Abstract
Restrictions on smoking in public places have become increasingly widespread in the United States, particularly since the year 2005. National-scale studies in Europe and local-scale studies in the United States have found decreases in hospital admissions for acute myocardial infarction (AMI) following smoking bans. The authors analyzed AMI admission rates for the years 1999–2008 in 387 US counties that enacted comprehensive smoking bans across 9 US states, using a study population of approximately 6 million Medicare enrollees aged 65 years or older. Effects of smoking bans on AMI admissions were estimated by using Poisson regression with linear and nonlinear adjustment for secular trend and random effects at the county level. Under the assumption of linearity in the secular trend of declining AMI, smoking bans were associated with a statistically significant ban-associated decrease in admissions for AMI in the 12 months following the ban. However, the estimated effect was attenuated to nearly zero when the assumption of linearity in the underlying trend was relaxed. This analysis demonstrates that estimation of potential health benefits associated with comprehensive smoking bans is challenged by the need to adjust for nonlinearity in secular trend.
Keywords: environmental tobacco smoke, mixed-effects models, secondhand smoke, smoking bans
Active smoking has long been identified as a cause of premature death and disease. Since the first Surgeon General's report in the year 1964, a growing list of diseases has been causally linked with smoking (1). The 1986 Surgeon General's report concluded that secondhand smoke causes lung cancer in nonsmokers and adverse effects in infants and children (2). Later investigations linked secondhand smoke exposure to increased risk for coronary heart disease (3, 4), with the 2006 report of the Surgeon General finding this link to be causal (5). Heart disease has historically been the leading cause of death attributable to secondhand smoke (6), with an estimated 21,800–75,100 deaths from coronary heart disease attributed to secondhand smoke annually between years 1999 and 2004 (7). Exposure to secondhand smoke can trigger a range of physiologic responses, including increased heart rate, decreases in skin microvascular dilatation (8), altered endothelial cell functioning, and other effects (5) that may have immediate consequences for cardiovascular health.
The dangers of secondhand smoke have prompted large-scale efforts to protect public health through smoking bans, largely legislated over the last decade in regions throughout the United States and the world. These bans usually prohibit smoking in restaurants, workplaces, and bars, although variation exists among jurisdictions (9). Nearly all national- and regional-scale studies in Europe and local-scale studies in North America have reported statistically significant evidence of decreases in admissions for acute myocardial infarction (AMI) following smoking bans in workplaces, restaurants, and bars (10–17). However, 2 recent studies of small US communities showed no ban-attributable decline (18, 19), and analyses conducted with models using nonlinear secular adjustments have found very small effects in England and Italy (20, 21). Interestingly, an Italian study from 2009 reported that the estimated effect of a smoking ban attenuated as model flexibility for trend increased (22). A recent meta-analysis of 17 studies (10 North American, 6 European, and 1 Australian) found statistically significant evidence of a 10% reduction in acute coronary events following the implementation of smoking bans (23). Studies also show that smoking bans reduce secondhand smoke exposure, as assessed by air monitoring and biomarkers (24).
However, several methodological issues need consideration when comparing results from the diverse studies of the impact of smoking bans. Studies to date have been heterogeneous in their designs, target populations, statistical analyses, choices of control groups, and types of smoking bans investigated. One particularly challenging issue is to carefully estimate the effect of a ban in the context of the ongoing trend of declining cardiovascular disease morbidity and mortality (25). If adequate adjustment is not made for the secular trend, the estimated health effects associated with the smoking ban may be biased. A committee of the US Institute of Medicine was recently tasked with assessing the evidence on the relation between secondhand smoke exposure and acute coronary events (25). The committee highlighted the importance of model choice in general, including the challenges in the adjustment for secular trend and the potential impact of the adjustment approach on results.
