpool Lung Project; NCI, National Cancer Institute; NELCS, New England Lung Cancer Study; New York, New York Multicenter Study; OR, odds ratio; RR, relative risk; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; Toronto, Samuel Lunenfeld Research Institute; UCLA, University of California, Los Angeles; UCSF, University of California, San Francisco; WSU, Wayne State University; WSU/KCI-1, Family Health Study; WSU/KCI-2, study of women's lung cancer epidemiology).
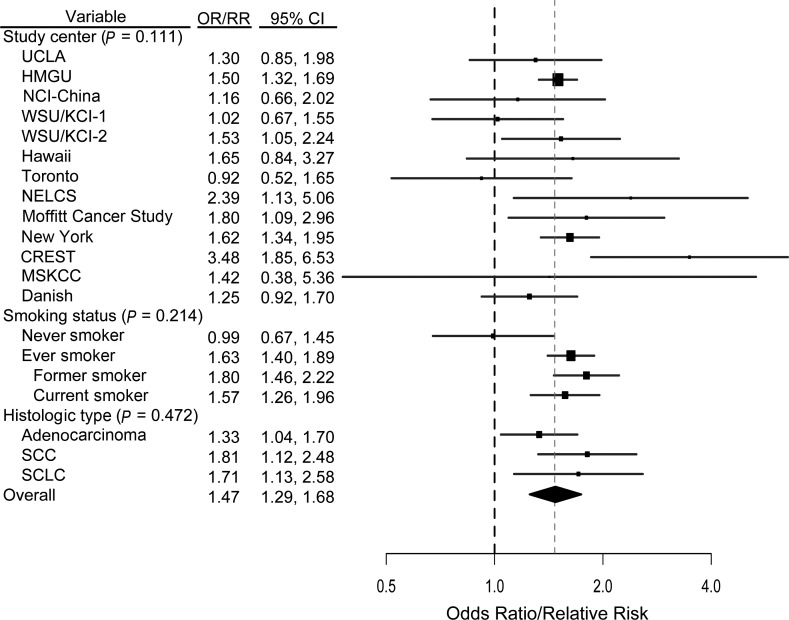
A previous diagnosis of chronic bronchitis was associated with increased risk overall, based on 13 studies (RR = 1.47, 95% CI: 1.29, 1.68; I2 = 33.91%), and among ever smokers (RR = 1.63, 95% CI: 1.40, 1.89; I2 = 40.79%) (Figure 2). There was no evidence of heterogeneity across the 13 studies (P = 0.111). After adjustment for other previous lung diseases, the risk ratio for chronic bronchitis was 1.25 (95% CI: 1.05, 1.56; I2 = 60.70%) (data not shown). When the effects of chronic bronchitis and emphysema were examined as a measure of COPD, the combined overall effect of COPD was a relative risk of 1.93 (95% CI: 1.48, 4.89; I2 = 89.54%) (data not shown).
Figure 2.
Results from a pooled analysis of chronic bronchitis as a risk factor for the development of lung cancer, International Lung Cancer Consortium, 1984–2011. The graph shows a forest plot of the association between chronic bronchitis and lung cancer risk by study center, smoking status, and histologic type. Models adjusted for age, sex, and pack-years of smoking. P values are from a test for heterogeneity across studies or across subgroups. (CI, confidence interval; CREST, CREST (Cancer of the Respiratory Tract) Biorepository; Danish, Danish Diet, Cancer, and Health Study; HMGU, Helmholtz Center Munich; KCI, Karmanos Cancer Institute; MSKCC, Memorial Sloan-Kettering Cancer Center; NELCS, New England Lung Cancer Study; New York, New York Multicenter Study; NCI, National Cancer Institute; OR, odds ratio; RR, relative risk; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; Toronto, Samuel Lunenfeld Research Institute; UCLA, University of California, Los Angeles; WSU, Wayne State University; WSU/KCI-1, Family Health Study; WSU/KCI-2, study of women's lung cancer epidemiology).
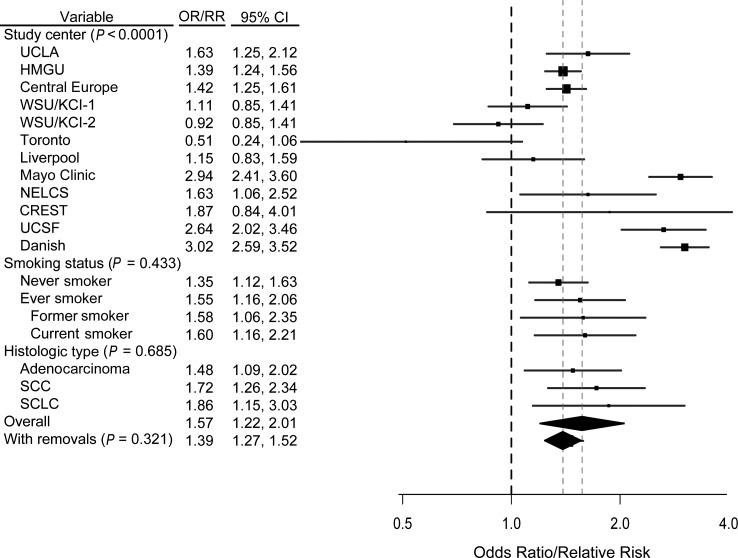
A previous diagnosis of pneumonia was associated with increased risk overall, based on 12 studies (RR = 1.57, 95% CI: 1.22, 2.01; I2 = 93.00%), and when stratified according to never (RR = 1.35, 95% CI: 1.12, 1.63; I2 = 23.01%) or ever (RR = 1.55, 95% CI: 1.16, 2.06; I2 = 93.18%) smoking (Figure 3). There was evidence of heterogeneity across studies that was not clearly explained by a single source (P < 0.001). When we removed the outlying studies (18, 20, 27, 29, 30) as indicated by the Galbraith plot (Web Figure 2), we observed a slight attenuation in the pooled effect estimate (RR = 1.39, 95% CI: 1.27, 1.52; I2 = 14.28%). After adjustment for other previous lung diseases, the relative risk for pneumonia was 1.42 (95% CI: 1.09, 1.86; I2 = 93.13%) (data not shown).
