Introduction
The concurrent rise of opiate abuse and undertreated pain continues to drive interest in non-invasive brain stimulation techniques that may diminish pain [1; 51; 68] One brain region that has emerged as a target of such stimulation is the dorsolateral prefrontal cortex (DLFPC). Studies have shown that stimulating DLPFC with transcranial direct current stimulation (tDCS) or repetitive transcranial magnetic stimulation (rTMS) reduces experimentally-induced pain [14; 54; 61] as well as chronic pain [5; 18; 46]. In two different postoperative studies, patients who received a single session of left DLPFC rTMS after gastric bypass surgery self-administered 40% less morphine than controls who received sham stimulation [17; 20]. Despite these positive results, there are conflicting reports about the extent to which rTMS produces a clinically relevant pain reduction [55]. An important step toward evaluating DLPFC rTMS as a potential analgesic intervention is to determine the pharmacological mechanisms by which it produces analgesia.
The periaqueductal gray (PAG) and rostral ventromedial medulla (RVM) are critical components of a supraspinal opioidergic circuit (SOC) that modulates pain, the subjective experience frequently linked to nociception [7; 8; 33; 49]. There are several lines of indirect evidence that cingulofrontal regions like DLPFC or ACC can drive top-down analgesia via gain modulation of the SOC. These include (a) anatomical connectivity as evidenced by retrograde tracing [4; 22] and fos immunoreactivity [48] in laboratory animals and diffusion tensor imaging [37] in humans, (b) physiological connectivity as evidenced by electrical or chemical stimulation in rodents, which alter PAG-RVM physiology and induce analgesia in an intensity- and dose-dependent manner [24; 39], (c) pharmacological connectivity as evidenced by the shared capacity for opioid release, opiate binding and opiate-mediated activation [10; 13; 60], and (d) functional connectivity as evidenced by naloxone reversible blood oxygen level dependent (BOLD) signal coupling during placebo analgesia [32; 57].
A recent study found that naloxone pretreatment significantly reduced right motor cortex (M1) rTMS-induced analgesia but did not affect right DLPFC rTMS-induced analgesia [26]. It is important to continue probing the pharmacological mechanisms of DLPFC-induced analgesia given the potential for a prefrontal-midbrain-brainstem circuit and the lack of clarity about functional lateralization in the prefrontal cortex (PFC). The purpose of the present study was to determine whether pretreatment with a μ-opioid antagonist inhibits left DLPFC rTMS-induced analgesia.
Materials and Methods
This study was approved by the Institutional Review Board at the Medical University of South Carolina. All participants signed a written informed consent prior to participation.
Study Design
Twenty-four (24) healthy volunteers between 18-45 years of age were recruited to participate in this double sham-controlled, double-blind, crossover study. All participants were naïve to TMS prior to enrollment. Qualified participants were randomly assigned to receive real or sham left DLPFC rTMS. Within each arm, participants were then randomized to receive IV naloxone (0.1 mg/kg) or saline during their first experimental trial. One week later, participants returned for a second experimental trial in which they received the same TMS treatment but the opposite IV infusion. The number of participants randomly assigned to each group was determined by power calculation based on data from a previous study in the lab [19].
Screening Procedures
Prospective participants (self-referred via flyers and e-mail announcements) were interviewed by the PI over the phone. Exclusion criteria were used to eliminate participants with conditions that might confound the research or put them at risk for an adverse event. Participants met the following criteria: (1) 18-45 years of age, (2) no seizure history (individual or family), (3) no history of depression, (4) no hospitalizations or surgeries in the previous 6 months, (5) not currently experiencing pain, (6) no history of chronic pain, (7) no metal implant above the waste (e.g. pacemakers, metal plates, wires, (8) not pregnant, (9) no alcohol dependence (10) no illicit drug use in the previous 6 months, (11) no known allergy to capsaicin, (12) no history of brain surgery or brain lesions, (13) no history of loss of consciousness (greater than 15 min), (14) no stimulants or medications that lower seizure threshold, (15) negative drug screen for opiate use. Individuals who phone qualified for the study were invited to a screening visit during which risks and benefits were explained. After providing written informed consent to participate, each participant submitted a urine sample tested for opiates. Female participants’ urine was also screened for pregnancy hormones.
Resting Motor Threshold Assessment
Resting motor threshold (rMT) assessment was performed using a Neuronetics TMS machine with an iron-core, solid-state figure-of-8 coil (Model 2100, Neuronetics Inc; Malvern, PA). The TMS machine was initially set to 65% of its maximal output. The coil was systematically moved until the area on the scalp that produced contraction of adbuctor pollicis brevis (APB) was identified. Custom-developed software that employs adaptive parameter estimation by sequential testing (PEST) data was used to determine rMT, or the minimum machine output necessary for visible APB contraction 50% of the time that pulses were delivered [15]. Once rMT was determined, the location on the scalp that corresponds to BA9 of left DLPFC was found using the Beam F3 method [9].
Behavioral Measures
Baseline pain assessments were performed using cutaneous hot and cold stimuli via a 30 × 30 mm ATS thermode on the Medoc Pathway System (Israel). There were two behavioral measures: quantitative sensory testing (QST) on untreated skin and blocks of pain on capsaicin-treated skin.