We report findings of a large retrospective cohort study on the public health benefits of US smoking bans. We use a population of 6 million Medicare enrollees, from 387 study counties across 9 US states (Illinois, Ohio, Minnesota, New York, Washington, New Jersey, Arizona, Massachusetts, and Delaware) where comprehensive smoking bans were implemented during the years 1999–2008. For each county, data were available at least 12 months before and after the implementation of the ban. Using a common statistical approach, we estimate the percent reduction in hospital admission rates for AMI following the implementation of comprehensive smoking bans for each county and overall for all the counties combined. Several models are used to adjust for potential confounding by the ongoing trend of declining cardiovascular disease morbidity: one using the assumption of linearity of secular trend common in the literature, and others using more flexible nonlinear trends. Finally, extensive sensitivity analyses are conducted to evaluate the robustness of these results to model specification.
MATERIALS AND METHODS
This analysis used monthly rates of hospital admissions for AMI during the years 1999–2008, derived from billing claims of Medicare enrollees from the National Claims History Files.
Our study sample was drawn from the Medicare Provider Analysis and Review inpatient claims database and the Medicare denominator file. Each billing claim includes age, sex, race, the dates of admission and discharge, disease classification in accordance with the International Classification of Diseases, Ninth Revision, and hospital information. The denominator file includes age, sex, race, state and county of residence, and information about Medicare plans. In 2006, there were 35.7 million Medicare enrollees aged 65 years or older, representing more than 90% of the US population older than 65 years. Approximately 85% of them were enrolled in the Medicare fee-for-service plan and were included in the inpatient claims. We linked the inpatient data to the denominator file by the unique beneficiary identification number and then determined admission for AMI using the principal discharge diagnosis code (410.xx, excluding 410.x2). We excluded patients that could not be linked with the denominator file. Patients discharged alive within 1 day of admission, not against medical advice and not transferred to a different facility, were also excluded, as these people are unlikely to have represented true cases of AMI. Hospital transfers occurring within 1 calendar day were linked as a single episode of care. Monthly time series of hospitalization rates were constructed for each county, summing the number of hospital admissions for each month. We examined eligibility for each beneficiary to account for new enrollment, disenrollment, or death during an index month. Finally, we restricted the sample to the 387 counties with comprehensive smoking bans.
Data concerning smoking bans were gathered from publicly available resources hosted by the Americans for Nonsmokers' Rights Foundation (9). The analysis was conducted by using counties with simultaneous bans on smoking in bars, restaurants, and workplaces that were enacted between January 2000 and December 2007, allowing a minimum of 12 months of hospitalization data both before the ban and 12 months after the ban, in states where 3 or more such counties existed. Counties were excluded if the comprehensive ban was preceded by any ban covering a spatial subset of the county. Web Appendix 1 available at http://aje.oxfordjournals.org/ lists the 387 study counties, which come from the following US states: Arizona, Delaware, Illinois, Massachusetts, Minnesota, New Jersey, New York, Ohio, and Washington. Web figures show the number of counties with bans (Web Figure 1) and spatial distribution of counties with bans (Web Figure 2).
State-level Poisson regression models with county-specific random effects were used to estimate change in AMI admission rates during the 12 months following implementation of a smoking ban compared with the months before the smoking ban, adjusting for demographic characteristics and seasonal and secular trends in admissions rates (26). Separate random-effects models were fit to counties within each state, because of the homogeneity of the tobacco control environment within states and the relative uniformity of secular trends at this level of aggregation. County-specific random effects for the AMI admission rate at time zero (i.e., December 1998) and for the linear component of the secular trend in AMI admissions rates were included in the model to account for the following: 1) correlation in observations among counties within each state; and 2) heterogeneity across counties in the linear component of the trend. County-specific random effects were also specified for the coefficient of the smoking ban indicator to allow for heterogeneity in the effect of the ban across counties within each state. The state-level Poisson regression model may be written as
 |
where c, t, a, and g represent county, time (month) indexed from 1 in January 1999 to 120 in December 2008, age groups a, and gender g. 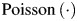 represents the Poisson distribution. The reference population is females, aged 65–74 years. This model includes the following: 1) the county-specific logarithm of the number of people at risk on a given month as an offset; 2) a state-level intercept and county-level random effects to account for differences in the overall admission rate; 3) a state-level linear function of time and county-level random effects to account for local trends; 4) in some models, an additional nonlinear, state-level smooth function for time (natural cubic splines, excluding the linear term) to flexibly account for secular trends in AMI admission rates; 5) a state-level indicator for each month to adjust for seasonality; 6) a state-level indicator for each age group (65–74 years, 75–84 years, >84 years); 7) a state-level indicator for gender; and 8) state-level fixed and county-level random effects for the ban effect, defined as the indicator for smoking ban that was 1 for months the ban was in effect and 0 otherwise. Random effects were assumed to follow a normal model with mean zero. The estimated fixed effect, β16, corresponding to model term 8 was the principal focus in evaluating the health effects associated with smoking bans.