Figure 3.
Results from a pooled analysis of pneumonia as a risk factor for the development of lung cancer, International Lung Cancer Consortium, 1984–2011. The graph shows a forest plot of the association between pneumonia and lung cancer risk by study center, smoking status, and histologic type. Models adjusted for age, sex, and pack-years of smoking. P values are from a test for heterogeneity across studies or across subgroups. “With removals” represents removal of the Toronto, WSU/KCI-2, UCSF, Mayo, and Danish studies. (CI, confidence interval; CREST, CREST (Cancer of the Respiratory Tract) Biorepository; Danish, Danish Diet, Cancer, and Health Study; HMGU, Helmholtz Center Munich; KCI, Karmanos Cancer Institute; Liverpool, Liverpool Lung Project; NELCS, New England Lung Cancer Study; OR, odds ratio; RR, relative risk; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; Toronto, Samuel Lunenfeld Research Institute; UCLA, University of California, Los Angeles; UCSF, University of California, San Francisco; WSU, Wayne State University; WSU/KCI-1, Family Health Study; WSU/KCI-2, study of women's lung cancer epidemiology).
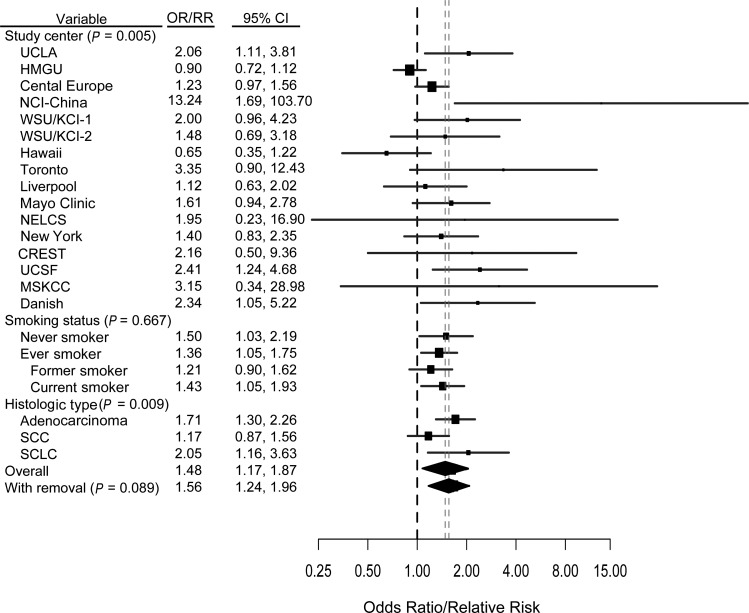
A previous diagnosis of tuberculosis was associated with increased risk overall, based on 16 studies (RR = 1.48, 95% CI: 1.17, 1.87; I2 = 54.27%), and among ever smokers (RR = 1.36, 95% CI: 1.05, 1.75; I2 = 47.96%) (Figure 4). We also observed an elevated risk among never smokers (RR = 1.50, 95% CI: 1.03, 2.19; I2 = 23.64%). There was evidence of heterogeneity across studies (P = 0.005); however, when we examined the Galbraith plot, it appeared that the heterogeneity was due to only 1 outlying study (40) (Web Figure 3). When this study was removed, a slight elevation in the pooled effect estimate was observed (RR = 1.56, 95% CI: 1.24, 1.96; I2 = 34.95%). After adjustment for other previous lung diseases, the relative risk for tuberculosis was 1.31 (95% CI: 1.03, 1.56; I2 = 50.99%) (data not shown).
Figure 4.
Results from a pooled analysis of tuberculosis as a risk factor for the development of lung cancer, International Lung Cancer Consortium, 1984–2011. The graph shows a forest plot of the association between tuberculosis and lung cancer risk by study center, smoking status, and histologic type. Models adjusted for age, sex, and pack-years of smoking. P values are from a test for heterogeneity across studies or across subgroups. “With removal” represents removal of the HMGU study. (CI, confidence interval; CREST, CREST (Cancer of the Respiratory Tract) Biorepository; Danish, Danish Diet, Cancer, and Health Study; HMGU, Helmholtz Center Munich; KCI, Karmanos Cancer Institute; Liverpool, Liverpool Lung Project; MSKCC, Memorial Sloan-Kettering Cancer Center; NCI, National Cancer Institute; NELCS, New England Lung Cancer Study; New York, New York Multicenter Study; OR, odds ratio; RR, relative risk; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; Toronto, Samuel Lunenfeld Research Institute; UCLA, University of California, Los Angeles; UCSF, University of California, San Francisco; WSU, Wayne State University; WSU/KCI-1, Family Health Study; WSU/KCI-2, study of women's lung cancer epidemiology).
