Abstract
Adipose tissue-derived stem cells (ADSC) secreted CXCL5 cytokine abundantly and higher passaged ADSC up to passage 6 (P6) secreted more CXCL5 than lower passaged ADSC. Higher passaged ADSC also appeared to express higher levels of CXCL5 receptor, i.e., CXCR2. Both CXCL5 and CXCR2 were localized in the tunica intima and tunica adventitia of blood vessels in adipose tissue. Colocalization with CD34 further indicates their association with the putative ADSC in tunica adventitia. Migration assay indicates chemoattractant effects of CXCL5 on ADSC and HUVEC endothelial cells. CXCL5 also enhanced matrigel-based endothelial tube-like formation of HUVEC.
Keywords: Adipose Tissue-Derived Stem Cells, CXCL5, CXCR2, Chemoattractant, Angiogenic
1. Introduction
The adipose tissue is now recognized as a bona fide endocrine organ that actively participates in a wide range of physiological and pathological processes [1]. This recognition is important because obesity is strongly associated with several significant health problems including diabetes, cancer, atherosclerosis, cardiovascular diseases, and urinary incontinence [2; 3].
Both the parenchyma and the stroma contribute to adipose tissue’s endocrine function [1]. The parenchyma, represented by adipocytes, secretes hormone-like adipokines such as leptin that regulates food intake and fat stores. The stroma, comprised of pre-adipocytes, fibroblasts, leukocytes, macrophages, and vascular cells (endothelial and smooth muscle cells), produces cytokines such as interlukins and interferons. Among the stromal cells, macrophages have attracted the most attention due to their proven ability to regulate both inflammatory and anti-inflammatory responses in association with the development of type 2 diabetes and cardiovascular diseases [4; 5]. Another population of stromal cells, which is commonly referred to as adipose tissue-derived stem cells (ADSC), originates from the adipose vasculature [6; 7; 8] and secretes a wide variety of cytokines and growth factors [9; 10].
ADSC are well known for their regenerative potentials [6]. But, whether their therapeutic efficacy is due to their ability to differentiate into desired cell types or due to their secretion of beneficial factors remains undefined [10]. In our recently published studies [11; 12; 13; 14], we have found that ADSC, while demonstrating high therapeutic efficacy, exhibited very limited cellular differentiation. Thus, we speculated that secretory factors are more likely responsible for ADSC’s therapeutic effects. To test this hypothesis, we initiated a study in which ADSC were compared to penile smooth muscle cells (PSMC) for their secreted cytokine profile. Among the 19 different cytokines examined, CXC ligand 5 (CXCL5, also known as ENA-78 and LIX in humans and rodents, respectively) was found to be secreted 8 times more abundantly by ADSC than by PSMC [15]. Interestingly, a recent study found that CXCL5 is an adipose tissue-derived factor that links obesity to insulin resistance [16]. Furthermore, based on immunoselection with anti-CD14 antibody and RT-PCR analysis, the authors concluded that adipose tissue resident macrophages were the source of CXCL5. In the present study we show that CD14 and CXCL5 were both expressed in tunica intima and tunica adventitia of adipose tissue blood vessels. We also show that CXCL5 exhibited chemoattractant and angiogenic properties.
2. Materials and methods
2.1. Quantification of CXCL5 secretion
Rat ADSC and PSMC were isolated and passaged as previously described [13; 17]. For quantification of secreted CXCL5, cells from passages 1 to 7 (P1 to P7) were seeded in DMEM with 10% FBS at 1 × 105 cells per well in 6-well culture plates. When 90% confluence was reached, the culture medium was removed and replaced with 1ml of DMEM without FBS. Twenty-four h later, the medium was harvested and centrifuged at 1200 rpm for 10 min. The supernatant was recovered and stored at -80 °C until use. To ensure unbiased assessment, all of the processed culture media (supernatants) were simultaneously subjected to enzyme-linked immunosorbent assay (ELISA) for CXCL5 with a commercial kit (R&D Systems, Minneapolis, MN, USA). To generate statistically relevant data, each cell medium was subjected to three independent ELISA experiments and each ELISA experiment included triplicates of each cell medium.