represents the Poisson distribution. The reference population is females, aged 65–74 years. This model includes the following: 1) the county-specific logarithm of the number of people at risk on a given month as an offset; 2) a state-level intercept and county-level random effects to account for differences in the overall admission rate; 3) a state-level linear function of time and county-level random effects to account for local trends; 4) in some models, an additional nonlinear, state-level smooth function for time (natural cubic splines, excluding the linear term) to flexibly account for secular trends in AMI admission rates; 5) a state-level indicator for each month to adjust for seasonality; 6) a state-level indicator for each age group (65–74 years, 75–84 years, >84 years); 7) a state-level indicator for gender; and 8) state-level fixed and county-level random effects for the ban effect, defined as the indicator for smoking ban that was 1 for months the ban was in effect and 0 otherwise. Random effects were assumed to follow a normal model with mean zero. The estimated fixed effect, β16, corresponding to model term 8 was the principal focus in evaluating the health effects associated with smoking bans.
To assess the sensitivity of the results to the degree of adjustment for the underlying nonlinear trend when the models were fitted to the full data, the degree of flexibility in the modeling of nonlinear trend for model term 4 was varied in increments of 0.1 df/year of data for the spline. The range of degrees of freedom considered was from 0 df for the nonlinear component (i.e., linearity) to 0.8 df/year to allow more flexibility. This approach was used because the total number of study years in each county and state depended on the times at which the smoking bans were implemented. Here, the Akaike Information Criterion, a measure of goodness of fit where a lower value corresponds to a better fitting model, was used to evaluate the appropriateness of each model. The likelihood ratio test was also used to formally evaluate the fit of competing models.
Furthermore, for each state, we tested for potential nonlinearity in the secular trends of preban monthly admission data using the same state-level Poisson model, less the ban effect term. Because there were no bans in effect for the monthly preban data, any statistically significant quadratic trend would provide evidence that models using a linear secular trend introduced a bias in the estimate of the ban effect. The estimated coefficient for a quadratic term orthogonal to the linear trend in these preban models was used as a measure of curvature, and t tests were used to evaluate statistical significance.
Additional sensitivity analyses were conducted to investigate robustness against amount of postban time studied and the use of random versus fixed effects. Details of the sensitivity analyses are available in Web Appendix 2, with results appearing in Web Figure 3.
An estimate of the smoking ban effect pooled across states was obtained by using a weighted average of the state-specific estimates, where weights were chosen as the inverse-square of the estimated county-specific standard errors (27). Thus, although states with fewer counties have less precise state-level estimates, they will contribute less to the pooled estimate.
The data were analyzed by using the lme4 package within the statistical software R, version 2.11.0 (28, 29). The study was reviewed and exempted by the Harvard School of Public Health Institutional Review Board.
RESULTS
For the 387 counties included in the study, there were approximately 64,000 annual admissions for AMI during the study period from January 1, 1999, through December 31, 2008. Figure 1 shows the monthly AMI hospitalization rates per 100,000 Medicare enrollees, obtained by averaging monthly rates across all counties within each state and all months within each year, separately for each state. Web Table 1 shows the annual number of enrollees and the admission rates for AMI. The mean AMI rate across states dropped about 28% during the years 1999–2008. A negative curvature in the secular trend was found in preban data for 8 of the 9 states, with 6 of the 9 states (Illinois, Ohio, Minnesota, Washington, New Jersey, Arizona) showing statistically significant downward curvature.
Figure 1.
