Abstract
Substantial progress has been made toward understanding the genetic architecture, cellular substrates, brain circuits and endophenotypic profiles of neuropsychiatric disorders, including autism spectrum disorders (ASD), schizophrenia and Alzheimer’s disease. Recent evidence implicates spiny synapses as important substrates of pathogenesis in these disorders. Although synaptic perturbations are not the only alterations relevant for these diseases, understanding the molecular underpinnings of spine pathology may provide insight into their etiologies and may reveal new drug targets. Here we discuss recent neuropathological, genetic, molecular and animal model studies that implicate structural alterations at spiny synapses in the pathogenesis of major neurological disorders, focusing on ASD, schizophrenia and Alzheimer’s disease as representatives of these categories across different ages of onset. We stress the importance of reverse translation, collaborative and multidisciplinary approaches, and the study of the spatio-temporal roles of disease molecules in the context of synaptic regulatory pathways and neuronal circuits that underlie disease endophenotypes.
In the mammalian forebrain, most glutamatergic excitatory synapses occur on small protrusions along dendrites called dendritic spines. During development and in adulthood, changes in dendritic spine number and morphology accompany synapse formation, maintenance and elimination, allowing the establishment and remodeling of connectivity within neuronal circuits. At the cellular level, spine structural plasticity is tightly coordinated with synaptic function and plasticity; for example, spine enlargement parallels long-term potentiation, whereas long-term depression is associated with spine shrinkage1. Spines undergo experience-dependent morphological changes in live animals2 and even subtle changes in dendritic spines may have marked effects on synaptic function and plasticity and patterns of connectivity in neuronal circuits. Notably, disease-specific disruptions in dendritic spine shape, size or number accompany a large number of brain disorders, suggesting that dendritic spines may serve as a common substrate for many neuropsychiatric disorders, particularly those that involve deficits in information processing.
Here we explore the idea that clinical findings should guide basic science’s approach to the relationship between dendritic spines and neurological disease. We use autism spectrum disorders (ASDs), schizophrenia and Alzheimer’s disease as example disorders, each of which can be characterized by severe information-processing deficits with impairments in neuronal connectivity and plasticity. We review recent postmortem neuropathological studies that reveal the pathological alterations in neuronal structure in the brains of affected individuals, genetic studies that address etiology, and animal and cellular studies that investigate the underlying neurobiological mechanisms. We discuss the strengths and weaknesses of both clinical and basic scientific evidence for the role of dendritic spine dysmorphogenesis in these disorders and suggest that future animal models, molecular mechanisms and genetic screens should be integrated with clinical and pathological findings in humans. This general approach will allow future research to determine the biological role of spine alterations in the pathophysiology of diseases, to model each of the diseases more accurately and to hasten the development of therapies that can target the biological processes and molecular mechanisms at work.
Neuropathological studies of spine morphology
ASD, schizophrenia and Alzheimer’s disease are neurological disorders that are characterized by marked disruptions in information processing and cognition, and recent studies support altered synaptic connectivity and plasticity in the brains of affected individuals3–6. Although Alzheimer’s disease is a definitive neurodegenerative disease, much evidence supports synaptic dysfunction as a preceding and contributing insult to eventual neuronal death7. Notably, the symptoms of each of these disorders manifest at distinct stages of life, suggesting that dysregulation of synaptic structure and function can coincide with unique deficits in cognition and behavior depending on when the disruptions occur across the lifespan (Fig. 1). ASDs, characterized by deficits in social interactions, disruption of verbal communication and the presence of repetitive behavior, affect 0.9% of children, with diagnosis usually occurring around 2–3 years of age8. Accumulating evidence from human neuropathological, genetic and model system studies suggests that autism may be conceptualized as a disease of the synapse8. Schizophrenia is a heterogeneous disorder affecting thought, perceptions of reality, affect and cognition, which affects approximately 0.5–1% of the population. Symptoms typically emerge in late adolescence or early adulthood. Finally, Alzheimer’s disease is a neurodegenerative disease, with a typical onset of age 65, marked by progressive loss of memory, critical reasoning and other cognitive abilities. Dementia affects an estimated 35.6 million people worldwide (http://www.alz.co.uk/research/worldreport/), with Alzheimer’s disease representing the most common form of dementia. Although amyloid plaques, neurofibrillary tangles and cell death remain defining characteristics of Alzheimer’s disease, findings from neuropathological and molecular studies provide strong support for synapse degeneration as having a central role in Alzheimer’s disease pathology7. Together, these disorders span the entire human life and comprise symptoms that have altered cognition in common, but with distinctions in socio-linguistic (ASD), perceptive (schizophrenia) and memory-related (Alzheimer’s disease) behaviors.
