Abstract
Although HIV-positive patients’ adherence to antiretroviral therapy (ART) is relatively high in African nations, as compared with industrialized nations, few studies have explored why. In the research presented here we aimed to understand the dynamics of good adherence to ART among patients receiving free ART and HIV-related services from a clinic in Arusha, Tanzania. We conducted individual semi-structured interviews with 6 health care providers and 36 patients at the study site. Interviews were conducted in Swahili using interview guides informed by social cognitive theory. All interviews were audio-recorded, transcribed in Kiswahili, translated into English and coded for themes and patterns with Atlas t.i. Of the 36 patients interviewed (mean time on ART 9.8 months; range 1–23 months), 32 reported perfect adherence in the previous month. Self-reported adherence was high despite economic hardship, depression, low rates of HIV disclosure and high perceived HIV-associated stigma. Five factors emerged to explain excellent adherence in the face of such barriers. First, all respondents experienced substantial improvements in their health after starting ART; this supported their confidence in the medication and motivated them to adhere. Second, their perceived need to be able to meet their family responsibilities motivated respondents to stay healthy. Third, respondents developed specific strategies to remember to take pills, particularly routinizing pill-taking by linking it with daily activities or events. Fourth, material and emotional support received from others facilitated adherence. Finally, respondents trusted the advice and instructions of their health care providers, who regularly emphasized adherence. The facilitating factors identified were consistent with the constructs of social cognitive theory and highlighted the importance of interventions that address multiple levels of influence on adherence.
INTRODUCTION
In 2007, 2.1 million people worldwide died from HIV and AIDS-related illnesses, with over 75% of those deaths occurring in Africa (UNAIDS, 2007). Antiretroviral therapy (ART) offers an unprecedented opportunity to avert deaths of people living with HIV/AIDS, and funds and political will have been mobilized to make ART available where it is most needed globally. In Tanzania, where approximately two million people (6.5% of the adult population) are living with HIV (UNAIDS, 2007), the government is committed to making free ART available to as many residents as possible. An estimated 136,000 people were receiving ART (31% of those in need) at over 200 sites across the country, as of the end of 2007 (World Health Organization, 2008).
The success of an African nation’s scale-up of ART will require bolstering the capacity and reach of the health care system and shifting the system’s orientation from an acute care model to a chronic care management model. At the same time, success will depend on patients’ abilities to adhere to medications, which are influenced by factors extending beyond the clinic environment. Poor adherence can lead to the virologic failure of patients using cheaper first-line treatment regimens and the spread of drug resistant forms of the virus, resulting in a public health calamity (Stevens, Kaye, & Corrah, 2004). Poor adherence can result in increased costs to health and society, in terms of direct financial costs of failed treatment and higher hospitalization rates. It can also entail indirect costs of lost productivity of patients and burden on family caregivers (Dunbar-Jacob, Erlen, Schlenk, Ryan, Sereika, & Doswell, 2000; Sokol, McGuigan, Verbrugge, & Epstein, 2005). The impact of sub-optimal adherence to ART is particularly concerning in countries that lack capacity for monitoring drug resistance and where second-line regimens are prohibitively expensive or unavailable (Cohen, 2007).
Initial findings about adherence to ART regimens in sub-Saharan Africa have been promising. A meta-analysis found that a pooled estimate of 77% of patients in African settings achieved adequate adherence (most often measured as taking 95% of prescribed pills), compared with just 55% of patients in North American settings (Mills, Nachega, Buchan, Orbinski, Attaran, Singh et al., 2006). The high levels of adherence have been observed in a subsequent multi-site study, including Tanzania (Hardon, Davey, Gerrits, Hodgkin, Irunde, Kgatlwane et al., 2006). At the same time as we have observed overall good adherence among African patients, evidence suggests that a large number of African patients may drop out of ART programs. A review of 33 patient cohorts taking ART in 13 African countries suggested only 60% of patients remain enrolled in programs after two years, with the ambiguous category of “loss to follow up” accounting for 56% of all attrition (Rosen, Fox, & Gill, 2007). The potentially high drop out levels suggest we need a better understanding of patients’ experiences taking ART. Understanding how patients integrate ART in the context of their daily lives and what strategies and motivations they use to adhere may contribute not only to supporting and sustaining good adherence, but also to keeping patients in care over time.
While quantitative studies have assessed factors that create challenges for adherence in Africa (Mills, Nachega, Bangsberg, Singh, Rachlis, Wu et al., 2006), only a few studies have explored factors that facilitate adherence, and how social and institutional contexts may contribute to optimal adherence (Crane, Kawuma, Oyugi, Byakika, Moss, Bourgois et al., 2006; Nam, Fielding, Avalos, Dickinson, Gaolathe, & Geissler, 2008). In a Uganda study, near-perfect adherence was motivated by a belief that ART was responsible for keeping them healthy and by a desire to stay alive to look after the well-being of family members, and concluded that these motivators outweighed the challenges patients faced securing funds to purchase ART (Crane et al., 2006). In a study in Botswana, patients who were able to achieve excellent adherence were those who had accepted their HIV status and engaged an encouraging confidante in their care (Nam et al., 2008). In North American studies, the factors that facilitate adherence to ART have been shown to span individual, inter-personal and institutional levels (Mills, Nachega, Bangsberg et al., 2006).