QST was performed via method of limits testing on the right thenar eminence. A program was designed to randomly order five hot and five cold stimuli with random durations (5-35 sec) between each trial. The ATS thermode began at 32°C and increased or decreased by 0.5°C per sec. Participants were instructed to press a button in order to signify three different measures: (1) sensory threshold, or the temperature at which the stimulus became noticeable, (2) pain threshold, or the temperature at which the stimulus became painful, and (3) pain tolerance, or the temperature at which the stimulus became unbearable. The device was set to stop heating at 51.5°C and stop cooling at 0°C to avoid tissue damage.
Block testing was done with a slightly adapted model of hot allodynia [56]. First, 1% capsaicin cream was applied to a 40 × 40 mm region of skin 12 cm away from the wrist on the right volar forearm. After 30 min, the capsaicin was cleaned off and cutaneous heat stimuli were applied to the capsaicin-treated skin via the ATS thermode. Pain was assessed by administering 22s blocks of fixed temperatures. After each trial, participants rated pain unpleasantness and intensity using an 11-point (0 to 10) rating scale. Multiple trials were conducted at baseline until the participant consistently rated one temperature as “7 out of 10” in intensity. Studies have shown that stimuli rated as 7 out of 10 produce highly reproducible results in terms of pain ratings and fMRI activation without posing significant risk to participants [27]. This temperature was administered in 3 separate 22 sec trials with 30 s between each trial 0, 20 and 40 min after TMS.
Intravenous Drug Infusion and TMS Procedure
After baseline pain measurements, a bolus of 0.1mg/kg naloxone or the equivalent amount of saline was blindly infused intravenously into the participant’s left arm. Naloxone was chosen over oral μ-opioid antagonists like naltrexone because naloxone has consistent bioavailability, immediate onset and short duration. Naloxone has also been shown to be safe to administer to healthy volunteers and drug-naïve subjects, producing very little obvious side effects [21; 40]. The saline infusion was designed to control for placebo effects. Five min after naloxone or saline infusion, participants blindly received either 20 min of left DLPFC TMS (10 Hz, 110% RMT, 5 s on, 10 s off) or 20 min of left DLPFC sham stimulation. The eSham system was implemented in conjunction with a specialized Neuronetics sham TMS coil. Two Thymapad Stimulus Electrodes (Somatics, LLC; Lake Bluff, IL) were placed on the scalp under the TMS coil. All participants had electrodes placed on the scalp location that corresponded to left DLPFC, but only those assigned to sham received electrical stimulation through them. Studies have shown that this sham system effectively blinds participants to TMS treatment (active versus sham) [16]. Following real or sham TMS, QST and block pain ratings were performed at 0, 20 and 40 min.
Unblinded nurses who were not present during TMS administration, data acquisition or data analysis prepared the saline or naloxone syringes according to a randomization chart. These individuals had minimal interaction with study participants. Similarly, the real or sham TMS coil was setup by a research assistant who was otherwise not involved in the study. This arrangement allowed the Principal Investigator (JJT), who collected all pain measurements and ran all study sessions, to remain blind to both medication and TMS allocation. The participants were also blinded and were not told of their TMS or medication randomization status. At the end of the experiment, each participant was asked to guess whether they had received real TMS or sham TMS or if they could not make a guess and had no idea which they had received.
Statistical Analyses
For all QST measures, the first hot and cold trial within each time point was discarded to control for novelty effects and reduce the variability associated with Aδ fiber activation [58]. All data were reviewed for quality prior to being locked for statistical analysis after unblinding. Results are expressed as mean temperature detected or tolerated (Celsius) or rating ± SD. Analysis of variance (ANOVA) tests were implemented using IBM SPSS Statistics Version 19 (New York, NY). One-way and mixed model ANOVAs were used to examine within- and between-group effects with Bonferroni adjustments for post hoc analyses.
Results
No reportable adverse events were produced by this protocol. One participant had to cease participation in the study after a brief syncopal episode during venipuncture prior to drug or TMS administration. Data from this individual were not used in the subsequent analyses.
Demographics
There were no significant differences between the real and sham TMS groups in terms of age (p = 0.42; student’s t-tests, two-tailed), gender (p = 0.44), handedness (p = 1.00), or race (p = 0.56; student’s t-tests, two-tailed) (Table 1).
Table 1.
Demographic data of the study sample. Twenty four (24) healthy volunteers were randomized into two groups that did not differ significantly from one another.
| Real TMS (n=12) Mean (SD) |
Sham TMS (n=12) Mean (SD) |
p | ||
|---|---|---|---|---|
| Age | 0.42 | |||
|
| ||||
| Average (years) | 24.33 (1.78) | 25.3 (3.80) | ||
| Range | 21-28 | 20-33 | ||
|
| ||||
| Sex | 0.44 | |||
|
| ||||
| Male | 5 | 7 | ||
| Female | 7 | 5 | ||
|
| ||||
| Handedness | 1.00 | |||
|
| ||||
| Right | 9 | 9 | ||
| Left | 3 | 3 | ||
|
| ||||
| Weight | 0.18 | |||
|
| ||||
| Average (lbs) | 156.58 (23.86) | 178.25 (48.04) | ||
| Range | 120-195 | 132-310 | ||
|
| ||||
| Race | 0.56 | |||
|
| ||||
| African American | 2 | 1 | ||
| Caucasian | 10 | 11 | ||
Resting Motor Threshold Assessment
There were no significant differences in resting motor threshold between the real and sham TMS groups (p = 0.86) or within the real TMS group between saline and naloxone pretreatment (p = 0.39; student’s t-tests, two-tailed).