Monthly acute myocardial infarction (AMI) hospitalization rates per 100,000 Medicare enrollees, averaged across all counties within each US state and all months within each year, 1999–2008. The symbol “x” is used to represent years where greater than 90% of the counties in the state had a simultaneous ban on smoking in restaurants, bars, and workplaces for at least 6 months. AZ, Arizona; DE, Delaware; IL, Illinois; MA, Massachusetts; MN, Minnesota; NJ, New Jersey; NY, New York; OH, Ohio; WA, Washington.
Among counties included in the study, 25% were covered by comprehensive smoking bans before January 2006, 50% before January 2007, 75% before November 2007, and all 387 were covered by the start of January 2008. Typically, smoking bans were enacted at the same time for all counties covered within a particular state (Web Figure 1). Web Figure 2 provides a national map with each study county colored to indicate when its simultaneous bans began.
For illustration, Figure 2 presents the secular trend, estimated under linear and nonlinear models (as described in Materials and Methods), for AMI rates in 90 counties with simultaneous bans in Illinois, the state with the largest number of counties. The estimated ban effect for Illinois, under the linear model for secular trend in AMI, is visible in the discontinuity of the straight line at the beginning of the year 2008. This estimated effect under the model of linear trend, visible as the size of the drop from the end of the straight line to the beginning of the dashed line with the same slope, was estimated as −5.4% (95% confidence interval (CI): −8.2, −2.5). However, with a nonlinear term for time, the estimated effect is no longer statistically significant. Similar patterns are observed in most of the other states as well.
Figure 2.
Four models for the secular trend in monthly acute myocardial infarction (AMI) rates along with the estimated ban effect, using data from Illinois, 1999–2008. A linear model (thick line) and splines with 0, 0.1, 0.3, and 0.5 df/year of data (thin lines) were used to estimate the nonlinear components of the spline; increasing flexible lines correspond to higher degrees of freedom. Lines showing estimated secular trend transition from solid to dashed in the month when comprehensive smoking bans are implemented. Estimated impacts of smoking bans on AMI rates are visible as discontinuities between solid and dashed lines.
Web Figure 4 shows the estimated percent reduction in AMI admissions associated with a comprehensive ban separately for each state. Results for 4 different models for trend are presented: a linear trend and nonlinear trends that included 0.1, 0.3, or 0.5 df for each year of data for that state. Allowing for any nonlinearity in the secular trend resulted in a pooled estimate that is not statistically significantly different from zero, both for all-age as well as age-stratified estimates. The pooled all-age ban effect was estimated to be −4.93% (95% CI: −6.26, −3.59) using a linear trend for adjustment, 0.62% (95% CI: −2.45, 3.79) using 0.1 df/year of data, 0.62% (95% CI: −1.61, 2.90) when using 0.3 df/year, and 0.58% (95% CI: −1.97, 3.20) when using 0.5 df/year. Under pooled age-stratified models, the estimated ban effect for enrollees aged 65–74 years was consistent across the linear and spline models, though not statistically significant. When applying the linear model for secular trend, we found the size of the estimated effect to be larger in older age groups. Stratified analyses of population subgroups were not studied because of concerns of loss of power.
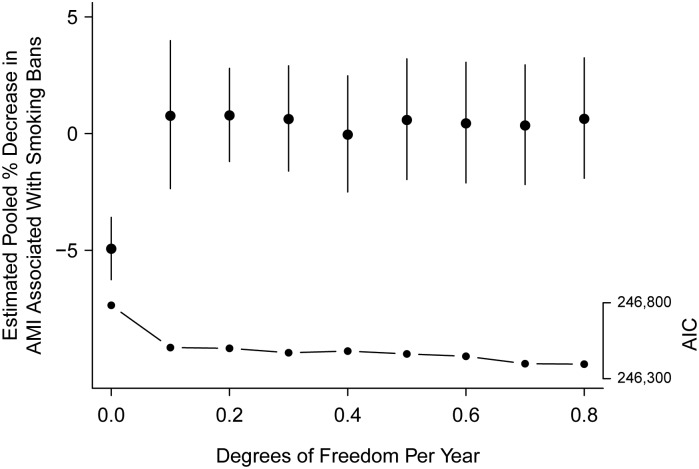
Figure 3 shows the estimated effect for all ages, pooled across states, for models under all 9 trends considered. Also shown is the Akaike Information Criterion for each model. Allowing even a small amount of flexibility in the model of secular trend in AMI leads to a substantially better fit. The likelihood ratio test rejected the null hypothesis (P < 0.0001) that a linear secular model fit the data as well as a spline model with 0.1 df/year.