Research about people’s experiences taking long-term medication thus far has lacked a theoretical basis and may benefit from the application of behavioral theory as an organizing framework (World Health Organization, 2001). Social cognitive theory (SCT) has been used as a theoretical lens for conducting observational adherence research and for developing and testing interventions to enhance adherence to ART (Diiorio, McCarty, Depadilla, Resnicow, Holstad, Yeager et al., 2007; Munro, Lewin, Swart, & Volmink, 2007). The organizing concept of SCT is reciprocal determinism, which asserts that personal factors, social factors and behavioral factors all interact to determine behavior. According to SCT, adherence is a function of one’s self efficacy to adhere, which is influenced, among other things, by positive reinforcements received to adhere, observational experiences of adherence, skills used to regulate adherence, and expectations of the results of faithfully taking medication (Baranowski, Perry, & Parcel, 2002).
In the study presented in this paper, we applied SCT to inform our examination of how factors in the clinic setting, as well as in HIV-infected patients’ lives outside the clinic, enhanced motivation and capacity to adhere to ART. It is the first study to document the perspectives of both patients and their HIV care providers on what facilitates optimal adherence to ART in a Tanzanian setting. Understanding the context in which patients in Tanzania take their ART as well as the motivations and techniques that patients employ to adhere to their medications within that context, and how these are reinforced in a health care setting, may provide important lessons for the expansion and sustainability of ART services in Tanzania and other developing nations.
STUDY SETTING
The clinic in which this study was conducted was founded in 1954 by the Lutheran Church of Tanzania. It includes a 120-bed full-service hospital located on the outskirts of the Arusha metropolitan area and an outpatient clinic in the city center that houses the ART program. The study clinic was among the first health care centers to respond to the HIV epidemic in Arusha, having established an AIDS Control Program in 1986. First oriented to providing HIV education and prevention, as well as support to orphans and affected families, the clinic began providing ART on a cost basis in 2003. In 2004, with the support of the United States’ President’s Emergency Plan for AIDS Relief (PEPFAR), the clinic began offering ART and related HIV services free to all eligible patients. At the time of data collection, approximately 700 adults were receiving ART at the study clinic.
Patients at the clinic are eligible to start ART if they have a CD4 count of less than 200 cells/mm3 or any symptoms representing a WHO clinical stage of IV (World Health Organization, 2007). Patients who meet clinical criteria must meet with a clinic counselor before initiating ART. At that appointment, the counselor provides information about the medications, including side effects and the importance of adherence. The counselor also addresses psychological issues, such as stigma and disclosure, and answers questions that the patient may have. Patients are encouraged to bring a friend or family member to the clinic for appointments.
The study clinic offers a peer support program for all ART patients, which it launched in 2005, making it unique among Tanzanian ART programs. The program pairs all patients with voluntary adherence counselors (VACs), who are HIV positive people trained to provide patients with counseling to address medication adherence and psycho-social issues. Patients meet with their VACs when they come for clinic appointments, and VACs may also visit patients in their homes to understand their living situations and provide supportive counseling to patients and their families.
METHODS
This study was part of a larger mixed-method investigation of adherence to ART. The qualitative data presented here were from in-depth interviews completed with six health care providers from the clinic and 36 patients who were taking ART at the time of the interview. Health care providers were eligible to participate in the study if the majority of their working time was spent providing direct care to patients who were preparing to take or were taking ART, as determined by the hospital director. Eligible providers included two physicians, two nurses, one counselor and one pharmacist who worked at the clinic during the time of the investigation. We introduced the study to health care providers at the clinic during a weekly meeting and then approached each eligible provider individually during working hours.
Patients were eligible to participate in the study if they had been taking ART for at least one month at the study clinic, were at least 18 years of age, and could give informed consent in Swahili. We used maximum variation sampling with quotas (Patton & Patton, 2002) to ensure that we included both men and women who had been taking ART for various lengths of time (<6 months, 6–12 months, >12 months). We set a minimum of at least four respondents for each combination of gender and time on ART, and continued recruitment and interviews until we reached a saturation of themes. Over three months, participants were recruited by two clinic counselors who routinely met with all patients coming to the clinic to pick up their monthly supply of ART. A member of the study team informed the recruited patients about the study and, if they were interested in participating, read them the informed consent form. No patients declined to participate in the interview.
Trained Tanzanian interviewers conducted the individual in-depth interviews with respondents in Swahili. Interviews took approximately 60 minutes and followed semi-structured interview guides. The interview guide for health care providers included questions and probes about the services they provided to patients and their impressions of patients’ experiences taking ART. Preliminary analysis of provider interviews was done to inform the content of the interview guide for patients, which included questions and probes about how ART fit into their daily routines, strategies used to adhere, motivations to adhere, and barriers faced in adhering. Patients were also asked about their adherence to ART over the past month with the question: “Many patients find it difficult to take all their pills as they are supposed to. Thinking back over the last month, how many of your ARV pills did you either forget to take or decide not to take?”. All patients and providers who participated in the interviews received 5,000 Tanzanian Shillings (approximately four US dollars).
The research staff audio-recorded the interviews, transcribed them in Swahili and translated them into English. Translated, transcribed interviews were coded by the first author using Atlas.ti (Atlas ti, 1997). In the first phase of coding, deductive codes were drawn from the interview guide and research questions. In the second phase, inductive codes were created and applied to identify additional themes, patterns and categories that emerged from the data (Patton & Patton, 2002). The data were summarized through descriptive text-based summaries and data display matrices. Provider and patient transcripts were coded and analyzed separately, and then compared for common themes. Representative, verbatim quotes were selected to illustrate key findings.
The study received ethical approval from the Institutional Review Board at the University of North Carolina’s School of Public Health and the National Institute for Medical Research in Tanzania.
RESULTS
The results are organized to first present the characteristics of the participants who took part in the study. Second, we present patients’ perspectives of the social context of taking ART, followed by health care providers’ perspectives on the clinical context. Next, we report the adherence levels of participants. Last, we present five factors that emerged as facilitating ART adherence in this sample of patients, supplemented by findings of how providers reinforced these facilitating factors with patients.