2.2. Immunofluorescence staining
Human adipose tissues [8] were fixed in cold 2% formaldehyde and 0.002% saturated picric acid in 0.1M phosphate buffer, pH 8.0, for 4 h, followed by overnight immersion in buffer containing 30% sucrose. The specimens were then embedded in OCT Compound (Sakura Finetek USA, Torrance, CA) and stored at –80°C until use. Fixed frozen tissue specimens were cut at 5 m, mounted onto SuperFrost-Plus charged slides (Fisher Scientific, Pittsburgh, PA, USA) and air-dried for 5 min. These slides were then placed in 0.3% H2O2/ice-cold methanol for 8 min, permeabilized with 0.05% Triton X-100 for 5 min, and blocked with 5% normal horse serum in PBS for 1 h at room temperature. Afterward, the tissue section was incubated for 2 h at room temperature with primary antibody, and after PBS rinses, further incubated for 1 h with FITC or Texas Red-conjugated anti-mouse or anti-rabbit secondary antibody, followed by staining for 5 min with 4′,6-diamidino-2-phenylindole (DAPI, for nuclear staining, 1 g/mL; Sigma-Aldrich, St. Louis, MO,USA). The stained tissue sections were examined with Nikon Eclipse E600 fluorescence microscope and the images recorded with Retiga 1300 Q-imaging camera. Primary antibodies were anti-CXCR2, anti-CD34, anti-CD14 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-CXCL5 (R&D Systems, Minneapolis, MN, USA), and anti-smooth muscle actin (Abcam Inc., Cambridge, MA, USA).
2.3. Western blot analysis
Rat ADSC and PSMC were seeded in 10-cm dishes and, when reached 90% confluence, lysed in a lysis buffer containing 1% IGEPAL, 0.5% sodium deoxycholate, 0.1% SDS, aprotinin (10 g/ml), leupeptin (10 g/ml), and PBS. The homogenate was centrifuged at 3000 rpm for 15 min, and the supernatant was recovered as protein sample, which was measured for protein concentration by the BCA method (Pierce Chemical Company, Rockford, IL, USA). Cell lysates containing 20 g of protein were electrophoresed in SDS-PAGE and then transferred onto PVDF membrane (Millipore Corp., Bedford, MA, USA). Detection of protein on the membrane was performed with the ECL kit (Amersham Life Sciences Inc., Arlington Heights, IL, USA) using anti-human CXCR2 (sc-30008,Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by exposure to X-ray films. The resulting image was analyzed with Chemilmager 4000 to determine the integrated density value of each protein band.
2.4. Matrigel-based capillary-like tube formation assay
HUVEC cells were purchased from Lonza Inc., (Walkersville, MD, USA) and maintained in EGM2 medium (CC-3162, Lonza). For tube formation assay, HUVEC cells were treated with or without 15 μg/ml CXCR2 antibody (sc-30008, Santa Cruz Biotechnology) for 1h. Afterward, the cells were trypsinized and 1.5 × 105 cells were seeded into 12-well culture plates that were pre-coated with 300 μl of growth factor reduced matrigel (MA 01730, BD Biosciences, Bedford, MA, USA). The cells were maintained in EGM2 medium supplemented with or without 50 ng/ml of human CXCL5 (R&D Systems Inc.), and then incubated in a 37°C incubator with 5% CO2 for 16 h to allow the formation of tubes. Endotubes were quantified by counting 9 random fields/sample under the microscope (x50). Each condition was assessed in triplicate.