Baseline Pain Comparisons
There were no significant differences in any of the baseline pain measures between saline-real TMS, saline-sham TMS, naloxone-real TMS, and naloxone-sham TMS treatment groups.
TMS Blind Integrity
As a group, participants were successfully blind to TMS treatment condition. When asked if they had received real or sham TMS, 6 participants guessed correctly, 7 participants guessed incorrectly and 11 had “no idea”. Of those who guessed about treatment condition,4 out of 8 participants successfully reported that they had received real TMS and 2 out of 5 participants successfully reported that they had received sham TMS [χ2(1) = 0.12, not significant]. Thus, the success rate for correctly identifying the TMS treatment condition was no better than chance.
Effect of Real versus Sham TMS
Acute Pain Model: Quantitative Sensory Testing
The mean hot pain threshold for participants in the saline-sham TMS group was 46.71 (2.34) at baseline, 46.26 (2.21) at time 0, 46.22 (2.38) at time 20 and 46.51 (2.30) at time 40. There were no significant within-group effects in the saline-sham TMS treatment group. The mean hot pain threshold for participants in the saline-real TMS treatment group was 44.84°C (2.76) at baseline, 46.13 (2.62) at time 0, 47.47 (1.70) at time 20, and 47.82 (1.82) at time 40. A within-group one-way ANOVA with Bonferroni correction revealed significant effects for real TMS on hot pain threshold at time 20 (p = 0.41) and time 40 (p = 0.014) when compared to baseline. A between-group mixed model ANOVA revealed a significant effect for time (F[3,66.29]= 9.34, p < 0.001) and a group*time interaction (F[3,66.29]= 13.52, p < 0.001) for hot pain threshold when saline-real TMS treatment was compared to saline-sham TMS treatment (Figure 1). Post hoc analyses with Bonferroni adjustment indicate that there were significant differences in hot pain threshold between the two treatment conditions when baseline was compared to time 20 (df = 66.29, p = 0.007) and time 40 (df = 66.29, p < 0.001). There were also significant differences in hot pain tolerance between the two treatment conditions when baseline was compared to time 20 (df = 65.97, p = 0.027) and time 40 (df = 65.97, p = 0.018).
Figure 1.
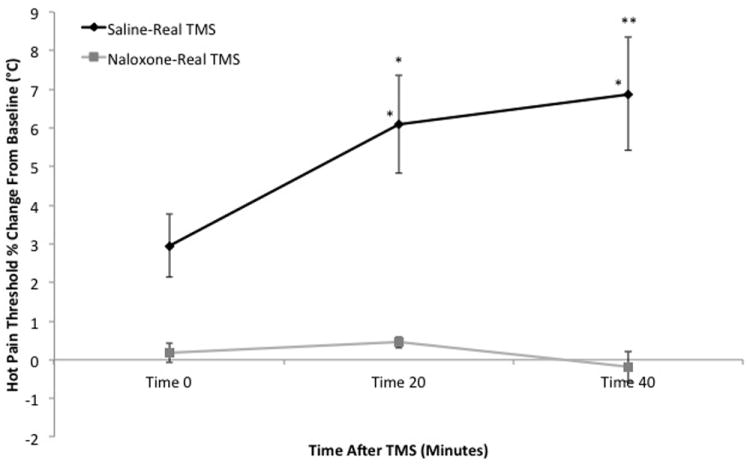
Comparison of hot pain thresholds (mean percent change from baseline ± SEM) over time. The black line represents saline-real TMS while the gray line represents naloxone-real TMS. Asterisks (* p < 0.05, ** p < 0.001) indicate significant baseline comparisons within-groups (immediately above data series) and between-groups (immediately above SEM bars).
Hot Allodynia Model: Block Testing
The saline-sham TMS group rated the mean temperature of 43.08 (3.26) at an intensity of 7.01 (0.25) at baseline, 6.86 (0.61) at time 0, 6.92 (0.43) at time 20 and 6.86 (0.33) at time 40. A within-group one-way ANOVA with Bonferroni correction found no significant within-group effects in the saline-sham TMS treatment group. In contrast, the saline-real TMS group rated the mean temperature of 42.33°C (2.64) at an intensity of 6.92 (0.78) at baseline, 4.42 (0.79) at time 0, 4.47 (0.95) at time 20 and 4.22 (0.75) at time 40. A within-group one-way ANOVA with Bonferroni correction revealed significant effects for real TMS on pain intensity at all three post-TMS time points (p < 0.001). Identical results were found with pain unpleasantness at each time point. A between-group mixed model ANOVA revealed a significant effect for group (F[1,22.00] = 66.40, p < 0.001), time (F[3,66.00] = 60.34, p < 0.001) and a group*time interaction (F[3,66.00] = 51.36, p < 0.001) for pain intensity when saline-real TMS treatment was compared to saline-sham TMS treatment. Post hoc analyses with Bonferroni adjustment indicate that there were significant differences in pain intensity between the two treatment conditions when baseline was compared to all three post-TMS time points (df = 66.00, p < 0.001). Identical results were found with pain unpleasantness. Thus, real TMS significantly reduced acute pain and hot allodynia ratings.