Figure 3.
The estimated percent decrease in acute myocardial infarction (AMI) admissions (and 95% confidence interval) associated with smoking bans, pooled across US states, 1999–2008, for 9 models with increasing degrees of smoothness in secular trend. The Akaike Information Criterion (AIC) under each of the 9 models is also shown as connected dots. The AIC scale is shown on the right, vertical axis. These models included adjustments for seasonality, gender, and age group.
Additional sensitivity analyses were performed to assess the functional form of the ban effect, the amount of time included after the implementation of the ban, and the impact on the estimated ban effect of using a state-level linear or nonlinear trend (refer to Web Appendix 2 for details). In each case, results remained substantively unchanged. Furthermore, modeling possible ban effects as a change in intercept facilitates comparison to the larger literature using that approach (10–13, 15–18, 20, 22, 30, 31). Overdispersion was carefully considered and found to be extremely limited. None of these sensitivity analyses led to systematic changes in the results reported above. Furthermore, the consistency of the results when using from 0.1 to 0.5 df/year for the nonlinear trend indicates the robustness of the findings; even in the quadratic case, where the trend is quite locally linear around the time of the ban, the ban effect attenuates to zero.
DISCUSSION
To assess the association between smoking bans and AMI admission rates, we used Medicare records to construct a large cohort of people in 387 US counties during the years 1999–2008. We fitted random-effects models to estimate the percentage reduction in AMI admissions associated with comprehensive county-wide bans, adjusting for seasonality and secular trends. Statistically significant reductions in hospital admissions rates for AMI were found when strict linearity of secular trends of AMI admission rates was assumed, but the estimated effect was attenuated to nearly zero under any relaxation of this assumption. This pattern of changing estimates suggests that the benefits of smoking bans may be overestimated if ongoing nonlinearity of trends in declining cardiovascular morbidity is not adequately taken into account. The findings further suggest that the benefits of smoking bans for cardiovascular disease may not extend to the elderly.
Central to the interpretation of these results is the extent to which short-term trends may be explained by alternative causes of a decrease in hospitalizations for AMI on the same timeframe as a postban reduction in cardiovascular morbidity, including the ongoing general decline in cardiovascular disease morbidity. National data show a curvilinear decline during the years of this study (32), with AMI hospitalization rates dropping by 23.4%, for years 2002 through 2007, among Medicare enrollees (33). However, studies addressing the linearity or nonlinearity in local and regional rates of decline are unavailable. Consequently, our modeling strategy tested for empirical evidence of departures from the assumption of linear secular trend in the 9 states following the ban. We assessed the sensitivity of our results to different modeling assumptions about the shape of underlying trends in admissions for AMI, finding that the effect depended primarily on the shape of the assumed time trend in the model. Although the curvilinear models could be attributing some of the effect of a ban to the general secular trend, these models provided the best fit for the secular trend observed in our data, even when considering preban data only. In addition, recent reports show that a linear model does not reflect the observed pattern of decline in the national population during the study period (32, 33). Flexible models were also found to be better fitting in a study of the impacts of smoking bans in Italy (22). However, most previous studies of smoking bans have assumed strict linearity in the secular trend without mention of the possibility of nonlinearity (25).
However, in our analysis, the estimated ban effect, pooled across states, is indistinguishable from zero under a nonlinear model of secular trend. Evidence based on the preban data suggests that the true temporal trend has a downward curvature in the age-pooled analysis. If the true temporal trend in hospital admissions was similar to a quadratic curve, then a linear model would result in a biased overestimate of the ban effect. Figure 2, which shows the downward curvature in AMI rates for Illinois, exemplifies such a scenario. Furthermore, the difference between the effects estimated in linear versus nonlinear models was especially large in states with significant quadratic trends in the preban data, suggesting a possible diagnostic tool. The possibility of attenuation under flexible modeling of secular trend has also been noted in a study of Italian smoking bans (22).