2.5. Cell migration assay
CXCL5-induced migration of human umbilical vein endothelial cells (HUVEC) was assessed in triplicate using 24-well BioCoat with an 8-mm pore size (#354578, BD Biosciences, Bedford, MA, USA). Briefly, 500 μl of Endothelial Cell Growth Medium-2 (EGM2) (Lonza Inc., Walkersville, MD, USA) containing 1% FBS and 0 ng/ml, 1 ng/ml, 10 ng/ml, or 100 ng/ml of human CXCL5 (254-XB/CF, R&D Systems, Inc., Minneapolis, MN, USA) was added to the lower chamber, and 300 μl of EGM2 containing 1 × 104 HUVEC was added to the upper chamber. After incubation for 24 h at 37 °C in a humidified atmosphere with 5% CO2, migrated cells, which adhered to the lower surface of a glass cover slide, were stained with Calcein (Invitrogen, Carlsbad, CA, USA) for 10 min and counted in 8 high-power microscopic fields. To demonstrate specificity of CXCL5-induced cell migration, the above-described cell migration assay was modified by the addition of 2.5 μg/ml of neutralizing anti-human CXCL5 antibody (MAB254, R&D Systems, Inc., Minneapolis, MN, USA) to the lower chamber that contained 10 ng/ml of CXCL5. In addition, rat ADSC and PSMC were also examined for CXCL5-induced migration; the procedure was the same as above except (1) DMEM was used in place of EGM2, (2) rat CXCL5 (543-RL, R&D Systems, Inc., Minneapolis, MN, USA) was used at 0 ng/ml, 5 ng/ml, 50 ng/ml, and 500 ng/ml, and (3) 4.0 μg/ml of anti-rat CXCL5 antibody (DY543, R&D Systems, Inc., Minneapolis, MN, USA) was used to neutralize 50 ng/ml of rat CXCL5.
2.6. Statistical Analysis
Data was analyzed with Prism 4 (GraphPad Software, Inc., San Diego, CA, USA) and expressed as mean ± standard error of the mean for continuous variables. The continuous data was compared among the groups using one-way analysis of variance. The Tukey-Kramer test was used for post-hoc comparisons. Statistical significance was set at p < 0.05.
3. Results
3.1. ADSC secrete CXCL5 and express CXCR2
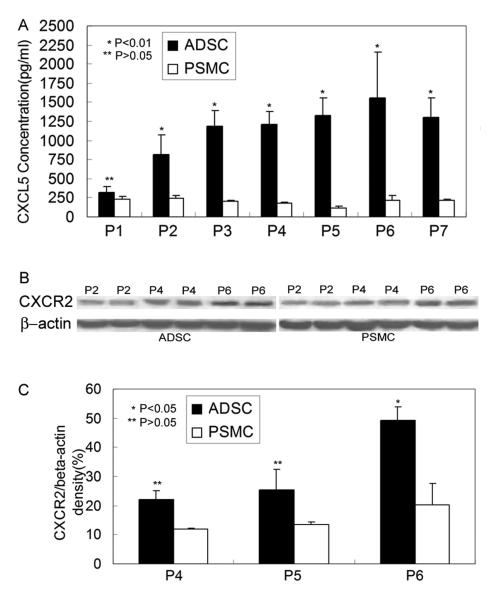
In cells cultured for up to the 7th passage (P7), CXCL5 was consistently more abundantly secreted by ADSC than by PSMC (Fig. 1A). In addition, while the level of secreted CXCL5 remained essentially unchanged in different passages of PSMC, it increased incrementally in successively passaged ADSC up to P6 (Fig. 1A). When compared at P6, ADSC secreted approximately 8 times more CXCL5 than PSMC (Fig. 1A). In addition, ADSC also expressed CXCR2 (receptor for CXCL5) at higher levels than PSMC, and higher passaged ADSC appeared to express higher levels of CXCR2 (Fig. 1B & C).
Fig. 1.
ADSC secrete CXCL5 and express CXCR2. Rat ADSC and PMSC were compared for CXCL5 secretion in culture media by ELISA (panel A) and for CXCR2 expression in cell extracts by western blot (panels B & C). P1 to P7 indicate passages 1 to 7.