Characteristics of study participants
The health care providers interviewed included all of the eligible clinic staff (two physicians, two nurses, one professional counselor and one pharmacist). All were Tanzanian nationals. Four were male and two female, ranging in age from 35 to 49.
The sample of patients taking ART included 19 females and 17 males. The demographic characteristics of the 36 ART patients who participated in in-depth interviews are presented in Table 2. All patients were taking Triomune (manufactured by Cipla in Mumbai, India), a single pill containing stavudine, lamivudine and nevirapine. The pill is intended to be taken twice per day at a 12 hour interval. Patients had been taking ART at the study clinic for a mean of 9.8 months (range 1–23 months), and all were ART naïve at the time of clinic initiation.
Patients’ Perspectives of the Social Context of ART Use
Patients described important social factors that influenced their experiences living with HIV and taking ART. These factors, which were common across the majority of patients, shed some light on the life experiences of patients in this setting. First, almost all patients reported economic insecurity, exacerbated in many cases by long periods of HIV-related illnesses, losing a spouse, or both. Although no patients mentioned lack of food or money for transportation as a reason for non-adherence, both came up repeatedly as concerns for patients. When asked what additional support they would like from the clinic, the most common responses were food support and small loans to initiate business ventures. Second, many patients expressed feeling depressed or hopeless after being diagnosed with HIV, often related to a belief that HIV was a death sentence. For most patients, as their health improved with ART, so did their emotional state. Third, almost all patients disclosed their HIV status very selectively in their social networks. Patients decided whether to disclose based on the emotional closeness of a relationship, the perceived ability of people to maintain confidentiality, and how they thought people would react to their HIV status. Patients reported that when they were around others to whom they hadn’t disclosed their HIV status, they either hid their pills or told people the pills were for something other than HIV. Lastly, most patients expressed a fear that people would treat them differently if they knew they were HIV positive, although less than half reported directly experiencing any stigma themselves. As one man said, “I was hearing that if you tell people about this problem, they will stop coming to my house or they will discriminate against me.”
Providers’ Perspectives of the Clinical Context of ART Use
Providers we interviewed emphasized that they respected the multiple challenges that patients faced living with HIV, and aimed to provide services that were comprehensive and wholistic. This approach was reflected in the way that health care providers talked about their interactions with patients. Counseling was viewed as an integral part of on-going care and was considered the responsibility of all health care providers. As one provider explained,
You need to understand a person. Therefore every person needs to be a counselor. Don’t say a patient is for [one health care worker or another]. A patient is for everyone. Everyone who faces the person should give advice properly. That is how we were told. (Female nurse)
Providers emphasized that the content of the counseling was not just about the clinical manifestations of the drugs, but also about the social context of patients’ lives. Health care providers gave us the following examples of the types of questions they posed to patients during their meetings: “What are the things [you] encounter that make [you] despair, or things that encourage [you]?”; “How is home? Do people at home know that you are using medicine?” Patients themselves confirmed having these types of conversations with providers.
The health care providers interviewed each talked about a personal motivation to serve patients, which was in part grounded in their own Christian faith and the faith-based orientation of the clinic. Although the clinic was not evangelical in its approach to patients, staff started each workday with a period of prayer and singing and closed all meetings with prayer. For at least two providers, their motivation to serve patients was related to the Christian ethic of the clinic, as one provider explained:
We enjoy serving [patients] because this is a church center. Therefore when you serve the patient you see them like you are a part of their problem. So when you serve them you do it with all your heart. (Male doctor)
Self-reported adherence
Despite the potential barriers to adherence patients faced as a result of the social context of their lives, the majority of patients reported that they had perfect adherence over the previous month. Of the 36 respondents, 32 said they had not missed any pills in the past month. Three patients said they had missed a single pill (equivalent to 98% adherence), giving the following reasons:
Busy with an evening church event (Female, 30, 4 months on ART)
Fell asleep early and missed an evening pill (Female, 35, 10 months on ART)
Busy in the morning and forgot (Female, 53, 13 months on ART)
One respondent had intentionally stopped taking her pills for ten days (Female, 31, 18 months on ART). The reason she gave for discontinuing therapy was that she had malaria and was vomiting. She said that when she explained this to the counselor at the next clinic appointment, she was advised to resume taking her pills and she did so.
Reasons for overall excellent adherence
Based on respondents’ narratives of their experiences taking ART, we identified five primary factors that facilitated adherence. Here we present these facilitating factors, along with providers’ perspectives on them.
1. Experiencing improved health on ART
Most patients had suffered severe and sustained illness before being diagnosed with HIV. As a result, most patients had vivid illness narratives and an understanding of the devastating impact of HIV on their bodies. These periods of illness contrasted sharply with the improvements in their health that they observed very soon after starting ART. These health improvements increased patients’ confidence in the medications.
Only about a third of respondents said they experienced side effects when starting ART (mostly abdominal symptoms), but this was always overshadowed by the marked improvements in their health on ART. Patients pointed to their increased weight, energy and the fact that they could return to work. The increase in strength and resumption of normal activities attributable to ART created a strong motivation to continue taking the drugs.
I have no such idea of stopping to take them for sure, because I was too much tortured before. I was sick for a very long time, I was just sleeping in bed. But now I am not. Therefore it’s not easy for me to stop them because they are the ones that made me better. (Female, 45, 17 months on ART)
The perceived benefits of adhering were closely linked in patients’ minds to perceived negative consequences of not adhering. When asked about the impact of missing pills or stopping ART altogether, most patients mentioned a fear that they would return to their previous condition or die. The association between adherence and avoidance of severe illness or death was evident even among patients who had been on ART for over a year and whose health had plateaued at a healthy state.