Effect of Naloxone versus Saline Pretreatment on Real TMS
Acute Pain Model: Quantitative Sensory Testing
The mean hot pain threshold for participants in the naloxone-real TMS treatment group was 45.25 (2.29) at baseline, 45.33 (2.43) at time 0, 45.45 (2.24) at time 20 and 45.17 (2.32) at time 40. A within-group one-way ANOVA with Bonferroni correction revealed no significant within-group effects in the naloxone-real TMS treatment (Figure 1). A between-group mixed model ANOVA revealed a significant effect for time (F[3,66.03]= 14.41, p < 0.001) and a group*time interaction (F[3,66.03]= 14.40, p < 0.001) for hot pain threshold when saline-real TMS treatment was compared to naloxone-real TMS treatment. Post hoc analyses with Bonferroni adjustment indicate that there were significant differences in hot pain threshold between the two treatment conditions when baseline was compared to time 0 (df = 66.03, p = 0.22), time 20 (df = 66.03, p < 0.001) and time 40 (df = 66.03, p < 0.001). There were also significant differences in hot pain tolerance between the two treatment conditions when baseline was compared to time 0 (df = 66.43, p = 0.002) and time 40 (df = 66.43, p = 0.009).
Hot Allodynia Model: Block Testing
In the naloxone-real TMS treatment group, the mean temperature of 42.58 (2.64) was rated at an intensity of 6.67 (1.06) at baseline, 6.56 (1.09) at time 0, 6.56 (1.05) at time 20 and 6.39 (0.99) at time 40. A within-group one-way ANOVA with Bonferroni correction revealed no significant within-group effects in the naloxone-real TMS treatment (Figure 2). A between-group mixed model ANOVA revealed a significant effect for group (F[1,22.06] = 16.28, p = 0.001), time (F[3,66.00] = 66.04, p < 0.001) and a group*time interaction (F[3,66.00] = 66.04, p < 0.001) for pain intensity when saline-real TMS treatment was compared to naloxone-real TMS treatment. Post hoc analyses with Bonferroni adjustment indicate that there were significant differences in pain intensity between the two treatment conditions when baseline was compared to all three post-TMS time points (df = 66.04, p < 0.001). Identical results were found with pain unpleasantness. Thus, naloxone pretreatment significantly reduces the analgesic effects of real TMS.
Figure 2.
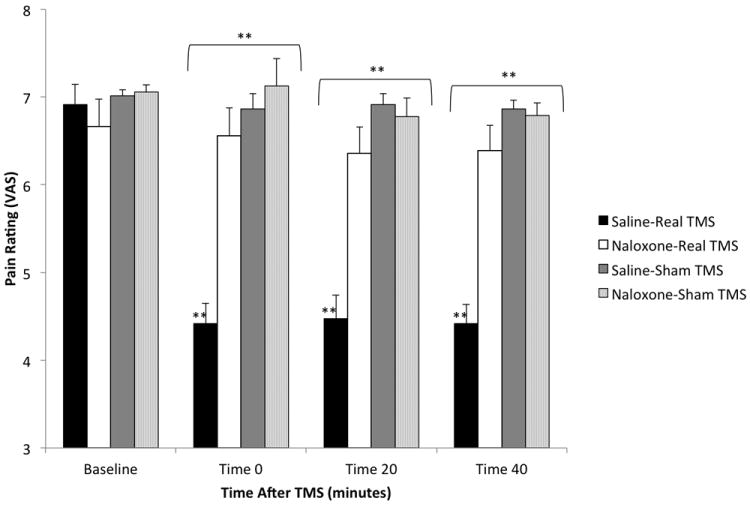
Comparison of block pain intensity ratings (mean ± SEM). Twenty-two (22) sec blocks of a fixed temperature reported as an intensity of “7” on a VAS (1-10) at baseline were applied to capsaicin-sensitized skin in three consecutive trials at 0, 20 and 40 min after TMS with pretreatment. Asterisks (* p < 0.05, ** p < 0.001) indicate significant baseline comparisons within groups (immediately above data series) and between-groups (immediately above brackets).
Effect of Naloxone versus Saline Pretreatment on Sham TMS
Acute Pain Model: Quantitative Sensory Testing
The mean hot pain threshold for participants in the naloxone-sham group was 46.78 (2.86) at baseline, 46.54 (2.30) at time 0, 46.76 (2.96) at time 20 and 46.55 (2.82) at time 40. There were no significant within-group effects in the naloxone-sham TMS treatment group, nor were there any significant between-group effects when this group was compared to the saline-sham TMS treatment group.
Hot Allodynia Model: Block Testing
In the naloxone-sham TMS treatment group, the mean temperature of 43.92 (3.26) was rated at an intensity of 7.06 (0.29) at baseline, 7.13 (1.09) at time 0, 6.78 (0.74) at time 20 and 6.79 (0.49) at time 40. There were no significant within-group effects in the naloxone-sham TMS treatment group, nor were there any significant between-group effects when this group was compared to the saline-sham TMS treatment. Thus, naloxone pretreatment had no effect on sham TMS.
Discussion
Naloxone Reduces Left DLPFC rTMS-induced Analgesia
The results of this study support the pre-study hypothesis that naloxone pretreatment would diminish the analgesic effects of left DLPFC rTMS. While the precise level of opioid blockade by naloxone is unknown, there is evidence to suggest that the dose used in this study was likely sufficient to block the behavioral and neurophysiological effects of endogenous opioids. A number of previous studies used comparatively smaller doses of naloxone to block endogenous opioid analgesia and induce BOLD signal changes in cortical and subcortical brain regions implicated in pain processing [21; 69]. In this study we found that naloxone pretreatment, at this dose and in a bolus and without redosing, effectively blocked TMS-induced analgesia.