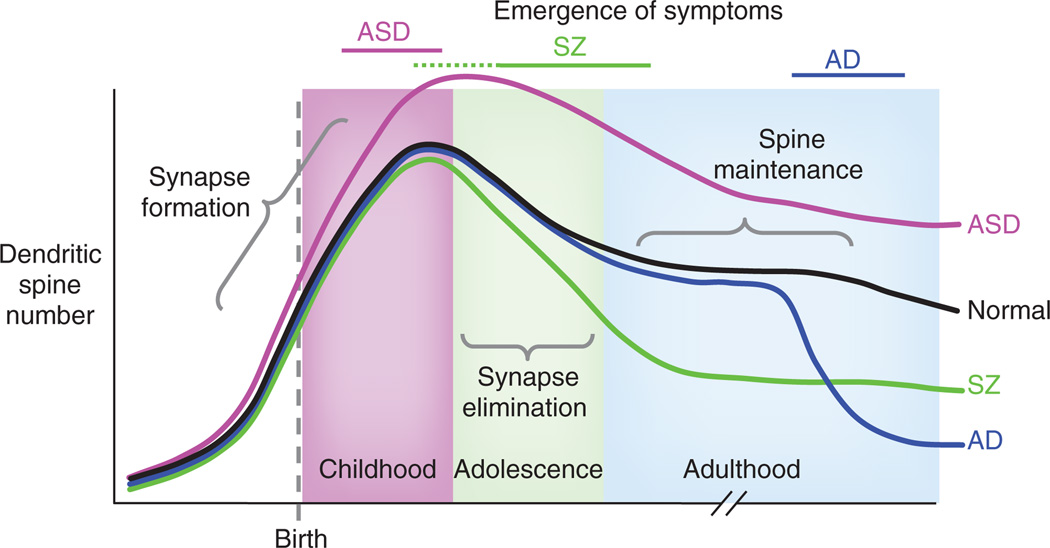
Figure 1.
Putative lifetime trajectory of dendritic spine number in the in a normal subject (black), in ASD (pink), in schizophrenia (SZ, green) and in Alzheimer’s disease (AD) (blue). Bars across the top indicate the period of emergence of symptoms and diagnosis. In normal subjects, spine numbers increase before and after birth; spines are selectively eliminated during childhood and adolescence to adult levels. In ASD, exaggerated spine formation or incomplete pruning may occur in childhood leading to increased spine numbers. In schizophrenia, exaggerated spine pruning during late childhood or adolescence may lead to the emergence of symptoms during these periods. In Alzheimer’s disease, spines are rapidly lost in late adulthood, suggesting perturbed spine maintenance mechanisms that may underlie cognitive decline.
Although dendritic spine alterations in other neurodevelopmental disorders, such as mental retardation, have been known for some time9, neuropathological data for spine dysmorphogenesis in ASD have only recently been provided4. This recent evidence from Golgi-impregnated post-mortem ASD human brain tissue revealed an increase in spine density on apical dendrites of pyramidal neurons from cortical layer 2 in frontal, temporal and parietal lobes and layer 5 only in the temporal lobe4. Spine density was inversely correlated with cognitive function. Such findings are consistent with an emerging hypothesis that the brains of individuals with ASD are characterized by hyperconnectivity in local circuits and hypoconnectivity between brain regions10. Further evidence of spine pathology is observed in tissue from individuals with diseases comorbid with autism. Similar to ‘pure’ autism, the fragile X brain is characterized by elevated spine density, which is thought to result from pruning deficits, with elongated, tortuous spine morphologies11, indicative of altered function. Together, these findings underscore the profound spine pathology exhibited by ASDs and comorbid disorders. It is possible that spine dysmorphology contributes to abnormalities in specific circuits, which in turn may underlie the socio-cognitive impairments characteristic of these disorders.