In those studies where multiple diseases were investigated, we examined the risk associated with having multiple lung diseases. There was a dose-response relation with increasing number of previous lung diseases (P-trend < 0.001). The relative risk associated with having 1 disease was 1.71 (95% CI: 1.61, 1.82); with having 2 diseases, it was 2.00 (95% CI: 1.80, 2.21); with having 3 diseases, 2.23 (95% CI: 1.76, 2.82); and with having all 4 diseases, 2.44 (95% CI: 0.92, 6.48) (only 8 controls and 15 cases had had all 4 diseases). We examined the effects of all 4 lung diseases separately among males and females and observed no differential effects by sex. (Full subgroup analyses are shown in Web Table 1.)
Population attributable fraction estimates for the diseases investigated ranged within the combined population from 0.9% for tuberculosis to 8.3% for pneumonia, with study-specific estimates varying according to population disease prevalence (tuberculosis, 0.29%–9.76%; chronic bronchitis, 3.63%–30.28%; emphysema, 1.20%–17.90%; pneumonia, 0.51%–44.11%). Among never smokers as a combined group, having had any of the previous lung diseases of interest conferred an attributable fraction of 5.91% (Web Table 2).
DISCUSSION
In this investigation into the effects of previous lung diseases on lung cancer risk, we found associations with increased cancer risk for each of the diseases of interest. Comparisons among all histologic subgroups were consistent with increases in risk observed overall, with the exception of squamous cell carcinoma among persons with tuberculosis. Risk estimates were consistent across smoking subgroups; estimates were elevated in all subgroups, with the exception of chronic bronchitis. Our results among never smokers suggest an effect of previous lung diseases on lung cancer risk independent of tobacco smoking, probably acting through the inflammatory response and pathogenesis associated with the diseases.
The results of this pooled analysis corroborate the results of the previous meta-analysis suggesting that there was a large difference in the prevalence of COPD/emphysema among cases and controls (16). This difference in prevalence among cases and controls may explain/confound the differential effects observed in genetic epidemiologic studies of lung cancer in which inconsistent effects have been observed among populations of similar genetic ancestry (41) or may act as mediators in the associations between the variants and lung cancer risk (42). Although chronic bronchitis and emphysema are commonly grouped together as COPD, we calculated detailed results for each condition separately in order to allow for differential effects of these two conditions, which have different pathologies and etiologies. Because we observed independent effects of both of these diseases when adjusting for the other in a fixed-effects analysis, we felt this to be a beneficial approach.
Reverse causality and the issue of temporality are paramount to the consideration of causality for these associations. It is certainly possible that some of the conditions were early manifestations or symptoms of lung cancer that were misdiagnosed, particularly for emphysema and chronic bronchitis. For pneumonia and tuberculosis, infections may have been the result of a weakened immune system due to lung cancer. In addition, tumors may have been interpreted as lesions from infections prior to cancer diagnosis. To address these issues, we conducted a latency analysis which found that diagnoses of the previous lung diseases more than 5 years and more than 10 years prior to cancer diagnosis were positively associated with lung cancer incidence. This suggests that reverse causality is not likely to fully explain these associations. For example, when the analysis was restricted to the conditions diagnosed 10 years prior to lung cancer, chronic bronchitis remained associated with an increased risk of lung cancer (RR = 1.45, 95% CI: 1.08, 1.95). Complete results of latency analyses are available in Web Table 3. Note that in the cohort study included in this analysis (20), both lung disease and smoking status were ascertained at baseline and the average follow-up time to diagnosis/censoring was approximately 7 years.
The use of self-reports for measuring previous lung diseases may have introduced misclassification bias into the studies included in the pooled analysis. Quantitative techniques for each of the previous lung diseases are presently available for improved diagnostic accuracy and disease classification; however, these were not employed in any of the component studies of the analysis. When effect estimates obtained using quantitative diagnostic tools for COPD (forced expiratory volume in 1 second, quantitative computed tomography, or radiographic evidence), pneumonia (microimmunofluorescence), and tuberculosis (radiography) were pooled in the previous meta-analysis (16), the risk estimates derived using quantitative techniques were consistent with those derived using self-reported diagnoses. The similarity between effect estimates from the cohort study included in the analysis and the pooled case-control estimates (results not shown) suggests that potential bias due to misclassification of exposure, recall bias, and reverse causality may not explain the associations completely. Although none of the studies contained in this analysis validated self-reports with medical records, self-reported COPD has been shown to have a high level of agreement with spirometry results (43, 44). Despite the reports of these previous studies, misclassification of exposure may have produced underestimation of the burden due to the exposures, since several investigations have shown that COPD/emphysema is present in many lung cancer patients who do not report a history of COPD (45–47).
For pneumonia, the question of persistence of inflammation arising from a condition with clinical transience should be addressed. Because this investigation did not contain information on the number of infections or the length and/or intensity of infection, it is difficult to conceptually include pneumonia with the other diseases in terms of persistence of inflammation. However, murine models have suggested that infection from Mycoplasma pneumoniae can lead to long-term changes in peribronchial histopathology (48), pulmonary airflow resistance, and elevated inflammatory biomarkers long after active infection clears (49). This suggests that inflammation resulting from pneumonia may be more long-term in nature than clinical symptoms may suggest.