What remains less clear is whether endogenous opioids are exclusively responsible for the maintenance of DLPFC rTMS-induced analgesia. The biological half-life of naloxone has historically been reported as 60-90 minutes [11; 35]. The fact that behavioral testing occurred within 60 minutes of pretreatment suggests that the majority of naloxone had not yet lost its pharmacologic activity. Nevertheless, there is a possibility that reduced opioid blockade explains the consistent yet statistically insignificant reduction in hot allodynia pain ratings in the naloxone-real group compared to naloxone-sham group (Figure 2). An alternative explanation for this residual analgesia is that a critical amount of endogenous opioid substrate was released, thereby partially displacing the competitive μ-opioid antagonist. The only published study to test the opioid sensitivity of PFC TMS featured a supplementary naloxone infusion following the initial bolus [26]. This technique was presumably intended to maintain constant plasma levels of naloxone for an extended period of time [62]. There are conflicting reports about the necessity of secondary infusions to achieve sustained opioid blockade [3; 21]. Moreover, the de Andrade et al. secondary infusion likely provided no long-term advantage because it was completed 60 minutes prior to 15 minutes of QST testing.
The lack of certainty about sustained opioid blockade also raises the possibility that residual analgesia in the naloxone-real group is explained by non-opioidergic mechanisms. In other words, endogenous opioid activity may be necessary but not sufficient for the maintenance of DLPFC rTMS-induced analgesia. This is not an unreasonable idea since neuropeptides are often co-localized with classic neurotransmitters and released in response to high frequency stimulation [67]. While it is difficult to speculate about the neuropharmacology that might work in conjunction with opioids to maintain DLFPC rTMS-induced analgesia, it is reasonable to suspect that anterior cingulate and insular cortex are involved given their role in pain processing and their PFC connectivity [6; 13; 27]
The Possibility of Functional Lateralization in DLPFC Pain Processing
This is the first study to demonstrate that endogenous opioids mediate PFC rTMS-induced analgesia. A previous study showed that inhibiting DLPFC with low-frequency rTMS blocks placebo analgesia, but there was no evidence that exciting DLPFC with the type of high-frequency rTMS that diminishes pain in clinical trials induces the release of endogenous opioids [44]. The de Andrade et al. study showed that naloxone pretreatment significantly reduced analgesia produced by right M1 stimulation but had no effect on analgesia produced by right DLPFC stimulation [26]. There are a number of differences between the de Andrade et al. study and the current study, including dose and location of TMS as well as type of pain measured. In the de Andrade et al. study, the authors delivered 62.5% fewer pulses (1500) at 20% lower intensity (80% rMT) than the dose of TMS previously shown to diminish postoperative morphine self-administration [17; 20]. The extent to which different TMS dosing paradigms produce different brain effects remains unclear. From a clinical standpoint, it is reasonable to suspect that larger TMS doses might be necessary to counteract postoperative sedation caused by decreased cortical, thalamic and brainstem activity [23]. As demonstrated by interleaved TMS-fMRI in healthy volunteers, larger TMS doses at suprathreshold intensity more effectively activate proximal targets and are more likely to propagate along efferent connections to distal targets like the SOC [12]. While inconclusive, these data raise the possibility that larger doses of DLPFC rTMS more effectively recruit the SOC or release endogenous opioids.
Another important difference between the de Andrade et al. study and the current study is laterality of stimulation. Naloxone pretreatment had no effect on right DLPFC rTMS-induced analgesia in the de Andrade et al. study but significantly reduced left DLPFC rTMS-induced analgesia in the current study. Taken together, these results suggest that opioidergic analgesia is mediated by left, but not right, DLPFC activation. It is difficult to evaluate the validity of this suggestion given the limited data on PFC functional lateralization. In one positron emission tomography (PET) study, elevated left DLPFC activity correlated negatively with perceived intensity and unpleasantness during heat allodynia. Further analyses revealed that left DLPFC activity was associated with diminished inter-regional correlation between midbrain and medial thalamus. Right DLPFC activity, by contrast, was associated with a weakened relationship between anterior insula and pain intensity [50]. This study provides some evidence that left DLPFC activity is linked to midbrain function during hot allodynia, but more experiments are needed to evaluate the laterality of pain processing.
The final difference between the de Andrade et al. study and the current study is the type of pain measured. Whereas the de Andrade et al. study measured acute cold pain, the current study measured acute hot and cold pain as well as hot allodynia. In the current study, real TMS had no significant effect on cold pain perception. This lack of significance is probably the result of floor effects and habituation rather than TMS selectively affecting hot pain perception. Differences in location and dose of stimulation, however, make it difficult to compare cold pain results between the two studies. Human and animal studies alike have implicated opioidergic midbrain and brainstem regions in allodynia and pain from hot stimuli lasting 20 seconds or longer [13; 29; 71], but these data do not explain why hot pain threshold and tolerance were affected by left DLPFC rTMS in the current study. It has been noted that the heterogeneity of experimentally induced pain between studies might explain the heterogeneity of data on DLPFC rTMS analgesia [34]. Clearly, more studies are needed to reveal how different types of pain are affected by TMS.