Neuropathological lines of evidence supporting synaptic pathology for schizophrenia and Alzheimer’s disease are far better characterized than for ASD. One of the defining neuropathological features of schizophrenia is gray matter loss, which is accelerated during periadolescence12. Several postmortem studies have examined spine density changes in brain regions showing the greatest indices of gray matter loss in schizophrenia and these results support the view that spine density changes directly contribute to gray matter loss in the disease3. The dorsolateral prefrontal cortex (DLPFC) shows severe dysfunction in schizophrenia, as affected individuals show reduced activity of this region during cognitive tasks13. Indeed, spine loss in the DLPFC has been reported, particularly in layer 3 neurons5. A reduction in superior temporal gyrus gray matter volume is one of the most consistently reported alterations in the schizophrenia brain14. At the cellular level, individuals with schizophrenia show a profound reduction in spine density on pyramidal neurons in the superior temporal gyrus, particularly in the primary auditory cortex15. Several studies have shown reductions in hippocampal volume and reduced spine density on subicular and CA3 dendrites in schizophrenia16,17. Collectively, these studies reveal strong associations between brain region–specific loss of gray matter, reduced spine density and functional hypoactivity in schizophrenia.
Unlike ASD and schizophrenia, Alzheimer’s disease research has benefited from over a century of neuropathological investigation. Studies analyzing postmortem tissue samples from patients diagnosed with Alzheimer’s disease consistently report prominent synapse loss7,18. Dendritic spine loss is observed in the hippocampus and throughout the cortex, the principal areas affected by Alzheimer’s disease–related pathology18,19. Dystrophic neurites are also associated with amyloid plaques in the brains of individuals with Alzheimer’s disease19,20. Notably, synapse and dendrite loss demonstrate a stronger correlation to cognitive decline than do neurofibrillary tangles or neuronal loss18. Detailed analysis of postmortem tissue has revealed synapse loss in mild cognitive impairment (MCI), but an even greater loss in Alzheimer’s disease, indicating that synapse loss occurs early in the progression of Alzheimer’s disease and worsens as the disease advances21. Furthermore, synapse loss often appears greater than what would be expected from neuronal death, underscoring synaptic dysgenesis as a prominent pathology of Alzheimer’s disease, rather than a byproduct, and a driving factor in cognitive decline19. That synapse deterioration begins early in Alzheimer’s disease highlights the need to develop better diagnostics and more thoroughly investigate the neurological changes that take place during prodromal stages of the disease, which is likely the most opportune time for intervention. The brain may possess an innate ability to forestall Alzheimer’s disease insults, as some studies note compensatory synaptic changes in Alzheimer’s disease, such as increased size in remaining spines22. Further research into these mechanisms is required as they may signify modifiable pathways capable of combating disease progression.
Layer-specific loss of spines in schizophrenia and ASD has intriguing implications for understanding disease etiology. Notably, in schizophrenia, loss of spines occurs in layer 3 of the DLPFC5, but not in layers 5 and 6 (ref. 23). This is interesting considering that layer 3 neurons undergo more substantial synaptic pruning during adolescence than layer 5/6 neurons in primates24. Although postmortem studies cannot identify the root cause of spine loss, it is likely that spine formation and stability are reduced or spine pruning is accelerated in schizophrenia (Fig. 1). Similarly, in ASD the increase in spine density found in layer 2 of the frontal and parietal lobe, which was not evident in layer 5, could indicate lamina-specific disruptions in synaptic formation and/or pruning.
In summary, neuropathological evidence points toward synapse and dendritic spine loss in schizophrenia and Alzheimer’s disease, whereas ASDs seem characterized by increased spine numbers. Several caveats must accompany studies of postmortem human tissue, such as postmortem interval, symptomatic heterogeneity and patient medical history. As these neuropathological studies are performed in an advanced stage of the disease, they reflect an endpoint of the disease process and do not distinguish between cause, consequence, compensation or confound. However, they are the best and sometimes only source of information about cellular alterations in the actual patient brain and, taken with the above caveats, should be considered a reference for neurobiological studies. Any technological advance that could improve in vivo characterization of substructures in humans will greatly aid the effort to understand fully the effects of each disease on synapse structure and connectivity in the affected brain.
Genetics and molecular mechanisms
The genetics of ASD, schizophrenia and Alzheimer’s disease have substantially driven our understanding of the etiology of each disorder. Although these diseases have varying degrees of heritability and a complex genetic architecture, genetic studies have guided research toward the molecular pathways that are relevant for pathology. Genome-wide association studies (GWASs), candidate gene resequencing, copy number variant (CNV) and single-nucleotide polymorphism (SNP) analyses have identified a multitude of common and rare variants that associate to varying degrees with these disorders. Many of these genes have defined roles in synapse regulation25 (Table 1).