It is also possible that our results, particularly among never smokers, may have been confounded from exposure to other agents such as secondhand smoke or other occupational exposures. Secondhand smoke has been associated with increased risk of lung cancer (50) and may be related to previous lung diseases (51). However, it is unlikely to fully explain the large effects associated with several of the previous lung diseases. When we adjusted for secondhand smoke in our analysis among never smokers, the results remained, with risk estimates changing only slightly. For example, the relative risk associated with pneumonia among never smokers changed marginally from 1.35 to 1.45. In addition, occupational exposures may have acted as confounders in the associations tested, as they have been associated with lung cancer (52, 53). We examined the inclusion of restricted cubic splines to check for nonlinearity in both age and smoking (pack-years) as covariates in the association models. As was previously observed (54), nonlinear components for age and smoking were significant in the models, suggesting a deviation from linear fit; however, the effect estimates for the lung diseases pooled across studies changed minimally. Therefore, we retained linear terms in the models to avoid overdispersion in small studies when examining the within-study effects.
For those instances where heterogeneity was observed in the overall estimates (emphysema, pneumonia, tuberculosis), removal of the outlying studies led to only slight differences in the pooled estimates. For emphysema and pneumonia, where more than one study was contributing to heterogeneity, meta-regression suggested that several sources, including continent, control type, and proportion of ever smokers, all accounted for some portion of the heterogeneity (results not shown). In subgroup analyses where more than 3 studies were included in a pooled estimate, the only major difference was seen for emphysema between Europe and North America (Web Table 1). For emphysema, differences by continent of study may be a product of different diagnostic practices across populations, since diagnostic criteria for COPD differ across continents. More specifically, the diagnostic guidelines of the British Thoracic Society and the American Thoracic Society lead to marked differences in the prevalence of COPD when applied to the same population (55). Diagnostic differences across study locations that are not discernable from questionnaires may also explain some portion of the heterogeneity. Although these differences in diagnostic practice should produce nondifferential variation in disease status classification across cases and controls, the potential of this to influence the results should not be precluded. Several studies included in this analysis displayed COPD (emphysema/chronic bronchitis) prevalence higher than that in the community at large (Web Table 2), where it is often largely undiagnosed (56). For emphysema, control source contributed significantly to heterogeneity, suggesting that the differences in diagnosis in population-based settings compared with hospital-based settings may affect the prevalence of disease reported and therefore the magnitude of estimates and associated population attribution measures.
Strengths of this investigation include the large sample size and the large number of exposed persons. The use of random-effect models, although it provides wider confidence intervals, reduces the likelihood of larger studies' overly affecting pooled estimates when combining data across studies by estimating both within- and across-study variance. The inclusion of prospective data is also a strength of this pooled analysis, although the number of cases collected prospectively was comparatively smaller, whereby the biases associated with case-control studies could be comparatively evaluated.
In conclusion, we observed elevated lung cancer risks associated with previous diagnoses of emphysema, chronic bronchitis, pneumonia, and tuberculosis in this pooled analysis of primary data. The observation of relatively consistent associations between several of the previous lung diseases and lung cancer risk across smoking groups, histologic subtypes, and study designs supports a direct association with lung cancer, reducing the likelihood of confounding by tobacco exposure. The most likely explanation for the increased risk associated with these diseases is the effect of the inflammatory response within lung tissue. Recent evidence has suggested that inflammation plays a pivotal role in the development of lung cancer (12, 57, 58). Inflammation may increase the risk of cancer development as an initiator or promoter through 3 processes: increased genetic mutation, antiapoptotic signaling (59), and angiogenesis (14).
Whether acting as promoters in the causal pathway or as causative agents, these diseases appear to be markers of risk for the development of lung cancer that are clinically relevant. Most importantly, when considered as a group, the lung diseases examined in this pooled analysis affect large numbers of persons. In the United States, the prevalence of emphysema is 18.5 per 1,000 persons, and the prevalence of chronic bronchitis is 43.0 per 1,000 (60). Although the incidence of pneumonia in the United States is unknown, there were approximately 1.4 million hospital discharges associated with pneumonia in 2005 (61). While the incidence of tuberculosis in North America is low (4.8 per 100,000 population per year) (62), in developing nations the disease affects millions. In Europe and Asia, these conditions collectively affect millions of persons, and thus the exposed population is large (63). Therefore, the positive associations between the lung diseases examined and lung cancer risk are of substantial public health importance, and the consistent associations suggest that a nontrivial proportion of all lung cancer cases are attributable to these other lung diseases or their underlying pathologies.
The previous lung diseases examined in this investigation are significant for both public health and clinical practice. The development of lung cancer risk prediction models (54, 64, 65) should continue to incorporate the lung diseases examined in this analysis for improved discriminatory ability among all patients, regardless of smoking history. The United Kingdom Lung Cancer Screening Trial, which uses computed tomography to screen for lung cancer, utilizes the lung cancer risk prediction model of the Liverpool Lung Project, which includes pneumonia as one of the factors (62) for selection of high-risk individuals for the trial (66). These diseases may be useful in determining who to monitor by providing a further resolution of risk stratification, particularly as new-era screening evaluations and initiatives advance (67, 68).