I will die if my previous situation comes again. I am worried about it. Therefore I carry pills anywhere; even now I have them in my bag, because I may go out and sometimes be late, so I must take them. (Male 55, 14 months on ART)
Health care providers said that they emphasized to patients the benefits of adhering to their ART for improving their health and prolonging their lives. One provider said that he sometimes held up examples of other patients or the VACs to illustrate the positive benefits of ART. “Sometimes we give them live examples, comparing a patient when they first started to their present condition. That helps them not to lose hope, to show them the medicine helps them.”
2. Having a sense of obligation to family
Patients’ desire to improve their health and prolong their lives was as much a social motivation as an individual one. HIV affects people at the height of their reproductive lives, and as a result the majority of respondents (31 of 35) had children under the age of 18. Patients’ experiences of near-death illnesses and their diagnosis with HIV, which many initially assumed was fatal, was often accompanied by a fear of not being able to support their families financially or leaving their children as orphans. One man explained that his immediate reaction to his HIV diagnosis was asking, “Who will I leave my wife and kids with? I really wanted to see them start and finish their studies, the least I could do for them.” Taking ART offered patients an opportunity to return to work, support their families, and be around to take care of their children. Children as a motivator was expressed by more men (7) than women (2). As one man said, “I could never give up because I have kids.”
Only one health care provider talked about drawing upon motivations for family obligations to support a patient’s adherence. She was advising a female patient who struggled to take her ART by saying to her, “Your weapon is medicine because your children are still young. If it were not for the medicine you wouldn’t be alive to support them.”
3. Making pill-taking part of the daily routine
The requirement that ART be taken at the same time every morning and evening meant that patients had to adapt their routines to incorporate taking their pills. Almost all patients explained that they had very consistent and predictable schedules, with little variation, which made it easier to incorporate pill-taking. This sentiment was strongest among women, who were more bound to responsibilities of the home. The following woman explained how taking her pills every morning and evening fit into her daily routine.
I arranged to take them at 10AM. I can just wake up and do my work. I do the dishes, mop, take a shower, then at about 9:45 I take my tea. I look at my watch and at 10am I take my medicines. …we normally fetch water at night. So after fetching water, the children have already eaten, and I sit down and eat... when it reaches 10PM I stop eating and take my drugs. After that I take a short rest and I go to sleep. (Female, 30, 6 months on ART)
Patients talked about their efforts to keep regular schedules and prioritize the time to take their pills. When we asked patients how they remembered to take their pills on time, many simply said that it’s “hard to forget” or “always on my mind.” One patient said “I take it as food, which you have to eat every day.” Patients also talked about strategies they used to remember to take their pills. Four respondents said they set alarms on watches or cell phones (cell phones were common in this setting). More often, however, patients anchored the time of taking their pills with other regular activities, such as meal or tea times, brushing their teeth, prayer, or radio or television programs.
The primary disruption in patients’ schedules, particularly among men, was traveling out of town for work or to visit family, or being busy and out of the house for long hours. When asked about this, respondents said that they prioritized pill taking and had strategies for carrying their pills, water and food. Patients who traveled out of town usually said that their pills were the first – and most important – thing they packed when getting ready for a trip.
Providers said they advised patients about different strategies they might use to remember to take their ART, such as using an alarm, or keeping the pill bottle next to something they use regularly (such as a toothbrush or a kettle).
4. Receiving material and emotional support from others
Social support facilitated adherence through reminders to take pills, material support of food and money, and emotional encouragement. Disclosure to family and friends of one’s HIV status was an important pathway of harnessing social support – in particular emotional support – but patients also talked about getting support without having to directly reveal their HIV status.
The most direct way that patients received support to adhere was through explicit reminders to take their pills. Fourteen respondents said they had someone who provided these reminders. When the patient’s spouse or partner was also taking ART (five respondents), they usually reminded each other. Ten respondents said that their children reminded them of the time to take their pills, even if they hadn’t told the children that the pills were to address HIV. Other respondents had family members call them or visit them to remind them to take their pills.
Most patients (24 of 36) reported receiving material support, primarily from family members, in the form of food and money. Respondents gave the impression that the supporters gave material support because of the general hardships the respondents were facing in their lives, not explicitly because of their HIV status or to help with taking their ART. Nevertheless, respondents spoke about the importance of this type of support for taking ART, as this respondent related: “If you want to enjoy taking these pills and have them help you, you must have some food. If you have some food, then there is no other problem.”
Although our questions about support first elicited responses about reminders and material support, probing also revealed that patients received important emotional support that made it easier for them to take their pills. Almost all respondents (29 of 36) talked about receiving some emotional support, often related to general encouragement or to helping them view HIV as a ‘normal’ illness by giving examples of other people with HIV who were living productive lives. One female patient who was taken in by her brother and his family after she became ill said that her brother and sister-in-law not only reminded her to take her pills, but also encouraged her to live positively with HIV, which seemed to increase her motivation to adhere to her ART regimen, and to be committed to it over the long term.
They really help me, give me advice. Even encourage me, because sometimes I feel heartbroken. To some extent they help to give me courage, so that I can see it as a normal thing. (Female, 30, 4 months on ART)
The voluntary adherence counselors (VACs) at the clinic also played an important role in providing support to patients. Almost all respondents (33 of 36) said they had a VAC assigned to them. Of those, 26 said that their VACs gave them important information relevant to adherence, and 14 said that their VACs gave them some form of emotional support. The fact that the VACs were also HIV positive made the information and emotional connection even more salient. As one woman said, “When you talk with a person with the same problem as you it’s easy to understand each other well.”
Health care workers spoke about their efforts to engage family members or friends in patients’ care. They did this by encouraging patients to bring their supporters to clinic appointments, counseling the supporters when they attended, and having VACs follow up with family members in the home. This doctor talked about the importance of engaging the patient’s support system.