3.2. Localization of CXCL5 and CXCR2 expression in adipose tissue
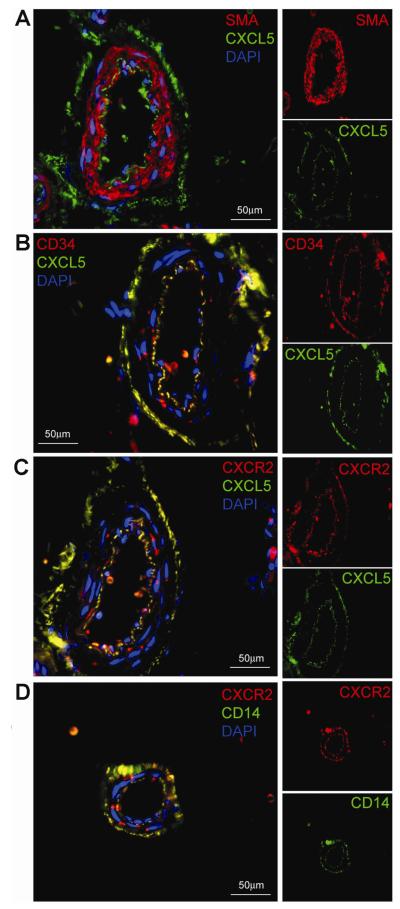
Previously we have shown that blood vessels in adipose tissue stained CD34 positive in both tunica intima (the endothelium) and tunica adventitia (progenitor cells, including ADSC) [8]. In the present study we found that these two vascular layers also stained positive for CXCL5 and CXCR2 (Fig. 2). When examined by double staining, CXCL5 and CD34 were near perfectly colocalized (Fig. 2B); the few exceptions were likely blood cells that invariably stained positive for CD34 [8]. Double staining for CXCL5 and CXCR2 also showed a large extent of colocalization although CXCR2+CXCL5- cells were readily visible in or near the tunica intima (Fig. 2C). Some CXCR2-CXCL5+ cells were also visible in the tunica adventitia (Fig. 2C).
Fig. 2.
Localization of CXCL5 and CXCR2 expression in adipose tissue. Human subcutaneous fat was examined by immunofluorescence staining. In each of the 4 composite images (panels A-D) 2 primary antibodies were employed, one labeled with FITC (green), the other Texas Red (red). A third fluor, DAPI, stains cell nuclei (blue). For clarity, each composite image is accompanied on its right by its original red- and green-stained images.
A previous study indicated that CXCL5 expression and secretion in adipose tissue were derived from macrophages, as determined by CD14 selection and RT-PCR analysis [16]. In the present study we found that, similar to the situations with CD34, CXCL5, CXCR2, expression of CD14 was localized to both tunica intima and tunica adventitia (Fig. 2D). Double staining for CD14 and CXCR2 further showed near perfect colocalization (Fig. 2D). Thus, the CXCL5-secreting CD14 positive cells are likely ADSC (See Discussion).
3.3. CXCL5 induces migration of ADSC and endothelial cells
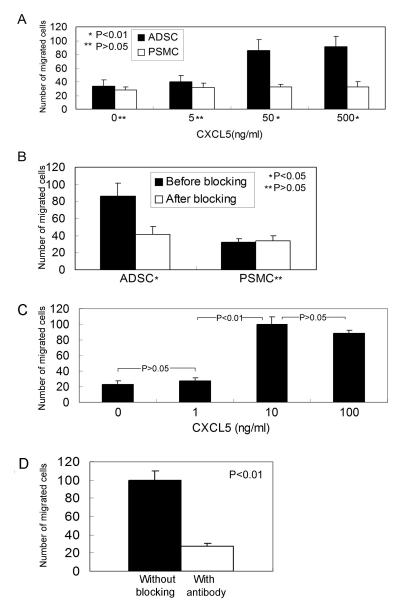
CXCL5 exerts chemoattraction through CXCR2, and, as described above (Fig. 1), ADSC expressed both CXCL5 and CXCR2. As such, we investigated whether ADSC responded to CXCL5 chemoattraction. The results show that ADSC migrated at an increasing rate in response to increasing concentrations of CXCL5 (Fig. 3A). In contrast, no such dose response was observed with PSMC (Fig. 3A). Importantly, the chemoattractant effects of CXCL5 on ADSC were abrogated by an anti-CXCL5 antibody (Fig. 3B), thus demonstrating specificity of ADSC’s migratory response to CXCL5. Moreover, since vascular endothelial cells were found to express CXCR2 (Fig. 2C & D), we also investigated whether CXCL5 induced endothelial migration through CXCR2. As shown in Fig. 3C & D, endothelial cells HUVEC migrated at significantly higher rates at 10 and 100 ng/ml than at 1 ng/ml of CXCL5, and this migratory response was blocked by anti-CXCL5 antibody.
Fig. 3.
CXCL5 attracts ADSC and endothelial cells. (A) Ability of CXCL5 to induce migration of ADSC and PMSC was assessed at 4 different concentrations (0, 5, 50, and 500 ng/ml). (B) The chemoattractant effect of CXCL5 on ADSC at the optimal concentration of 50 ng/ml was blocked by anti-CXCL5 antibody. (C & D) The same assays were applied to HUVEC, except (1) the CXCL5 concentrations were tested at 0, 1, 10, and 100, and (2) the chemoattractant effect of CXCL5 at the optimal concentration of 10 ng/ml was blocked by anti-CXCL5 antibody.