ACKNOWLEDGMENTS
Author affiliations: Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (Darren R. Brenner, John R. McLaughlin, Rayjean J. Hung); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (Darren R. Brenner, John R. McLaughlin, Rayjean J. Hung); Institute for Translational Epidemiology, Mount Sinai School of Medicine, New York, New York (Paolo Boffetta); International Prevention Research Institute, Lyon, France (Paolo Boffetta); Unit of Nutrition, Environment and Cancer, Cancer Epidemiology Research Program, Catalan Institute of Oncology, Barcelona, Spain (Eric J. Duell); Department of Genetic Epidemiology, Medical School, Georg-August University of Göttingen, Göttingen, Germany (Heike Bickeböller, Albert Rosenberger); International Agency for Research on Cancer, Lyon, France (Valerie McCormack, Paul Brennan); Division of Epidemiology, Penn State Cancer Institute, Pennsylvania State University, State College, Pennsylvania (Joshua E. Muscat); Departments of Pharmacology and Health Evaluation Sciences, Penn State Cancer Institute, Pennsylvania State University, State College, Pennsylvania (Philip Lazarus); Epidemiology and Genetics of Lung Cancer Research Program, Mayo Clinic, Rochester, Minnesota (Ping Yang); Institute of Epidemiology, Helmholtz Center Munich, German Research Center for Environmental Health, Neuherberg, Germany (H.-Erich Wichmann, Irene Brueske-Hohlfeld); Department of Oncology, School of Medicine, Wayne State University, Detroit, Michigan (Ann G. Schwartz, Michele L. Cote); Karmanos Cancer Institute, Detroit, Michigan (Ann G. Schwartz, Michele L. Cote); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (Anne Tjønneland, Søren Friis); University of Hawaii Cancer Center, Honolulu, Hawaii (Loic Le Marchand); Department of Epidemiology, School of Public Health, University of California, Los Angeles, Los Angeles, California (Zuo-Feng Zhang); Departments of Epidemiology and Environmental Health Sciences, School of Public Health, University of Michigan, Ann Arbor, Michigan (Hal Morgenstern); Comprehensive Cancer Center, University of Michigan, Ann Arbor, Michigan (Hal Morgenstern); Department of Epidemiology, Institute of Occupational Medicine, Lodz, Poland (Neonila Szeszenia-Dabrowska); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (Jolanta Lissowska); Institute of Carcinogenesis, Cancer Research Centre, Moscow, Russia (David Zaridze); Fodor József National Center for Public Health, National Institute of Environmental Health, Budapest, Hungary (Peter Rudnai); Department of Occupational Health, Specialized State Health Institute, Banska Bystrica, Slovakia (Eleonora Fabianova); Department of Cancer Epidemiology and Genetics, Masaryk Memorial Cancer Institute, Brno, Czech Republic (Lenka Foretova); Department of Preventive Medicine, Faculty of Medicine, Palacky University, Olomouc, Czech Republic (Vladimir Janout); Institute of Hygiene and Epidemiology, First Faculty of Medicine, Charles University, Prague, Czech Republic (Vladimir Bencko, Miriam Schejbalova); University of Medicine and Pharmacy Carol Davila, Bucharest, Romania (Ioan N. Mates); Roy Castle Lung Cancer Research Programme, Department of Molecular and Clinical Cancer Medicine, University of Liverpool Cancer Research Centre, Liverpool, United Kingdom (John K. Field, Olaide Raji); Ontario Cancer Institute, Princess Margaret Hospital, Toronto, Ontario, Canada (Geoffrey Liu); Department of Epidemiology and Biostatistics, School of Medicine, University of California, San Francisco, San Francisco, California (John Wiencke); Unit of Clinical and Molecular Epidemiology, IRCCS (Istituto di Ricovero e Cura a Carattere Scientifico) San Raffaele Pisana, Rome, Italy (Monica Neri); Units of Epidemiology and Biostatistics, University of Genoa, Genoa, Italy (Donatella Ugolini); Units of Epidemiology, Biostatistics, and Clinical Trials, National Cancer Research Institute, Genoa, Italy (Donatella Ugolini); Unit of Biostatistics and Epidemiology, Department of Community and Family Medicine, Norris Cotton Cancer Center, Dartmouth Medical School, Lebanon, New Hampshire (Angeline S. Andrew); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland (Qing Lan, Wei Hu); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York (Irene Orlow); and Department of Surgery, Memorial Sloan-Kettering Cancer Center, New York, New York (Bernard J. Park).