It is a benefit, especially for adherence to the medication. It is believed that if they involve a second person, it reduces stigma first and then it improves adherence to the medication. And these people can also assist them to take medication, like telling them that they forgot this medication today.
5. Trusting the advice of service providers
Lastly, patients expressed trust in the advice of service providers at the study clinic, which motivated them to adhere. Patients consistently said that counselors, doctors and VACs all emphasized the importance of adherence in their interactions, and they accepted this advice without reservation. Having trust in the advice of service providers allowed patients to overcome any lack of knowledge about the impact or long-term nature of ART.
I will not stop, even if there are side effects, because I was told to use it. If I stop for some reason, it will be bad. If the doctor who gave me the pills tells me that I’m supposed to stop, I will stop, but apart from that I will use it, even if there are side effects. (Male, 49, 4 months on ART)
Overall, patients felt very positively about the services they received at the study clinic, particularly comparing the study clinic to other clinical settings they had experienced. The things they appreciated about the clinic were the information they received on taking ART and the openness of staff to addressing their problems.
Trusting health care providers may in part have to do with the relationships the providers at the study clinic tried to build with patients through direct interactions as well as indirectly through VACs. One provider explained how he related to patients: “I make jokes sometimes to make patients feel comfortable, so you can explore their insights and they can tell you how they feel…. You feel free to chat with patients and they feel free to chat with you.” Another provider recognized the importance of building such relationships when she said, “It is important that they trust you a lot. So we are trying to build good relationships between the patients and the staff.”
DISCUSSION
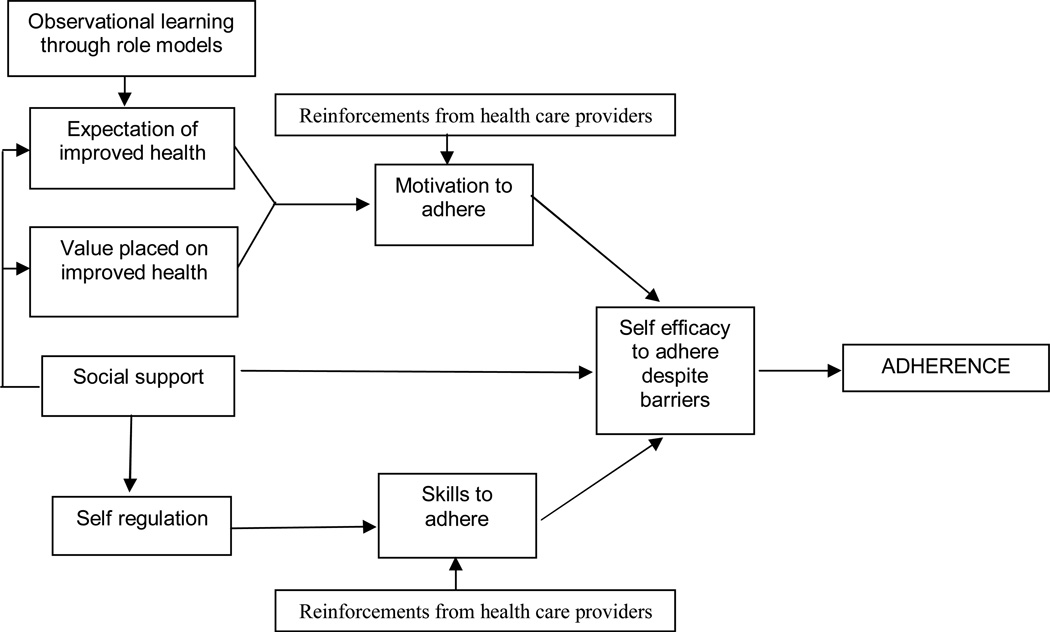
Findings from this inquiry suggest that the social contexts of patients’ lives means that they come to their diagnosis and treatment with potential barriers to ART adherence, as have been observed in other studies. These include poverty, emotional distress, low rates of HIV disclosure, and high levels of perceived HIV-associated stigma (Hardon, Akurut, Comoro, Ekezie, Irunde, Gerrits et al., 2007; Mills, Nachega, Bangsberg et al., 2006; Sankar, Golin, Simoni, Luborsky, & Pearson, 2006). Despite these potential barriers, most patients reported that they had perfect adherence over the past month. This begs the question of how these patients have been able to overcome potential barriers. Through individual patient narratives and provider interviews in this study, several factors emerged as facilitating adherence to ART in this setting resonated with the constructs of social cognitive theory (Baranowski et al., 2002). Factors at the individual, inter-personal and institutional levels interacted to positively influence adherence (Figure 1).
Figure 1.
Explanatory model of ART adherence facilitators
Similar to other studies (Golin, Isasi, Bontempi, & Eng, 2002; Remien, Hirky, Johnson, Weinhardt, Whittier, & Le, 2003), a primary factor that facilitated adherence in our sample was a belief in the efficacy of the medications to improve health and prolong life. This belief came from patients’ lived experiences transitioning from debilitating illnesses before starting ART to improved health after initiating therapy. The clinic environment, including the VACs as role models, likely reinforced the benefits of ART for patients and enhanced their motivation to adhere to the medication regimen and live with HIV long-term.
A desire to be alive to support their families also motivated patients to adhere, as has been documented in other studies (Golin et al., 2002; Remien et al., 2003; Richter, Sowell, & Pluto, 2002; Wood, Tobias, & McCree, 2004). While most of these other studies documented patients’ desire to be around to “see their children grow up,” respondents in this study spoke more about their responsibility for the economic well-being of their children, particularly to ensure that their children received an education. In addition, while other studies have documented children as a facilitating factor in adherence primarily among women, in this study it was a more salient theme among male patients. This contrast must be understood within the context of the patrilineal system of northern Tanzania, where men are responsible for children’s education and helping them become established as economically independent.