Evidence for Functional Connectivity between DLPFC and the SOC
The evidence for connectivity between DLPFC and the SOC in humans is limited to PET and functional magnetic resonance imaging (fMRI) studies that demonstrate time-correlated elevations in tracer or BOLD signal, respectively, between these two regions. Placebo analgesia, for example, is strongly associated with a naloxone-reversible increase in DLPFC and PAG activity [32; 65; 66]. This finding is corroborated by a meta-analysis of 162 neuroimaging studies in which co-activation of BA9 and PAG was found to be essential for assigning emotional valence, a process that contributes to noxious stimulus interpretation [42]. While these types of imaging studies are useful, they fail to provide the level of compelling circuit-based evidence found in animal studies because they rely on nonspecific cognitive processes instead of electrical or chemical stimulation to drive changes in surrogate markers of neuronal activity. Without focal stimulation to test directionality, knowledge of brain networks is limited to functional connectivity based on time correlations. Even authors that use such evidence to argue that DLPFC exerts a top-down influence on subcortical opioidergic regions acknowledge the need for an “interventional approach” [50]. Interleaved TMS-fMRI studies are needed to ultimately confirm or reject the involvement of the SOC in DLPFC rTMS-induced analgesia
Prefrontal rTMS: An Interventional Approach to Pain Circuitry
From a basic science standpoint, the current results reveal important clues about the interface between cognition, nociception and pain. Attention, mood and expectations are among the executive functions associated with DLPFC that tailor pain in a context-dependent manner [2; 50; 63]. Before this study, however, there was limited evidence that DLPFC stimulation drove opioidergic pain relief in the human brain. The capacity for the highly evolved PFC to recruit the phylogenetically conserved SOC [32; 53; 71] might be the evolutionary substrate for low fidelity pain perception. From a translational standpoint, the current results support existing theories that endogenous opioids play a role in pain conditions [38; 41; 43; 47] and stress-related psychiatric disorders like depression, post-traumatic stress disorder and panic disorder [25; 28; 30; 31; 36; 45; 52; 59] that frequently involve DLPFC. Using TMS as an interventional tool to understanding the circuitry underlying these conditions may ultimately reveal new therapeutic applications of TMS. From a clinical standpoint, the current results lend credibility to the use of DLPFC rTMS as an adjunctive therapy for pain. More studies are needed to determine if left DLPFC rTMS produces a synergistic effect with opiates or if it can be used in opiate addiction treatment. The importance of these clinical possibilities cannot be overstated given rising opiate abuse and the extent to which chronic and postoperative pain remains undertreated [64; 70].
Acknowledgments
The authors would like to thank Matt Schmidt, MS, Kathryn Beaver, RN, Tanya Miller, RN, and Colleen Hanlon, PhD, for their assistance in conducting this study.
Footnotes
The authors declare no conflict of interest related to this study.
References
- 1.Emergency department visits involving nonmedical use of selected prescription drugs - United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2010;59(23):705–709. [PubMed] [Google Scholar]
- 2.Akitsuki Y, Decety J. Social context and perceived agency affects empathy for pain: an event-related fMRI investigation. Neuroimage. 2009;47(2):722–734. doi: 10.1016/j.neuroimage.2009.04.091. [DOI] [PubMed] [Google Scholar]
- 3.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(1):484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An X, Bandler R, Ongur D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401(4):455–479. [PubMed] [Google Scholar]
- 5.Arul-Anandam AP, Loo C, Martin D, Mitchell PB. Chronic neuropathic pain alleviation after transcranial direct current stimulation to the dorsolateral prefrontal cortex. Brain stimulation. 2009;2(3):149–151. doi: 10.1016/j.brs.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978;4(5):451–462. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- 8.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 9.Beam W, Borckardt JJ, Reeves ST, George MS. An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul. 2009;2(1):50–54. doi: 10.1016/j.brs.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becerra L, Harter K, Gonzalez RG, Borsook D. Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volunteers. Anesth Analg. 2006;103(1):208–216. doi: 10.1213/01.ane.0000221457.71536.e0. table of contents. [DOI] [PubMed] [Google Scholar]
- 11.Berkowitz BA. The relationship of pharmacokinetics to pharmacological activity: morphine, methadone and naloxone. Clin Pharmacokinet. 1976;1(3):219–230. doi: 10.2165/00003088-197601030-00004. [DOI] [PubMed] [Google Scholar]
- 12.Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Subthreshold high-frequency TMS of human primary motor cortex modulates interconnected frontal motor areas as detected by interleaved fMRI-TMS. Neuroimage. 2003;20(3):1685–1696. doi: 10.1016/j.neuroimage.2003.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Bingel U, Tracey I. Imaging CNS modulation of pain in humans. Physiology (Bethesda) 2008;23:371–380. doi: 10.1152/physiol.00024.2008. [DOI] [PubMed] [Google Scholar]
- 14.Boggio PS, Zaghi S, Lopes M, Fregni F. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur J Neurol. 2008;15(10):1124–1130. doi: 10.1111/j.1468-1331.2008.02270.x. [DOI] [PubMed] [Google Scholar]