Table 1.
Candidate molecules for susceptibility to ASD, schizophrenia or Alzheimer’s disease and their role in spine morphogenesis
| Protein | Functional role | Disease association |
Evidence for disease association |
References for disease association |
Role in dendritic spine morphogenesis |
Evidence for spine effects |
References for dendritic spine effects |
|---|---|---|---|---|---|---|---|
| Neuroligin-3 | Postsynaptic adhesion protein | ASD | Rare variants | Reviewed in ref. 28 | ↑ spine density | Cell culture | 29 |
| Neuroligin-4 | Postsynaptic adhesion protein | ASD | Rare variants | Reviewed in ref. 28 | ↑ spine density | Cell culture | 29 |
| Neurexin1 | Presynaptic adhesion protein | ASD | Rare variants | Reviewed in ref. 28 | ↑ spine density | Transgenic mouse | 30 |
| Shank3 | Postsynaptic scaffold | ASD | Rare variants | 31 | ↑ spine density | Cell culture | 33 |
| Shank2 | Postsynaptic scaffold | ASD | Rare variants | 32 | ↑ spine size | Cell culture | 34 |
| Epac2 | Rap GEF | ASD | Rare variants | 35 | ↓ spine size and stability | Cell culture | 36 |
| FMRP | Regulator of protein synthesis | ASD comorbid (Fragile X syndrome) |
Trinucleotide repeat-induced gene silencing | Reviewed in ref. 42 | ↑ spine density | Transgenic mouse | Reviewed in ref. 42 |
| MeCP2 | Transcription factor | ASD comorbid | Mutations | 43 | ↑ synapse density | Transgenic mouse; | 43 |
| (Rett syndrome) | ↑ spine length | cell culture | |||||
| ↓ spine breadth | |||||||
| Ube3A | E3 ubiquitin ligase | ASD comorbid | Chromosomal duplications | 44 | ↓ spine density and length | Transgenic mouse; cell culture | 45 |
| TSC1 | Tumor suppressor protein | ASD comorbid | Mutations | Reviewed in ref. 37 | ↓ spine size and ↑ density | Cell culture | 39 |
| TSC2 | Tumor suppressor protein | ASD comorbid | Mutations | Reviewed in ref. 37 | ↓ spine size and ↑ density | Cell culture | 39 |
| PTEN | Tyrosine phosphatase | ASD comorbid | Mutations | Reviewed in ref. 37 | ↓ spine density | Transgenic mouse | 40 |
| DISC1 | Scaffold | SZ | Translocation or SNPs | 52, 53 | ↑ or ↓ spine size and density | Cell culture, transgenic mouse | 54, 90 |
| NRG1 | Secreted trophic factor | SZ | High-risk SNPs | Reviewed in ref. 47 | ↑ spine size and density | Transgenic mouse | 48 |
| ErbB4 | Postsynaptic receptor tyrosine kinase | SZ | Rare mutations, SNPs, CNV expression changes | Reviewed in ref. 47 | ↑ spine size and density | Cell culture | 49 |
| Kalirin | Rac GEF | SZ, AD | ↓ Expression, missense mutations | 56, 57, 72 | ↑ spine size and density | Cell culture, transgenic mouse | 71, 92 |
| ApoE ε4 | Lipoprotein metabolism and transport | AD | Presence of ApoE-ε4 allele (SNPs) | Reviewed in ref. 65 | ↓ spine density | Transgenic mouse, human tissue | 66 |
| Presenilin-1 | Part of γ-secretase protease complex | AD | Mutations | Reviewed in ref. 61 | ↑ spine size and density | Transgenic mouse | 96 |
| Drebrin A | F-actin-binding protein | AD | ↓ expression | Reviewed in ref. 20 | ↑ spine size and density | Cell culture | 20 |
| Calcineurin (PP2B) | Calcium-sensitive phosphatase | AD | ↑ activation | 73 | ↓ spine density | Cell culture, transgenic mouse | 76 |
ASD-associated proteins: neuroligin-3, neuroligin-4, neurexin1, Shank3, Shank2, Epac2, FMRP, MeCP2, Ube3A, TSC1, TSC2, PTEN; schizophrenia (SZ)-associated proteins: DISC1, NRG1, Erb4; Alzheimer’s disease (AD)-associated proteins: ApoE ε4, presenilin-1, Drebrin A, calcineurin. Kalirin is associated with both schizophrenia and Alzheimer’s disease. Shank3, SH3 and multiple ankyrin repeat domains 3; Epac2, exchange protein directly activated by cAMP; TSC1, tuberous sclerosis protein 1; TSC2, tuberous sclerosis protein 2; FMRP, fragile X mental retardation protein; MeCP2, methyl CpG binding protein 2; PTEN, phosphatase and tensin homolog; DISC1, disrupted-in-schizophrenia 1; NRG1, neuregulin 1; ErbB4, v-erb-a erythroblastic leukemia viral oncogene homolog 4; ApoE ε4, ε4 allele of apolipoprotein E.