Patients in this study reported consistent and predictable daily routines, which made it easier to integrate pill-taking into their daily lives. North American studies have documented unstable lives, shifting routines, and the fact that “every day is different” as one of the primary challenges to taking ART on schedule (Adam, Maticka-Tyndale, & Cohen, 2003; Golin et al., 2002; Ryan & Wagner, 2003). Because patients in this sample had consistent routines, they could anchor their pill-taking to daily events like morning tea, brushing their teeth, or bedtime. A simple medication regimen (one pill twice a day) may also have contributed to greater ease in integrating pill-taking into daily routines (Mills, Nachega, Bangsberg et al., 2006).
The positive influence of social support on adherence has been documented in previous studies using qualitative methods (Malcolm, Ng, Rosen, & Stone, 2003; Nam et al., 2008; Remien et al., 2003) as well as quantitative methods (Diabate, Alary, & Koffi, 2007; Murphy, Marelich, Hoffman, & Steers, 2004). Social support arose as an important facilitating factor among this sample, taking the form of direct reminders, financial help and emotional backing. Health care providers in the study clinic recognized the influential nature of patients’ support networks on adherence and encouraged patients to involve their supporters in routine care. The VACs at the clinic also provided information and emotional support that particularly resonated because of the VACs’ personal experience living with HIV.
Finally, patients’ trust in the advice of health care providers and VACs facilitated adherence in this sample. Patients perceived these providers as referent authorities whose advice they should follow without question, and the advice they were being provided was to adhere to their medications. The influence of patient-provider relationships on adherence is consistent with findings from other qualitative studies (Abel & Painter, 2003; Golin et al., 2002; Malcolm et al., 2003; Murphy, Roberts, Hoffman, Molina, & Lu, 2003; Remien et al., 2003; Roberts, 2002). Providers in the study clinic recognized the importance of building trusting relationships with patients, and did so by engaging in thoughtful conversations with patients and attempting to reduce the social distance between provider and patient.
The clinic environment itself likely had a positive influence on adherence in this setting. The health care providers we spoke with all expressed a personal motivation to do their work, grounded in an ethic of service that seemed in part influenced by the faith-based orientation of the clinic. The fact that the study clinic aimed to provide a continuum of care to patients by offering a range of services for people living with and affected by HIV/AIDS reflected a broader commitment to seeing patients as whole people with needs and influences outside of the clinic setting. Strong patient-provider relationships are essential components of a chronic illness management model that can support and sustain adherence over time (Swartz & Dick, 2002; World Health Organization, 2002). Although this study was not a formal evaluation of the VAC program, its findings do suggest a positive influence of this peer support program for people taking ART.
The excellent adherence reported by patients in this study must be interpreted cautiously in light of the possibility of self-reporting bias, which likely over-estimates adherence due to recall bias and social desirability (Chesney, Morin, & Sherr, 2000). This is particularly a concern given that interviews were conducted in the clinic setting, and respondents may have been unwilling to admit lapses in adherence for fear that they might be excluded from the ART program. Self-reported adherence measurements have been shown consistently to be associated with viral load counts (Fletcher, Testa, Brundage, Chesney, Haubrich, Acosta et al., 2005), but viral load data was not available for the study patients to examine its correlation with patients’ reported adherence. In addition to the possibility of self-reporting bias, other explanations of excellent adherence may be attributable to the patient pool at the clinic. Patients interviewed were only those who returned for clinical care and therefore excluded those who had either temporarily or permanently dropped out of care. Respondents had only been on ART for a short time due to the relative newness of ART services at the clinic, and adherence might change as patients are on ART for longer periods. Additionally, the fact that ART and related care were free at the clinic likely also facilitated patients’ adherence, given that research conducted in nearby Moshi found that self-funding of ART care was associated with poor adherence (Ramadhani, Thielman, Landman, Ndosi, Gao, Kirchherr et al., 2007).
The themes of adherence facilitators reported in this article are limited to the issues that individual respondents chose to discuss in-depth during their interviews. Although the depth of discussion on the topics probed was not standardized across interviewers, the interview guide included specific probes for follow-up. As is the case with qualitative interviews, not all providers and patients were asked the same questions in the exact same way, but all interviewers received intensive training, practice, and constructive feedback. Finally, as applies to any qualitative study, the results are not generalizable to a larger population. Instead, the findings illuminate the rich details of experience and context of a clinic in Tanzania that may be transferrable to others.
The findings presented in this article highlight the motivators and strategies that HIV-infected patients use to adhere to ART and live positively with the disease. Health care providers need to have the time, motivation and skills to recognize and address patients’ own facilitators through effective counseling. Motivational interviewing may be a useful tool for providers to use in this setting (Cooperman & Arnsten, 2005). The value of social support in facilitating adherence points to the need to engage patients’ natural supporters (family and friends) in long-term ART care, and to promote peer support programs, such as VACs. Finally, the findings point to the need to employ comprehensive interventions that are grounded in the unique social context of their settings, as have been demonstrated in rural Haiti (Mukherjee, Ivers, Leandre, Farmer, & Behforouz, 2006).
Future research on this topic may benefit from observations of patient-provider interactions that would provide more information about the nature of communication between these groups and validate self-reported findings from providers. To understand how patients’ support systems influence adherence, studies should explore the role that family and friends play in patients’ clinical and social care, and seek the perspectives not only of patients but also of their supporters. Because adherence is a dynamic process that may change over time, multiple contacts with respondents over time may be more useful than single interviews (Conrad, 1990). Although few gender differences in adherence facilitators emerged in this study, this issue should be explored further, particularly given the different social influences of men and women and the fact that all four patients who reported missed pills in the past month were women.