- 15.Borckardt JJ, Nahas Z, Koola J, George MS. Estimating resting motor thresholds in transcranial magnetic stimulation research and practice: a computer simulation evaluation of best methods. J ECT. 2006;22(3):169–175. doi: 10.1097/01.yct.0000235923.52741.72. [DOI] [PubMed] [Google Scholar]
- 16.Borckardt JJ, Reeves ST, Beam W, Jensen MP, Gracely RH, Katz S, Smith AR, Madan A, Patterson D, George MS. A randomized, controlled investigation of motor cortex transcranial magnetic stimulation (TMS) effects on quantitative sensory measures in healthy adults: evaluation of TMS device parameters. Clin J Pain. 2011;27(6):486–494. doi: 10.1097/AJP.0b013e31820d2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borckardt JJ, Reeves ST, Weinstein M, Smith AR, Shelley N, Kozel FA, Nahas Z, Byrne KT, Morgan K, George MS. Significant analgesic effects of one session of postoperative left prefrontal cortex repetitive transcranial magnetic stimulation: a replication study. Brain Stimul. 2008;1(2):122–127. doi: 10.1016/j.brs.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borckardt JJ, Smith AR, Reeves ST, Madan A, Shelley N, Branham R, Nahas Z, George MS. A pilot study investigating the effects of fast left prefrontal rTMS on chronic neuropathic pain. Pain Med. 2009;10(5):840–849. doi: 10.1111/j.1526-4637.2009.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borckardt JJ, Smith AR, Reeves ST, Weinstein M, Kozel FA, Nahas Z, Shelley N, Branham RK, Thomas KJ, George MS. Fifteen minutes of left prefrontal repetitive transcranial magnetic stimulation acutely increases thermal pain thresholds in healthy adults. Pain Res Manag. 2007;12(4):287–290. doi: 10.1155/2007/741897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borckardt JJ, Weinstein M, Reeves ST, Kozel FA, Nahas Z, Smith AR, Byrne TK, Morgan K, George MS. Postoperative left prefrontal repetitive transcranial magnetic stimulation reduces patient-controlled analgesia use. Anesthesiology. 2006;105(3):557–562. doi: 10.1097/00000542-200609000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Borras MC, Becerra L, Ploghaus A, Gostic JM, DaSilva A, Gonzalez RG, Borsook D. fMRI measurement of CNS responses to naloxone infusion and subsequent mild noxious thermal stimuli in healthy volunteers. J Neurophysiol. 2004;91(6):2723–2733. doi: 10.1152/jn.00249.2003. [DOI] [PubMed] [Google Scholar]
- 22.Bragin EO, Yeliseeva ZV, Vasilenko GF, Meizerov EE, Chuvin BT, Durinyan RA. Cortical projections to the periaqueductal grey in the cat: a retrograde horseradish peroxidase study. Neurosci Lett. 1984;51(2):271–275. doi: 10.1016/0304-3940(84)90563-9. [DOI] [PubMed] [Google Scholar]
- 23.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363(27):2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calejesan AA, Kim SJ, Zhuo M. Descending facilitatory modulation of a behavioral nociceptive response by stimulation in the adult rat anterior cingulate cortex. Eur J Pain. 2000;4(1):83–96. doi: 10.1053/eujp.1999.0158. [DOI] [PubMed] [Google Scholar]
- 25.Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, Manji HK, Drevets WC. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C] DASB; comparison with bipolar disorder. Biol Psychiatry. 2007;62(8):870–877. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 26.de Andrade DC, Mhalla A, Adam F, Texeira MJ, Bouhassira D. Neuropharmacological basis of rTMS-induced analgesia: the role of endogenous opioids. Pain. 2011;152(2):320–326. doi: 10.1016/j.pain.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 27.deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JD, Mackey SC. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci U S A. 2005;102(51):18626–18631. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del-Ben CM, Graeff FG. Panic disorder: is the PAG involved? Neural Plast. 2009;2009:108135. doi: 10.1155/2009/108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derbyshire SW, Osborn J. Offset analgesia is mediated by activation in the region of the periaqueductal grey and rostral ventromedial medulla. Neuroimage. 2009;47(3):1002–1006. doi: 10.1016/j.neuroimage.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 30.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1-2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13(8):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63(4):533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5(7):565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 34.Fierro B, De Tommaso M, Giglia F, Giglia G, Palermo A, Brighina F. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) during capsaicin-induced pain: modulatory effects on motor cortex excitability. Exp Brain Res. 2010;203(1):31–38. doi: 10.1007/s00221-010-2206-6. [DOI] [PubMed] [Google Scholar]
- 35.Fishman J, Roffwarg H, Hellman L. Disposition of naloxone-7,8,3H in normal and narcotic-dependent men. J Pharmacol Exp Ther. 1973;187(3):575–580. [PubMed] [Google Scholar]
- 36.Graeff FG, Del-Ben CM. Neurobiology of panic disorder: from animal models to brain neuroimaging. Neurosci Biobehav Rev. 2008;32(7):1326–1335. doi: 10.1016/j.neubiorev.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Hadjipavlou G, Dunckley P, Behrens TE, Tracey I. Determining anatomical connectivities between cortical and brainstem pain processing regions in humans: a diffusion tensor imaging study in healthy controls. Pain. 2006;123(1-2):169–178. doi: 10.1016/j.pain.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 38.Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60(1):214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutchison WD, Harfa L, Dostrovsky JO. Ventrolateral orbital cortex and periaqueductal gray stimulation-induced effects on on- and off-cells in the rostral ventromedial medulla in the rat. Neuroscience. 1996;70(2):391–407. doi: 10.1016/0306-4522(95)00372-x. [DOI] [PubMed] [Google Scholar]