It is becoming clear that disorders such as ASD and schizophrenia could be best described by a ‘common disease, many rare mutations’ model25, with mutations being individually rare or even ‘private’ (only accounting for a minority of cases), but highly penetrant. This model highlights the importance of determining the signaling pathways in which disease risk genes function, with the aim of identifying new candidate genes for deep sequencing and new therapeutic targets by identifying more druggable proteins in the pathway.
Although disease candidate genes may reveal causal factors, molecules with altered expression in synapses of diseased brains or that functionally interact with established risk genes could be yet unidentified etiological factors in need of genetic confirmation. They might also be modulators of genetic susceptibility or mediators of the brain’s adaptive response to the original insults. As such, recent studies have begun to examine the expression of disease genes in postmortem tissue and the molecular interactions of disease molecules with spine regulators.
The high degree of heritability of ASDs has sparked a great deal of genetic research over the last decade (reviewed in ref. 26). Notably, multiple reports have identified rare mutations and CNVs in genes encoding synaptic proteins in autistic individuals, supporting the hypothesis that synaptic dysfunction may be important in the etiology of ASDs8. Although each of these mutations may not account for a large percentage of cases, consistent with a ‘common disease, many rare alleles’ model25, they provide valuable insight into the molecular pathways underlying pathogenesis (Fig. 2).
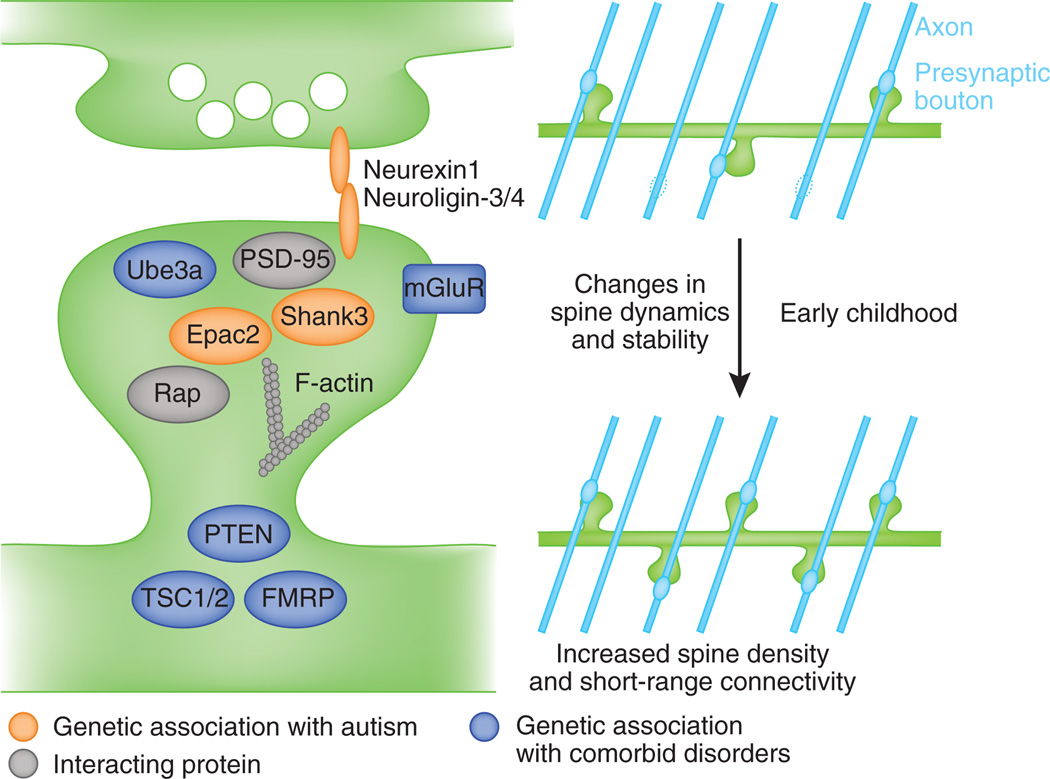
Figure 2.