Despite its limitations, this is the first qualitative study in Tanzania on factors that facilitate patients’ adherence to ART. As such, it increases our understanding of why adherence might be high in a low-resource setting despite the presence of potential barriers.
Table 1.
Demographic characteristics of Tanzanian patients interviewed (N=36)
| N | |
|---|---|
| Age | |
| 20–29 | 5 |
| 30–39 | 13 |
| 40–49 | 11 |
| 50 or older | 7 |
| Education | |
| No education | 2 |
| Some primary | 3 |
| Completed primary | 26 |
| Post-primary | 5 |
| Religion | |
| Christian | 28 |
| Muslim | 8 |
| Marital status | |
| Never married | 5 |
| Currently married | 14 |
| Separated/divorced | 6 |
| Widowed | 11 |
| Main economic activity | |
| Petty trading | 9 |
| Farming/ livestock | 7 |
| Formal employment | 7 |
| No economic activity | 13 |
| Time from home to clinic | |
| < 1 hour | 26 |
| > 1 hour | 10 |
REFERENCES
- Abel E, Painter L. Factors that influence adherence to HIV medications: perceptions of women and health care providers. J Assoc Nurses AIDS Care. 2003;14(4):61–69. doi: 10.1177/1055329003252879. [DOI] [PubMed] [Google Scholar]
- Adam BD, Maticka-Tyndale E, Cohen JJ. Adherence practices among people living with HIV. AIDS Care. 2003;15(2):263–274. doi: 10.1080/0954012031000068407. [DOI] [PubMed] [Google Scholar]
- Baranowski T, Perry C, Parcel G. How individuals, environments, and health behavior interact: Social Cognitive Theory. In: Glanz K, Rimer B, Lewis FM, editors. Health Behavior and Health Education: Theory, research and practice. 3rd ed. San Francisco: Jossey-Bass; 2002. pp. 165–184. [Google Scholar]
- Chesney MA, Morin M, Sherr L. Adherence to HIV combination therapy. Soc Sci Med. 2000;50(11):1599–1605. doi: 10.1016/s0277-9536(99)00468-2. [DOI] [PubMed] [Google Scholar]
- Cohen GM. Access to diagnostics in support of HIV/AIDS and tuberculosis treatment in developing countries. Aids. 2007;21(Suppl 4):S81–S87. doi: 10.1097/01.aids.0000279710.47298.5c. [DOI] [PubMed] [Google Scholar]
- Conrad P. Qualitative research on chronic illness: a commentary on method and conceptual development. Soc Sci Med. 1990;30(11):1257–1263. doi: 10.1016/0277-9536(90)90266-u. [DOI] [PubMed] [Google Scholar]
- Cooperman NA, Arnsten JH. Motivational interviewing for improving adherence to antiretroviral medications. Curr HIV/AIDS Rep. 2005;2(4):159–164. doi: 10.1007/s11904-005-0010-x. [DOI] [PubMed] [Google Scholar]
- Crane JT, Kawuma A, Oyugi JH, Byakika JT, Moss A, Bourgois P, Bangsberg DR. The price of adherence: qualitative findings from HIV positive individuals purchasing fixed-dose combination generic HIV antiretroviral therapy in Kampala, Uganda. AIDS Behav. 2006;10(4):437–442. doi: 10.1007/s10461-006-9080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabate S, Alary M, Koffi CK. Determinants of adherence to highly active antiretroviral therapy among HIV-1-infected patients in Cote d'Ivoire. Aids. 2007;21(13):1799–1803. doi: 10.1097/QAD.0b013e3282a5667b. [DOI] [PubMed] [Google Scholar]
- Diiorio C, McCarty F, Depadilla L, Resnicow K, Holstad MM, Yeager K, Sharma SM, Morisky DE, Lundberg B. Adherence to Antiretroviral Medication Regimens: A Test of a Psychosocial Model. AIDS Behav. 2007 doi: 10.1007/s10461-007-9318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar-Jacob J, Erlen JA, Schlenk EA, Ryan CM, Sereika SM, Doswell WM. Adherence in chronic disease. Annu Rev Nurs Res. 2000;18:48–90. [PubMed] [Google Scholar]
- Fletcher CV, Testa MA, Brundage RC, Chesney MA, Haubrich R, Acosta EP, Martinez A, Jiang H, Gulick RM. Four measures of antiretroviral medication adherence and virologic response in AIDS clinical trials group study 359. J Acquir Immune Defic Syndr. 2005;40(3):301–306. doi: 10.1097/01.qai.0000180078.53321.6a. [DOI] [PubMed] [Google Scholar]
- Golin C, Isasi F, Bontempi JB, Eng E. Secret pills: HIV-positive patients' experiences taking antiretroviral therapy in North Carolina. Aids Education And Prevention. 2002;14(4):318–329. doi: 10.1521/aeap.14.5.318.23870. [DOI] [PubMed] [Google Scholar]
- Hardon AP, Akurut D, Comoro C, Ekezie C, Irunde HF, Gerrits T, Kglatwane J, Kinsman J, Kwasa R, Maridadi J, Moroka TM, Moyo S, Nakiyemba A, Nsimba S, Ogenyi R, Oyabba T, Temu F, Laing R. Hunger, waiting time and transport costs: time to confront challenges to ART adherence in Africa. AIDS Care. 2007;19(5):658–665. doi: 10.1080/09540120701244943. [DOI] [PubMed] [Google Scholar]
- Hardon AP, Davey S, Gerrits T, Hodgkin C, Irunde HF, Kgatlwane J, Kinsman J, Nakiyemba A, Laing R. From access to adherence: The challenges of antiretroviral treatment: Studies from Botswana, Tanzania and Uganda. Geneva: World Health Organization; 2006. [Google Scholar]
- Malcolm SE, Ng JJ, Rosen RK, Stone VE. An examination of HIV/AIDS patients who have excellent adherence to HAART. Aids Care-Psychological And Socio-Medical Aspects Of Aids/Hiv. 2003;15(2):251–261. doi: 10.1080/0954012031000068399. [DOI] [PubMed] [Google Scholar]