The percentage decrease in AMI rates postban compared with preban, pooled across states, was estimated to be approximately 5% when using a linear trend, which is smaller than estimates from previous studies in the general population. The largest effects have been observed in small US studies; a 27% reduction in AMI admissions was found in Pueblo, Colorado (13), a county of approximately 150,000 people, and a 40% reduction in AMI admission rates was associated with a smoking ban in Helena, Montana (17). European studies at the regional or national scale have found effects on the order of 7%–15% (10–12). To date, the largest US study on the effects of smoking bans in 62 counties within the state of New York found an 8% reduction in admissions for AMI (15). Two recent meta-analyses found reductions of approximately 17% in AMI admissions and 10% in admissions for acute coronary events (23, 30, 34).
We consider 2 potential factors that may contribute to the apparently discrepant findings in the cohort of Medicare enrollees, compared with prior reports. First, for older persons, the various places covered by smoking bans may contribute little to total personal exposure to secondhand smoke. Time spent in workplaces, bars and restaurants, and public places is generally more limited for older versus younger persons. In an Italian study, an 11% decline in AMI rates was found for persons aged under 60 years, but no statistically significant effect was found for those 60 years of age or older (10).
Second, publication bias may have led to the publication of some studies with larger and statistically significant effects. Many previous studies involved much smaller populations, and their results had large standard errors in comparison to the current study. Indeed, there has been a general trend of smaller estimated effects among more recent and larger studies (23). Some evidence of publication bias has also been observed (34), and, even if there were no impact of smoking bans, some of the large effects reported during early work could be due to chance (31).
We considered using data from other states, beyond the 9 in the present analysis, to provide additional support in accounting for unmeasured confounding. However, secular trends varied (almost always statistically significantly) from state to state. Furthermore, 15 of the 42 states (including the District of Columbia (DC)) not included in the analysis had Medicare populations below 200,000 enrollees. Finally, half of the states had partial bans during the study period or a ban prior to the study period. Taken collectively, these issues created significant challenges to identify counties that could act as a “control” (12, 13) and motivated us to use an interrupted time-series approach (11, 14, 15, 17, 21, 22, 31) instead by applying flexible models to only those states with comprehensive bans.
Our study has several strengths, particularly in comparison to prior studies. By studying a population of 6 million Medicare enrollees with a place of residence in the 387 counties that have implemented a smoking ban, we eliminate concerns inherent to small sample size. Also, by restricting the analysis to county-wide comprehensive bans that were not preceded by a ban covering any spatial subset within the county, we avoid any dilution of the effect by a prior ban. Finally, we carefully assessed the sensitivity of results to the assumptions made in the statistical analysis.
Although our results do not provide statistically significant evidence of a smoking ban-related decrease in AMI hospital admission rates in the Medicare population, there is already substantial evidence that smoking bans can benefit the general public health (25). This study, using a Medicare-based cohort, suggests that any reduction of risk for AMI following a ban may be limited among older Americans.
ACKNOWLEDGMENTS
Author affiliations: Biostatistics Department, Harvard School of Public Health, Boston, Massachusetts (Christopher D. Barr, David M. Diez, Yun Wang, Francesca Dominici); and Department of Preventative Medicine, University of Southern California Keck School of Medicine, Los Angeles, California (Jonathan M. Samet).
Funding for C. D. B., D. M. D., Y. W., and F. D. was provided by grants RD83362201 and RD83241701 from the US Environmental Protection Agency and by grant R01 ES012054 from the National Institutes of Health/National Institute of Environmental Health Sciences. Funding for C. D. B., D. M. D., and F. D. was provided by award P01CA134294 from the National Cancer Institute.
The authors would like to acknowledge useful comments from Dr. Nan M. Laird and Dr. Sebastien J. P. A. Haneuse. Drs. Laird and Haneuse are professors of biostatistics at the Harvard School of Public Health; they were not compensated for their input.