Model of molecular mechanisms of spine pathology in ASD. Proteins with genetic associations with ASD and comorbid disorders participate in pathways that regulate spine morphogenesis. Their disruption may alter spine dynamics and stability, leading to an increase in spine density and increased connectivity with nearby axons (blue lines) during early childhood.
Several molecular and genetic themes in ASD synaptic pathology are emerging that include small GTPase and adhesion-related signaling pathways8,27. Rare variants in the genes for synaptic cell adhesion proteins neuroligin 3 and 4 (NLGN3, NLGN4) and their presynaptic ligand neurexin1 (NRXN1) have been genetically linked with autism28. Point mutants in NLGN3 and NLGN4, a truncation of NLGN4, and a promoter mutation in NLGN4 have been identified. Data gleaned from cell biological studies have shown that NLGN3 or NLGN4 increase excitatory synapse number in hippocampal neurons and the NLGN3 Arg451Cys variant prevents this induction of synapse formation29, whereas α-neurexin loss reduces dendrite length and total spine number in cortex30. Members of the Shank family of postsynaptic scaffolding proteins have also been linked with ASD susceptibility. Autism-associated mutations in SHANK3 (ref. 31) include a variety of frameshift, truncation and missense mutations, and more recently, de novo CNVs and inherited point mutation have been identified in the SHANK2 gene in patients with ASD and mental retardation32. Shank3 controls spine maintenance in forebrain33, whereas Shank2 has been implicated in activity-dependent spine remodeling34. Finally, rare structural variants of RAPGEF4, encoding the synaptically localized Rap guanine nucleotide exchange factor (GEF) Epac2, have been identified in autistic individuals35. Epac2 missense mutations increase dendritic spine number (Epac2-T809S) and area (Epac2-V646F)36. Notably, NLGN3 also complexes with Epac2 and enhances its signaling activity36 and Shank3 may complex with and signal downstream of NLGNs37. Thus, NLGN3, NRXN1, Epac2 and the Shank proteins constitute members of a synaptic regulatory pathway, disruption of which could confer ASD-like synapse pathology.
In addition to genetic analysis of individuals with ASD, genetic and molecular studies investigating monogenic disorders comorbid with ASDs may also shed light on ASD etiology. Individuals with mutations of tuberous sclerosis proteins 1 and 2 (TSC1, TSC2) or the phosphatase and tensin homolog (PTEN) gene, which causes macroencephaly, are frequently diagnosed with autism38 and each of these proteins regulates synaptic structure39,40. Their mutation or loss yields deficits in neuronal morphology and connectivity. PTEN deficiency results in dendritic hypertrophy and elevated spine density41 and TSC1 or TSC2 loss causes enlarged spines39. Fragile X syndrome results from transcriptional silencing of the FMR1 gene, which in turn causes an upregulation in global dendritic translation rates that may contribute to the elevated spine density observed in the brains of affected individuals42. MeCP2, a transcriptional regulator that is mutated in Rett syndrome, controls the function of excitatory synapses and spine morphology in an activity-dependent manner43. Lastly, maternal duplications of chromosome 15q11–q13, a region that encompasses the Angelman syndrome gene UBE3A, are associated with autism44. UBE3A encodes an E3 ubiquitin ligase whose maternal deficiency reduces dendritic spine density and length in cerebellar and hippocampal pyramidal neurons45, suggesting a potential link between Angelman syndrome, autism and altered synaptic structure. These disorders, which have an autistic phenotype, can be useful in determining relevant molecular mechanisms in ASD pathogenesis. However, as these disorders are pathologically and genetically distinct from ‘pure’ autism, the relevance of specific molecular mechanisms and cellular alterations to pure autism should be considered with caution.
Over 240 gene variants have been associated with schizophrenia. Of these, a handful of genes have shown consistent associations with schizophrenia, including, but not limited to, NRG1, ERBB4 and DISC1 (Fig. 3). In addition, rare but highly penetrant CNVs have been found in many genes encoding synaptic proteins46.