- Mills EJ, Nachega JB, Bangsberg DR, Singh S, Rachlis B, Wu P, Wilson K, Buchan I, Gill CJ, Cooper C. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3(11):e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, Rachlis B, Wu P, Cooper C, Thabane L, Wilson K, Guyatt GH, Bangsberg DR. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. Jama. 2006;296(6):679–690. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- Mukherjee JS, Ivers L, Leandre F, Farmer P, Behforouz H. Antiretroviral therapy in resource-poor settings. Decreasing barriers to access and promoting adherence. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S123–S126. doi: 10.1097/01.qai.0000248348.25630.74. [DOI] [PubMed] [Google Scholar]
- Munro S, Lewin S, Swart T, Volmink J. A review of health behaviour theories: how useful are these for developing interventions to promote long-term medication adherence for TB and HIV/AIDS? BMC Public Health. 2007;7:104. doi: 10.1186/1471-2458-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DA, Marelich WD, Hoffman D, Steers WN. Predictors of antiretroviral adherence. AIDS Care. 2004;16(4):471–484. doi: 10.1080/09540120410001683402. [DOI] [PubMed] [Google Scholar]
- Murphy DA, Roberts KJ, Hoffman D, Molina A, Lu MC. Barriers and successful strategies to antiretroviral adherence among HIV-infected monolingual Spanish-speaking patients. AIDS Care. 2003;15(2):217–230. doi: 10.1080/0954012031000068362. [DOI] [PubMed] [Google Scholar]
- Nam SL, Fielding K, Avalos A, Dickinson D, Gaolathe T, Geissler PW. The relationship of acceptance or denial of HIV-status to antiretroviral adherence among adult HIV patients in urban Botswana. Soc Sci Med. 2008;67(2):301–310. doi: 10.1016/j.socscimed.2008.03.042. [DOI] [PubMed] [Google Scholar]
- Patton MQ, Patton MQ. Qualitative research and evaluation methods. 3 ed. Thousand Oaks, Calif.: Sage Publications; 2002. [Google Scholar]
- Ramadhani HO, Thielman NM, Landman KZ, Ndosi EM, Gao F, Kirchherr JL, Shah R, Shao HJ, Morpeth SC, McNeill JD, Shao JF, Bartlett JA, Crump JA. Predictors of incomplete adherence, virologic failure, and antiviral drug resistance among HIV-infected adults receiving antiretroviral therapy in Tanzania. Clin Infect Dis. 2007;45(11):1492–1498. doi: 10.1086/522991. [DOI] [PubMed] [Google Scholar]
- Remien RH, Hirky AE, Johnson MO, Weinhardt LS, Whittier D, Le GM. Adherence to medication treatment: A qualitative study of facilitators and barriers among a diverse sample of HIV+ men and women in four US cities. Aids And Behavior. 2003;7(1):61–72. doi: 10.1023/a:1022513507669. [DOI] [PubMed] [Google Scholar]
- Richter DL, Sowell RL, Pluto DM. Attitudes toward antiretroviral therapy among African American women. Am J Health Behav. 2002;26(1):25–33. doi: 10.5993/ajhb.26.1.3. [DOI] [PubMed] [Google Scholar]
- Roberts KJ. Physician-patient relationships, patient satisfaction, and antiretroviral medication Adherence among HIV-infected adults attending a public health clinic. AIDS Patient Care STDS. 2002;16(1):43–50. doi: 10.1089/108729102753429398. [DOI] [PubMed] [Google Scholar]
- Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-saharan Africa: a systematic review. PLoS Med. 2007;4(10):e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan GW, Wagner GJ. Pill taking 'routinization': a critical factor to understanding episodic medication adherence. AIDS Care. 2003;15(6):795–806. doi: 10.1080/09540120310001618649. [DOI] [PubMed] [Google Scholar]
- Sankar A, Golin C, Simoni JM, Luborsky M, Pearson C. How qualitative methods contribute to understanding combination antiretroviral therapy adherence. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S54–S68. doi: 10.1097/01.qai.0000248341.28309.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- Stevens W, Kaye S, Corrah T. Antiretroviral therapy in Africa. BMJ. 2004;328(7434):280–282. doi: 10.1136/bmj.328.7434.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz L, Dick J. Managing chronic diseases in less developed countries. Bmj. 2002;325(7370):914–915. doi: 10.1136/bmj.325.7370.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. 2007 AIDS Epidemic Update. Geneva: UNAIDS; 2007. [Google Scholar]
- Wood SA, Tobias C, McCree J. Medication adherence for HIV positive women caring for children: in their own words. AIDS Care. 2004;16(7):909–913. doi: 10.1080/09540120412331290158. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Adherence to Long-Term Therapies: Policy for Action. Geneva: World Health Organization; 2001. [Google Scholar]
- World Health Organization. Innovative care for chronic conditions: building blocks for action. Geneva: WHO: World Health Organization; 2002. [Google Scholar]
- World Health Organization. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva: World Health Organization; 2007. [Google Scholar]
- World Health Organization. Toward universal access: Scaling up priority HIV/AIDS interventions in the health sector. Geneva: WHO; 2008. [Google Scholar]