3.4. CXCL5 induces capillary-like tube formation
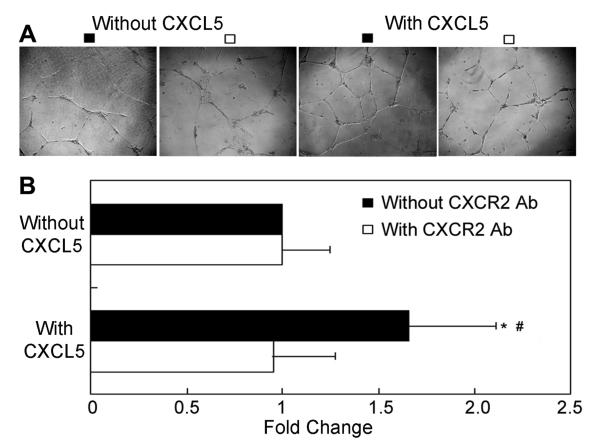
To examine whether CXCL5 is angiogenic, we performed a standard tube formation test. As shown in Fig. 4, CXCL5 significantly upregulated HUVEC’s ability to form endothelial-like tubes, and this effect was completely blocked by anti-CXCR2 antibody.
Fig. 4.
CXCL5/CXCR2 axis promotes endothelial-like tube formation in HUVEC. HUVEC cells were treated with or without 15 μg/ml CXCR2 antibody and with or without 50 ng/ml of CXCL5. (A) Representative photographs of tube-like structures. (B) Differences in the number of endotubes between different treatments are presented as fold change. * denotes P<0.01 when compared to without CXCL5/without CXCR2 Ab. # denotes P<0.01 when compared to with CXCL5/with CXCR2 antibody.
4. Discussion
The adipose tissue exerts numerous biological functions by the secretion of a wide variety of cytokines from both the parenchyma and the stroma. Within the stroma, the vasculature is the most prominent structure and is the origin of ADSC, which is increasingly recognized as one of the most promising cell types for regenerative medicine [6]. However, despite numerous reports demonstrating ADSC’s therapeutic potential, it remains unclear how ADSC exerts its therapeutic effects. In our recent studies, which showed ADSC’s remarkable ability to restore urinary and erectile functions, we have observed only limited differentiation of the transplanted cells [11; 12; 13; 14]. Therefore, in consideration of the paracrine pathway, we have begun to explore which cytokines or growth factors are responsible for ADSC’s therapeutic effects.
In the present study we first showed that ADSC secreted large amounts of CXCL5 when compared to PSMC, and longer passaged ADSC up to P6 secreted more CXCL5. The increase in CXCL5 expression/secretion during cell passage is probably due to selection of a specific cell population or as a result of increased gene expression. Further studies are needed to discriminate these two possible mechanisms. Interestingly, ADSC also expressed CXCR2 and higher passaged ADSC appeared to express higher levels of CXCR2. Such a concomitant expression of a cytokine and its receptor in ADSC and the parallel increased expression of both in longer passaged ADSC (up to P6) suggest an interdependent functional relationship. In any case, immunofluorescence analysis of adipose tissue showed that both CXCL5 and CXCR2 were identifiable in both tunica intima and tunica adventitia of blood vessels (Fig. 2). Importantly, colocalization with CD34 (Fig. 2B) indicated that CXCL5 positive cells in tunica adventitia were probably ADSC, and since standard ADSC isolation and plating procedure readily excludes endothelial cells [18], all CXCL5 positive cells in a standard ADSC culture should originate from the adventitia.