- 40.Jefferys DB, Volans GN. An investigation of the role of the specific opioid antagonist naloxone in clinical toxicology. Hum Toxicol. 1983;2(2):227–231. doi: 10.1177/096032718300200209. [DOI] [PubMed] [Google Scholar]
- 41.Khan S, Javed S, Park N, Gill SS, Patel NK. A magnetic resonance imaging-directed method for transventricular targeting of midline structures for deep brain stimulation using implantable guide tubes. Neurosurgery. 2010;66(6 Suppl Operative):234–237. doi: 10.1227/01.NEU.0000369648.71236.17. discussion 237. [DOI] [PubMed] [Google Scholar]
- 42.Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong J, Tu PC, Zyloney C, Su TP. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krummenacher P, Candia V, Folkers G, Schedlowski M, Schonbachler G. Prefrontal cortex modulates placebo analgesia. Pain. 2010;148(3):368–374. doi: 10.1016/j.pain.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 45.Kung JC, Chen TC, Shyu BC, Hsiao S, Huang AC. Anxiety- and depressive-like responses and c-fos activity in preproenkephalin knockout mice: oversensitivity hypothesis of enkephalin deficit-induced posttraumatic stress disorder. J Biomed Sci. 2010;17:29. doi: 10.1186/1423-0127-17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lefaucheur JP. Use of repetitive transcranial magnetic stimulation in pain relief. Expert Rev Neurother. 2008;8(5):799–808. doi: 10.1586/14737175.8.5.799. [DOI] [PubMed] [Google Scholar]
- 47.Leith JL, Koutsikou S, Lumb BM, Apps R. Spinal processing of noxious and innocuous cold information: differential modulation by the periaqueductal gray. J Neurosci. 2010;30(14):4933–4942. doi: 10.1523/JNEUROSCI.0122-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim LW, Temel Y, Visser-Vandewalle V, Blokland A, Steinbusch H. Fos immunoreactivity in the rat forebrain induced by electrical stimulation of the dorsolateral periaqueductal gray matter. J Chem Neuroanat. 2009;38(2):83–96. doi: 10.1016/j.jchemneu.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 49.Loeser JD, Treede RD. The Kyoto protocol of IASP Basic Pain Terminology. Pain. 2008;137(3):473–477. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 50.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126(Pt 5):1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 51.McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123(2):119–130. doi: 10.3810/pgm.2011.03.2270. [DOI] [PubMed] [Google Scholar]
- 52.Merenlender-Wagner A, Dikshtein Y, Yadid G. The beta-endorphin role in stress-related psychiatric disorders. Curr Drug Targets. 2009;10(11):1096–1108. doi: 10.2174/138945009789735147. [DOI] [PubMed] [Google Scholar]
- 53.Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317(5841):1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nahmias F, Debes C, de Andrade DC, Mhalla A, Bouhassira D. Diffuse analgesic effects of unilateral repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers. Pain. 2009;147(1-3):224–232. doi: 10.1016/j.pain.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 55.O’Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. A report of a Cochrane systematic review and meta-analysis. Eur J Phys Rehabil Med. 2011;47(2):309–326. [PubMed] [Google Scholar]
- 56.Petersen KL, Rowbotham MC. A new human experimental pain model: the heat/capsaicin sensitization model. Neuroreport. 1999;10(7):1511–1516. doi: 10.1097/00001756-199905140-00022. [DOI] [PubMed] [Google Scholar]
- 57.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 58.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3(1):57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 59.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu YH, Wu XY, Xu H, Sackett D. Neuroimaging study of placebo analgesia in humans. Neurosci Bull. 2009;25(5):277–282. doi: 10.1007/s12264-009-0907-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosen AC, Ramkumar M, Nguyen T, Hoeft F. Noninvasive transcranial brain stimulation and pain. Curr Pain Headache Rep. 2009;13(1):12–17. doi: 10.1007/s11916-009-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schoell ED, Bingel U, Eippert F, Yacubian J, Christiansen K, Andresen H, May A, Buechel C. The effect of opioid receptor blockade on the neural processing of thermal stimuli. PLoS One. 2010;5(8):e12344. doi: 10.1371/journal.pone.0012344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22(7):2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. Lancet. 2011;377(9784):2226–2235. doi: 10.1016/S0140-6736(11)60402-9. [DOI] [PubMed] [Google Scholar]
- 65.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 66.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci U S A. 2007;104(26):11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagner JJ, Caudle RM, Neumaier JF, Chavkin C. Stimulation of endogenous opioid release displaces mu receptor binding in rat hippocampus. Neuroscience. 1990;37(1):45–53. doi: 10.1016/0306-4522(90)90190-f. [DOI] [PubMed] [Google Scholar]
- 68.Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999-2006. NCHS Data Brief. 2009;(22):1–8. [PubMed] [Google Scholar]
- 69.Willer JC, Dehen H, Cambier J. Stress-induced analgesia in humans: endogenous opioids and naloxone-reversible depression of pain reflexes. Science. 1981;212(4495):689–691. doi: 10.1126/science.6261330. [DOI] [PubMed] [Google Scholar]
- 70.Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet. 2011;377(9784):2215–2225. doi: 10.1016/S0140-6736(11)60245-6. [DOI] [PubMed] [Google Scholar]
- 71.Zambreanu L, Wise RG, Brooks JC, Iannetti GD, Tracey I. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain. 2005;114(3):397–407. doi: 10.1016/j.pain.2005.01.005. [DOI] [PubMed] [Google Scholar]