The Toronto study (18) was funded by the Canadian Cancer Society Research Institute (grant 020214). The New England Lung Cancer Study (25) was funded by the National Center for Research Resources, US National Institutes of Health (grant P20RR018787). The Liverpool Lung Project (35) was funded by the Roy Castle Lung Cancer Foundation. The study from Memorial Sloan-Kettering Cancer Center (33) was funded by Steps for Breath, the Labrecque Foundation, and the Society of Memorial Sloan-Kettering Cancer Center. The Central Europe study (23) was funded by the World Cancer Research Fund and the European Commission's INCO-COPERNICUS Program (contract IC15-CT96-0313). The Warsaw portion of the Central Europe study was funded by the Polish State Committee for Scientific Research (grant SPUB-M-COPERNICUS/P-05/DZ-30/99/2000). The Family Health Study (22) and the study of women's lung cancer epidemiology (30), conducted by Wayne State University and the Karmanos Cancer Institute, were funded by the US National Institutes of Health (grants R01CA060691, R01CA87895, N01-PC35145, and P30CA22453). The study at the University of California, San Francisco (29) was funded by the US National Institute of Environmental Health Sciences (grant ES06717) and the US National Cancer Institute (grant CA-113710 to S. S. O.). The Danish Diet, Cancer, and Health Study (20) is funded by the Danish Cancer Society. The Helmholtz Center Munich lung cancer study (39, 40, 69, 70) was funded by the German Federal Ministry of Education, Science, Research and Technology, the State of Bavaria, the National Genome Research Network, the German Research Foundation (grants BI 576/2-1 and BI 576/2–2), the Helmholtz Association, and the German Federal Office for Radiation Protection (grant STSch4454).
R. J. H. holds a Cancer Care Ontario Chair in Population Studies. D. B. holds a Canadian Institutes of Health Research Canada Graduate Scholarship.
Conflict of interest: none declared.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Cancer Society's Steering Committee. Canadian Cancer Statistics 2010. Toronto, Ontario, Canada: Canadian Cancer Society; 2010. [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Samet JM, Avila-Tang E, Boffetta P, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res. 2009;15(18):5626–5645. doi: 10.1158/1078-0432.CCR-09-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peek RM, Jr, Mohla S, DuBois RN. Inflammation in the genesis and perpetuation of cancer: summary and recommendations from a National Cancer Institute-sponsored meeting. Cancer Res. 2005;65(19):8583–8586. doi: 10.1158/0008-5472.CAN-05-1777. [DOI] [PubMed] [Google Scholar]
- 6.Ballaz S, Mulshine JL. The potential contributions of chronic inflammation to lung carcinogenesis. Clin Lung Cancer. 2003;5(1):46–62. doi: 10.3816/CLC.2003.n.021. [DOI] [PubMed] [Google Scholar]
- 7.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9(1):57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 8.Weitzman SA, Gordon LI. Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood. 1990;76(4):655–663. [PubMed] [Google Scholar]
- 9.Rutgers SR, Postma DS, ten Hacken NH, et al. Ongoing airway inflammation in patients with COPD who do not currently smoke [abstract] Chest. 2000;117(5 suppl 1):262S. doi: 10.1378/chest.117.5_suppl_1.262s. [DOI] [PubMed] [Google Scholar]
- 10.Moldoveanu B, Otmishi P, Jani P, et al. Inflammatory mechanisms in the lung. J Inflamm Res. 2009;(2):1–11. [PMC free article] [PubMed] [Google Scholar]
- 11.Brody JS, Spira A. State of the art. Chronic obstructive pulmonary disease, inflammation, and lung cancer. Proc Am Thorac Soc. 2006;3(6):535–537. doi: 10.1513/pats.200603-089MS. [DOI] [PubMed] [Google Scholar]
- 12.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Rev Anticancer Ther. 2008;8(4):605–615. doi: 10.1586/14737140.8.4.605. [DOI] [PubMed] [Google Scholar]
- 13.Heikkilä K, Harris R, Lowe G, et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20(1):15–26. doi: 10.1007/s10552-008-9212-z. [DOI] [PubMed] [Google Scholar]
- 14.Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev. 2008;11(1):1–15. doi: 10.1080/10937400701436460. [DOI] [PubMed] [Google Scholar]
- 15.Punturieri A, Szabo E, Croxton TL, et al. Lung cancer and chronic obstructive pulmonary disease: needs and opportunities for integrated research. J Natl Cancer Inst. 2009;101(8):554–559. doi: 10.1093/jnci/djp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLoS ONE. 2011;6(3):e17479. doi: 10.1371/journal.pone.0017479. ( doi:10.1371/journal.pone.0017479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung RJ, Christiani DC, Risch A, et al. International Lung Cancer Consortium: pooled analysis of sequence variants in DNA repair and cell cycle pathways. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3081–3089. doi: 10.1158/1055-9965.EPI-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenner DR, Hung RJ, Tsao MS, et al. Lung cancer risk in never-smokers: a population-based case-control study of epidemiologic risk factors. BMC Cancer. 2010;10:285. doi: 10.1186/1471-2407-10-285. ( doi:10.1186/1471-2407-10-285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ugolini D, Neri M, Canessa PA, et al. The CREST biorepository: a tool for molecular epidemiology and translational studies on malignant mesothelioma, lung cancer, and other respiratory tract diseases. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3013–3019. doi: 10.1158/1055-9965.EPI-08-0524. [DOI] [PubMed] [Google Scholar]