The funding agencies/sponsors listed above had no involvement in the design and conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript. This study has not been subjected to the US Environmental Protection Agency's required peer and policy review and, therefore, it does not necessarily reflect the views of the agency and no official endorsement should be inferred. The contents of this article are solely the responsibility of the authors and do not necessarily reflect the views and policies of the National Cancer Institute or the National Institute of Environmental Health Sciences of the National Institutes of Health.
Conflict of interest: none declared.
REFERENCES
- 1.US Public Health Service. Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington, DC: Office of the Surgeon General, US Public Health Service; 1964. (DHEW publication no. (PHS) 1103) [Google Scholar]
- 2.US Department of Health and Human Services. The Health Consequences of Involuntary Smoking: A Report of the Surgeon General. Rockville, MD: Office of Smoking and Health, Department of Health and Human Services; 1986. [Google Scholar]
- 3.Law MR, Morris JK, Wald NJ. Environmental tobacco smoke exposure and ischaemic heart disease: an evaluation of the evidence. BMJ. 1997;315:973–980. doi: 10.1136/bmj.315.7114.973. doi:10.1136/bmj.315.7114.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. doi:10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: Office of Smoking and Health, US Department of Health and Human Services; 2006. [PubMed] [Google Scholar]
- 6.Glantz SA, Parmley WW. Passive smoking and heart disease. Circulation. 1991;83(1):1–12. doi: 10.1161/01.cir.83.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Lightwood JM, Coxson PG, Bibbins-Domingo K, et al. Coronary heart disease attributable to passive smoking: CHD policy model. Am J Prev Med. 2009;36(1):13–20. doi: 10.1016/j.amepre.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Argacha JF, Adamopoulos D, Gujic M, et al. Acute effects of passive smoking on peripheral vascular function. Hypertension. 2008;51(6):1506–1511. doi: 10.1161/HYPERTENSIONAHA.107.104059. [DOI] [PubMed] [Google Scholar]
- 9.American Nonsmokers’ Rights Foundation. Berkeley, CA: American Nonsmokers’ Rights Foundation; 2011. US tobacco control laws database: research applications. (http://www.no-smoke.org/pdf/USTobaccoControlLawsDatabase2010.pdf. ). (Accessed May 5, 2011) [Google Scholar]
- 10.Barone-Adesi F, Vizzini L, Merletti F, et al. Short-term effects of Italian smoking regulation on rates of hospital admission for acute myocardial infarction. Eur Heart J. 2006;27(20):2468–2472. doi: 10.1093/eurheartj/ehl201. [DOI] [PubMed] [Google Scholar]
- 11.Cesaroni G, Forastiere F, Agabiti N, et al. Effect of the Italian smoking ban on population rates of acute coronary events. Circulation. 2008;117:1183–1188. doi: 10.1161/CIRCULATIONAHA.107.729889. doi:10.1161/CIRCULATIONAHA.107.729889. [DOI] [PubMed] [Google Scholar]
- 12.Pell JP, Haw S, Cobbe S, et al. Smoke-free legislation and hospitalizations for acute coronary syndrome. N Engl J Med. 2008;359(5):482–491. doi: 10.1056/NEJMsa0706740. [DOI] [PubMed] [Google Scholar]
- 13.Bartecchi C, Alsever RN, Nevin-Woods C, et al. Reduction in the incidence of acute myocardial infarction associated with a citywide smoking ordinance. Circulation. 2006;114(14):1490–1496. doi: 10.1161/CIRCULATIONAHA.106.615245. [DOI] [PubMed] [Google Scholar]
- 14.Dove MS, Dockery DW, Mittleman MA, et al. The impact of Massachusetts’ smoke-free workplace laws on acute myocardial infarction deaths. Am J Public Health. 2010;100(11):2206–2212. doi: 10.2105/AJPH.2009.189662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juster HR, Loomis BR, Hinman TM, et al. Declines in hospital admissions for acute myocardial infarction in New York State after implementation of a comprehensive smoking ban. Am J Public Health. 2007;97(11):2035–2039. doi: 10.2105/AJPH.2006.099994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemstra M, Neudorf C, Opondo J. Implications of a public smoking ban. Can J Public Health. 