To our knowledge, only one previous study has looked into CXCL5 expression in adipose tissue. This study first established that CXCL5 was predominantly expressed in the stroma, and subsequently determined through CD14 selection that CXCL5 was secreted by macrophages [16]. However, CD14 is expressed in many cell types, including endothelial cells [19]. In the present study we confirmed CD14 expression in the endothelium (Fig. 2D). More importantly, we showed that CD14 was also expressed in the adventitia where ADSC probably reside [7; 8; 14], and these CD14 positive adventitial cells also expressed CXCR2 (Fig. 2D). Whether CD14 and CXCL5 are colocalized could not be determined due to the lack of compatible antibodies (both anti-CD14 and anti-CXCL5 are mouse antibodies). However, based on colocalization of CXCL5 and CXCR2 (Fig. 2C), it can be inferred that CD14 and CXCL5 are also colocalized. Thus, based on ELISA studies that showed abundant secretion of CXCL5 in cultured ADSC and on immunofluorescence studies that showed colocalization of CD14 and CXCL5, we believe that CXCL5-secreting cells isolated from adipose tissue through CD14 selection should have a sizable representation by ADSC. In addition, it should be noted that while the adipose macrophage study suggested an association between high CXCL5 expression and insulin resistance [16], a recent survey of 3225 people failed to find such an association [20].
The significance of CXCL5/CXCR2 coexpression (Fig. 2C) is presently unknown although migratory response to CXCL5 stimulation (Fig. 3A & C) suggests autoregulation of ADSC through the CXCL5/CXCR2 axis. Whether such an autoregulated migratory response plays a role in normal physiological or in cell-based therapy remains to be seen. On the other hand, migratory response of endothelial cells to CXCL5 (Fig. 3D) would suggest ability of ADSC to recruit endothelial cells, as during angiogenesis and vasculogenesis. Previous studies have shown that CXCL5/CXCR2 axis promotes angiogenesis [21; 22], and this was confirmed in the present study (Fig. 4). Thus, through CXCL5 expression ADSC appears to be able to recruit endothelial cells and encourage these cells to form new blood vessels. While the physiological significance of these findings remains to be seen, it appears that CXCL5 expression/secretion could contribute to ADSC’s therapeutic potential by promoting angiogenesis in ischemic tissues. While increased vascularity has been considered as the possible mechanism for functional improvements in various disease models treated with ADSC [11; 12; 13; 14; 23; 24], the present study is the first to show that the CXCL5/CXCR2 axis is likely responsible for the therapeutic effects via angiogenesis.
Acknowledgments
This work was supported by Mr. Arthur Rock, the Rock Foundation, and the National Institutes of Health (DK64538, DK045370, and DK069655).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–39. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- [2].Catenacci VA, Hill JO, Wyatt HR. The obesity epidemic. Clin Chest Med. 2009;30:415–44. doi: 10.1016/j.ccm.2009.05.001. vii. [DOI] [PubMed] [Google Scholar]
- [3].Vachharajani V, Granger DN. Adipose tissue: a motor for the inflammation associated with obesity. IUBMB Life. 2009;61:424–30. doi: 10.1002/iub.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol Lett. 2007;112:61–7. doi: 10.1016/j.imlet.2007.07.003. [DOI] [PubMed] [Google Scholar]
- [5].Mathieu P, Lemieux I, Despres JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther. 2010;87:407–16. doi: 10.1038/clpt.2009.311. [DOI] [PubMed] [Google Scholar]
- [6].Zuk PA. The Adipose-derived stem cell: looking back and looking ahead. Mol Biol Cell. 2010;21:1783–7. doi: 10.1091/mbc.E09-07-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lin CS, Xin ZC, Deng CH, Ning H, Lin G, Lue TF. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25:807–15. doi: 10.14670/HH-25.807. [DOI] [PubMed] [Google Scholar]
- [8].Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–63. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zvonic S, Lefevre M, Kilroy G, Floyd ZE, DeLany JP, Kheterpal I, Gravois A, Dow R, White A, Wu X, Gimble JM. Secretome of primary cultures of human adipose-derived stem cells: modulation of serpins by adipogenesis. Mol Cell Proteomics. 2007;6:18–28. doi: 10.1074/mcp.M600217-MCP200. [DOI] [PubMed] [Google Scholar]