- 20.Tjønneland A, Olsen A, Boll K, et al. Study design, exposure variables, and socioeconomic determinants of participation in Diet, Cancer and Health: a population-based prospective cohort study of 57,053 men and women in Denmark. Scand J Public Health. 2007;35(4):432–441. doi: 10.1080/14034940601047986. [DOI] [PubMed] [Google Scholar]
- 21.Cui Y, Morgenstern H, Greenland S, et al. Dietary flavonoid intake and lung cancer—a population-based case-control study. Cancer. 2008;112(10):2241–2248. doi: 10.1002/cncr.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz AG, Cote ML, Wenzlaff AS, et al. Racial differences in the association between SNPs on 15q25.1, smoking behavior, and risk of non-small cell lung cancer. J Thorac Oncol. 2009;4(10):1195–1201. doi: 10.1097/JTO.0b013e3181b244ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brennan P, Crispo A, Zaridze D, et al. High cumulative risk of lung cancer death among smokers and nonsmokers in Central and Eastern Europe. Am J Epidemiol. 2006;164(12):1233–1241. doi: 10.1093/aje/kwj340. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher CJ, Muscat JE, Hicks AN, et al. The UDP-glucuronosyltransferase 2B17 gene deletion polymorphism: sex-specific association with urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol glucuronidation phenotype and risk for lung cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(4):823–828. doi: 10.1158/1055-9965.EPI-06-0823. [DOI] [PubMed] [Google Scholar]
- 25.Heck JE, Andrew AS, Onega T, et al. Lung cancer in a U.S. population with low to moderate arsenic exposure. Environ Health Perspect. 2009;117(11):1718–1723. doi: 10.1289/ehp.0900566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muscat JE, Stellman SD, Wynder EL. Insulation, asbestos, smoking habits, and lung cancer cell types. Am J Ind Med. 1995;27(2):257–269. doi: 10.1002/ajim.4700270210. [DOI] [PubMed] [Google Scholar]
- 27.Yang P, Bamlet WR, Ebbert JO, et al. Glutathione pathway genes and lung cancer risk in young and old populations. Carcinogenesis. 2004;25(10):1935–1944. doi: 10.1093/carcin/bgh203. [DOI] [PubMed] [Google Scholar]
- 28.Le Marchand L, Murphy SP, Hankin JH, et al. Intake of flavonoids and lung cancer. J Natl Cancer Inst. 2000;92(2):154–160. doi: 10.1093/jnci/92.2.154. [DOI] [PubMed] [Google Scholar]
- 29.Wrensch MR, Miike R, Sison JD, et al. CYP1A1 variants and smoking-related lung cancer in San Francisco Bay Area Latinos and African Americans. Int J Cancer. 2005;113(1):141–147. doi: 10.1002/ijc.20537. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz AG, Cote ML, Wenzlaff AS, et al. Chronic obstructive lung diseases and risk of non-small cell lung cancer in women. J Thorac Oncol. 2009;4(3):291–299. doi: 10.1097/JTO.0b013e3181951cd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallagher CJ, Ahn K, Knipe AL, et al. Association between haplotypes of manganese superoxide dismutase (SOD2), smoking, and lung cancer risk. Free Radic Biol Med. 2009;46(1):20–24. doi: 10.1016/j.freeradbiomed.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Field RW, Smith BJ, Platz CE, et al. Lung cancer histologic type in the Surveillance, Epidemiology, and End Results registry versus independent review. J Natl Cancer Inst. 2004;96(14):1105–1107. doi: 10.1093/jnci/djh189. [DOI] [PubMed] [Google Scholar]
- 33.Orlow I, Park BJ, Mujumdar U, et al. DNA damage and repair capacity in patients with lung cancer: prediction of multiple primary tumors. J Clin Oncol. 2008;26(21):3560–3566. doi: 10.1200/JCO.2007.13.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lan Q, He X, Costa DJ, et al. Indoor coal combustion emissions, GSTM1 and GSTT1 genotypes, and lung cancer risk: a case-control study in Xuan Wei, China. Cancer Epidemiol Biomarkers Prev. 2000;9(6):605–608. [PubMed] [Google Scholar]
- 35.Field JK, Smith DL, Duffy S, et al. The Liverpool Lung Project research protocol. Int J Oncol. 2005;27(6):1633–1645. [PubMed] [Google Scholar]
- 36.Greenland S, Robins JM. Conceptual problems in the definition and interpretation of attributable fractions. Am J Epidemiol. 1988;128(6):1185–1197. doi: 10.1093/oxfordjournals.aje.a115073. [DOI] [PubMed] [Google Scholar]
- 37.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galbraith R. Graphical display of estimates having different standard errors. Technometrics. 1988;30(3):271–281. [Google Scholar]
- 39.Sauter W, Rosenberger A, Beckmann L, et al. Matrix metalloproteinase 1 (MMP1) is associated with early-onset lung cancer. LUCY-Consortium. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1127–1135. doi: 10.1158/1055-9965.EPI-07-2840. [DOI] [PubMed] [Google Scholar]
- 40.Kreuzer M, Heinrich J, Wölke G, et al. Residential radon and risk of lung cancer in eastern Germany. Epidemiology. 2003;14(5):559–568. doi: 10.1097/01.ede.0000071410.26053.c4. [DOI] [PubMed] [Google Scholar]
- 41.Yang P, Li Y, Jiang R, et al. A rigorous and comprehensive validation: common genetic variations and lung cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(1):240–244. doi: 10.1158/1055-9965.EPI-09-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Spitz MR, Amos CI, et al. Mediating effects of smoking and chronic obstructive pulmonary disease on the relation between the CHRNA5-A3 genetic locus and lung cancer risk. Cancer. 2010;116(14):3458–3462. doi: 10.1002/cncr.25085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radeos MS, Cydulka RK, Rowe BH, et al. Validation of self-reported chronic obstructive pulmonary disease among patients in the ED. Am J Emerg Med. 2009;27(2):191–196. doi: 10.1016/j.ajem.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barr RG, Herbstman J, Speizer FE, et al. Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. Am J Epidemiol. 2002;155(10):965–971. doi: 10.1093/aje/155.10.965. [DOI] [PubMed] [Google Scholar]