2008;99(1):62–65. doi: 10.1007/BF03403743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sargent RP, Shepard RM, Glantz SA. Reduced incidence of admissions for myocardial infarction associated with public smoking ban: before and after study. MJ. 2004;328:977–980. doi: 10.1136/bmj.38055.715683.55. doi:10.1136/bmj.38055.715683.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruintjes G, Bartelson BB, Hurst P, et al. Reduction in acute myocardial infarction hospitalization after implementation of a smoking ordinance. Am J Med. 2011;124(7):647–654. doi: 10.1016/j.amjmed.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Gupta R, Luo J, Anderson RH, et al. Clean indoor air regulation and incidence of hospital admissions for acute coronary syndrome in Kanawha County, West Virginia. Prev Chronic Dis. 2011;8(4):A77. http://www.cdc.gov/pcd/issues/2011/jul/10_0200.htm. ). (Accessed May 5, 2011) [PMC free article] [PubMed] [Google Scholar]
- 20.Sims M, Maxwell R, Bauld L, et al. Short term impact of smoke-free legislation in England: retrospective analysis of hospital admissions for myocardial infarction. BMJ. 2010;340:c2161. doi: 10.1136/bmj.c2161. doi:10.1136/bmj.c2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barone-Adesi F, Gasparrini A, Vizzini L, et al. Effects of Italian smoking regulation on rates of hospital admission for acute coronary events: a country-wide study. PLoS ONE. 2011;6(3):e17419. doi: 10.1371/journal.pone.0017419. doi:10.1371/journal.pone.0017419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasparrini A, Gorini G, Barchielli A. On the relationship between smoking bans and incidence of acute myocardial infarction. Eur J Epidemiol. 2009;24(10):597–602. doi: 10.1007/s10654-009-9377-0. [DOI] [PubMed] [Google Scholar]
- 23.Mackay DF, Irfan MO, Haw S, et al. Meta-analysis of the effect of comprehensive smoke-free legislation on acute coronary events. Heart. 2010;96(19):1525–1530. doi: 10.1136/hrt.2010.199026. [DOI] [PubMed] [Google Scholar]
- 24.Farrelly MC, Nonnemaker JM, Chou R, et al. Changes in hospitality workers’ exposure to secondhand smoke following the implementation of New York's smoke-free law. Tob Control. 2005;14(4):236–241. doi: 10.1136/tc.2004.008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Institute of Medicine. Secondhand Smoke Exposure and Cardiovascular Effects: Making Sense of the Evidence. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 26.Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Pharm Ther. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Bates D, Maechler M. Berkeley, CA: Comprehensive R Archive Network, University of California; 2010. lme4: linear mixed-effects models using S4 classes, R package version 0.999375-34. (http://cran.r-project.org/src/contrib/Archive/lme4/ ). (Accessed May 5, 2011) [Google Scholar]
- 29.R Development Core Team. R Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 30.Lightwood JM, Glantz SA. Declines in acute myocardial infarction after smoke-free laws and individual risk attributable to secondhand smoke. Circulation. 2009;120(14):1373–1379. doi: 10.1161/CIRCULATIONAHA.109.870691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shetty KD, DeLeire T, White C, et al. Changes in U.S. hospitalization and mortality rates following smoking bans. J Policy Anal Manage. 2011;30(1):6–28. doi: 10.1002/pam.20548. [DOI] [PubMed] [Google Scholar]
- 32.Luepker RV, Berger AK. Is acute myocardial infarction disappearing? Circulation. 2010;121(11):1280–1282. doi: 10.1161/CIR.0b013e3181d98478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Normand ST, Wang Y, et al. Recent declines in hospitalizations for acute myocardial infarction for Medicare fee-for-service beneficiaries. Circulation. 2010;121:1322–1328. doi: 10.1161/CIRCULATIONAHA.109.862094. doi:10.1161/CIRCULATIONAHA.109.862094. [DOI] [PubMed] [Google Scholar]
- 34.Meyers DG, Neuberger JS, He J. Cardiovascular effect of bans on smoking in public places. J Am Coll Cardiol. 2009;54(14):1249–1255. doi: 10.1016/j.jacc.2009.07.022. [DOI] [PubMed] [Google Scholar]