- [10].Salgado AJ, Reis RL, Sousa N, Gimble JM. Adipose Tissue Derived Stem Cells Secretome: Soluble Factors and Their Roles in Regenerative Medicine. Curr Stem Cell Res Ther. 2009 doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- [11].Garcia MM, Fandel TM, Lin G, Shindel AW, Banie L, Lin CS, Lue TF. Treatment of erectile dysfunction in the obese type 2 diabetic ZDF rat with adipose tissue-derived stem cells. J Sex Med. 2010;7:89–98. doi: 10.1111/j.1743-6109.2009.01541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Huang YC, Ning H, Shindel AW, Fandel TM, Lin G, Harraz AM, Lue TF, Lin CS. The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J Sex Med. 2010;7:1391–400. doi: 10.1111/j.1743-6109.2009.01697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huang YC, Shindel AW, Ning H, Lin G, Harraz AM, Wang G, Garcia M, Lue TF, Lin CS. Adipose derived stem cells ameliorate hyperlipidemia associated detrusor overactivity in a rat model. J Urol. 2010;183:1232–40. doi: 10.1016/j.juro.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lin G, Wang G, Banie L, Ning H, Shindel AW, Fandel TM, Lue TF, Lin CS. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy. 2010;12:88–95. doi: 10.3109/14653240903350265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang H, Yang R, Wang Z, Lin G, Lue TF, Lin CS. Adipose Tissue-Derived Stem Cells Secrete CXCL5 Cytokine with Neurotrophic Effects on Cavernous Nerve Regeneration. J Sex Med. doi: 10.1111/j.1743-6109.2010.02128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chavey C, Lazennec G, Lagarrigue S, Clape C, Iankova I, Teyssier J, Annicotte JS, Schmidt J, Mataki C, Yamamoto H, Sanches R, Guma A, Stich V, Vitkova M, Jardin-Watelet B, Renard E, Strieter R, Tuthill A, Hotamisligil GS, Vidal-Puig A, Zorzano A, Langin D, Fajas L. CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metab. 2009;9:339–49. doi: 10.1016/j.cmet.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yang R, Huang YC, Lin G, Wang G, Hung S, Dai YT, Sun ZY, Lue TF, Lin CS. Lack of direct androgen regulation of PDE5 expression. Biochem Biophys Res Commun. 2009;380:758–62. doi: 10.1016/j.bbrc.2009.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–41. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- [19].Jersmann HP. Time to abandon dogma: CD14 is expressed by non-myeloid lineage cells. Immunol Cell Biol. 2005;83:462–7. doi: 10.1111/j.1440-1711.2005.01370.x. [DOI] [PubMed] [Google Scholar]
- [20].Yang Z, Zhang Z, Wen J, Wang X, Lu B, Yang Z, Zhang W, Wang M, Feng X, Ling C, Wu S, Hu R. Elevated serum chemokine CXC ligand 5 levels are associated with hypercholesterolemia but not a worsening of insulin resistance in Chinese people. J Clin Endocrinol Metab. 2010;95:3926–32. doi: 10.1210/jc.2009-2194. [DOI] [PubMed] [Google Scholar]
- [21].Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Morris SB, Xue YY, Burdick MD, Glass MC, Iannettoni MD, Strieter RM. Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J Clin Invest. 1998;102:465–72. doi: 10.1172/JCI3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Matsuo Y, Raimondo M, Woodward TA, Wallace MB, Gill KR, Tong Z, Burdick MD, Yang Z, Strieter RM, Hoffman RM, Guha S. CXC-chemokine/CXCR2 biological axis promotes angiogenesis in vitro and in vivo in pancreatic cancer. Int J Cancer. 2009;125:1027–37. doi: 10.1002/ijc.24383. [DOI] [PubMed] [Google Scholar]
- [23].Shoji T, Ii M, Mifune Y, Matsumoto T, Kawamoto A, Kwon SM, Kuroda T, Kuroda R, Kurosaka M, Asahara T. Local transplantation of human multipotent adipose-derived stem cells accelerates fracture healing via enhanced osteogenesis and angiogenesis. Lab Invest. 90:637–49. doi: 10.1038/labinvest.2010.39. [DOI] [PubMed] [Google Scholar]
- [24].Rubina K, Kalinina N, Efimenko A, Lopatina T, Melikhova V, Tsokolaeva Z, Sysoeva V, Tkachuk V, Parfyonova Y. Adipose stromal cells stimulate angiogenesis via promoting progenitor cell differentiation, secretion of angiogenic factors, and enhancing vessel maturation. Tissue Eng Part A. 2009;15:2039–50. doi: 10.1089/ten.tea.2008.0359. [DOI] [PubMed] [Google Scholar]