- 45.Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34(2):380–386. doi: 10.1183/09031936.00144208. [DOI] [PubMed] [Google Scholar]
- 46.Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178(7):738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Torres JP, Bastarrika G, Wisnivesky JP, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007;132(6):1932–1938. doi: 10.1378/chest.07-1490. [DOI] [PubMed] [Google Scholar]
- 48.Hardy RD, Jafri HS, Olsen K, et al. Mycoplasma pneumoniae induces chronic respiratory infection, airway hyperreactivity, and pulmonary inflammation: a murine model of infection-associated chronic reactive airway disease. Infect Immun. 2002;70(2):649–654. doi: 10.1128/iai.70.2.649-654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardy RD, Jafri HS, Olsen K, et al. Elevated cytokine and chemokine levels and prolonged pulmonary airflow resistance in a murine Mycoplasma pneumoniae pneumonia model: a microbiologic, histologic, immunologic, and respiratory plethysmographic profile. Infect Immun. 2001;69(6):3869–3876. doi: 10.1128/IAI.69.6.3869-3876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stayner L, Bena J, Sasco AJ, et al. Lung cancer risk and workplace exposure to environmental tobacco smoke. Am J Public Health. 2007;97(3):545–551. doi: 10.2105/AJPH.2004.061275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reardon JZ. Environmental tobacco smoke: respiratory and other health effects. Clin Chest Med. 2007;28(3):559–573. doi: 10.1016/j.ccm.2007.06.006. vi. [DOI] [PubMed] [Google Scholar]
- 52.Moulin JJ. A meta-analysis of epidemiologic studies of lung cancer in welders. Scand J Work Environ Health. 1997;23(2):104–113. doi: 10.5271/sjweh.187. [DOI] [PubMed] [Google Scholar]
- 53.Cassidy A, ‘t Mannetje A, van Tongeren M, et al. Occupational exposure to crystalline silica and risk of lung cancer: a multicenter case-control study in Europe. Epidemiology. 2007;18(1):36–43. doi: 10.1097/01.ede.0000248515.28903.3c. [DOI] [PubMed] [Google Scholar]
- 54.Tammemagi CM, Pinsky PF, Caporaso NE, et al. Lung cancer risk prediction: Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial models and validation. J Natl Cancer Inst. 2011;103(13):1058–1068. doi: 10.1093/jnci/djr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lindberg A, Jonsson AC, Rönmark E, et al. Prevalence of chronic obstructive pulmonary disease according to BTS, ERS, GOLD and ATS criteria in relation to doctor's diagnosis, symptoms, age, gender, and smoking habits. Respiration. 2005;72(5):471–479. doi: 10.1159/000087670. [DOI] [PubMed] [Google Scholar]
- 56.Young RP, Hopkins RJ, Hay BA, et al. Lung cancer susceptibility model based on age, family history and genetic variants. PLoS ONE. 2009;4(4):e5302. doi: 10.1371/journal.pone.0005302. ( doi:10.1371/journal.pone.0005302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fitzpatrick FA. Inflammation, carcinogenesis and cancer. Int Immunopharmacol. 2001;1(9-10):1651–1667. doi: 10.1016/s1567-5769(01)00102-3. [DOI] [PubMed] [Google Scholar]
- 59.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117(5):1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Epidemiology and Statistics Unit, American Lung Association. Trends in COPD (Chronic Bronchitis and Emphysema): Morbidity and Mortality. Washington, DC: American Lung Association; 2007. [Google Scholar]
- 61.DeFrances CJ, Hall MJ. 2005 National Hospital Discharge Survey. Adv Data. 2007;(385):1–19. [PubMed] [Google Scholar]
- 62.Epidemiology and Statistics Unit, American Lung Association. Trends in Tuberculosis: Morbidity and Mortality. Washington, DC: American Lung Association; 2007. [Google Scholar]
- 63.World Health Organization. Global Burden of Disease. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 64.Spitz MR, Etzel CJ, Dong Q, et al. An expanded risk prediction model for lung cancer. Cancer Prev Res (Phila) 2008;1(4):250–254. doi: 10.1158/1940-6207.CAPR-08-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cassidy A, Myles JP, van Tongeren M, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer. 2008;98(2):270–276. doi: 10.1038/sj.bjc.6604158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baldwin DR, Duffy SW, Wald NJ, et al. UK Lung Screen (UKLS) nodule management protocol: modelling of a single screen randomised controlled trial of low-dose CT screening for lung cancer. Thorax. 2011;66(4):308–313. doi: 10.1136/thx.2010.152066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aberle DR, Berg CD, Black WC, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243–253. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6 suppl):273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 69.Kreienbrock L, Kreuzer M, Gerken M, et al. Case-control study on lung cancer and residential radon in western Germany. Am J Epidemiol. 2001;153(1):42–52. doi: 10.1093/aje/153.1.42. [DOI] [PubMed] [Google Scholar]
- 70.Holle R, Happich M, Löwel H, et al. KORA—a research platform for population based health research. Gesundheitswesen. 2005;67(suppl 1):S19–S25. doi: 10.1055/s-2005-858235. [DOI] [PubMed] [Google Scholar]