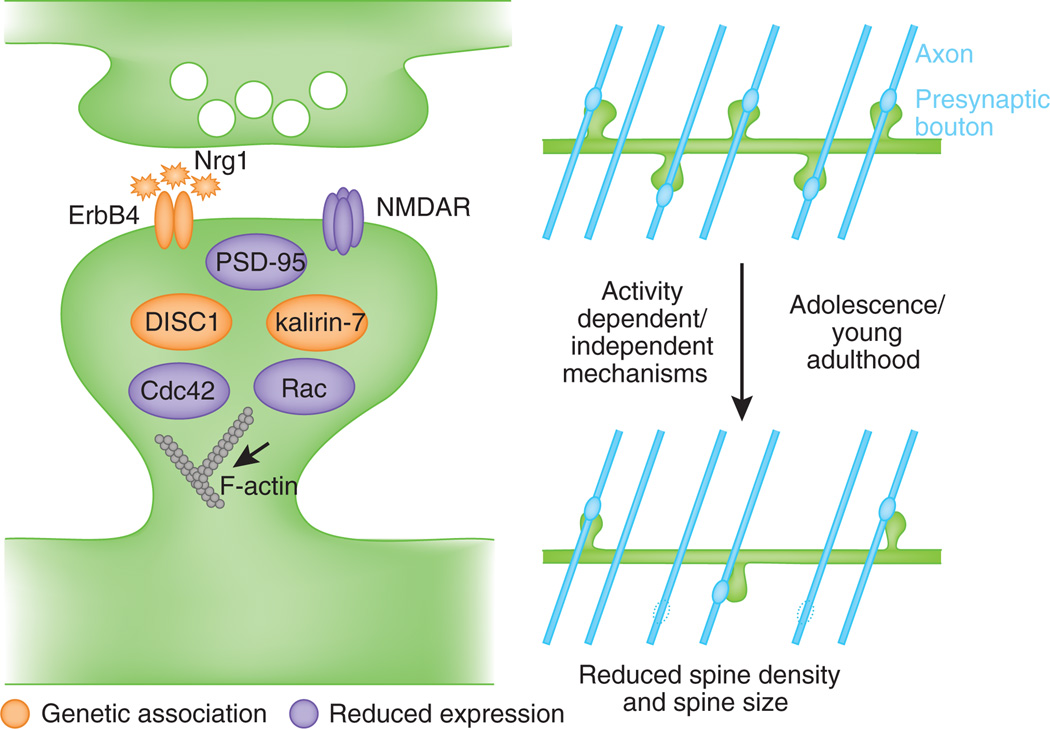
Figure 3.
Model of molecular mechanisms contributing to spine dysfunction in schizophrenia. Molecules genetically or neuropathologically implicated in schizophrenia interact with regulators of spine plasticity and maintenance. Their disruption may lead to exaggerated spine loss and loss of connectivity with axons (blue lines) in late adolescence or early adulthood.
Polymorphisms in NRG1 are associated with schizophrenia47. Neuregulins are trophic factors that exist in both membrane-bound and soluble form. ErbB receptors are postsynaptic receptor tyrosine kinases and are activated on neuregulin binding47. ErbB4 is thought to be the predominant receptor for NRG1. Notably, a rare CNV for ERBB4 has been identified in schizophrenia46. This mutation is a deletion that would result in a protein lacking most of its intracellular kinase domain, akin to a dominant-negative protein. ErbB4 is expressed in interneurons and, less abundantly, in cortical pyramidal cells and in spines. NRG1 and erbB4 regulate spine structure and function; long-term NRG1 treatment increases pyramidal neuronal spine density and the preponderance of spines with mature phenotypes48. ErbB4 overexpression increases spine density, area and excitatory synaptic transmission49. Conversely, erbB4 knockdown reduces spine density and size in a cell-autonomous fashion49.
The 22q11.2 microdeletion syndrome is the most common CNV associated with schizophrenia, accounting for 1–2% of cases50. Primary hippocampal neurons from mice engineered to carry the 1.3-Mb orthologous chromosomal region (Df(16)A+/−) showed reduced spine density and sizes51. Loss of either of two genes in this region (ZDHHC8 and DGCR8) was sufficient to impair spine and dendrite morphology50,51. ZDHHC8 is a palmitoyl transferase that palmitoylates the postsynaptic density scaffolding molecule PSD-95; ZDHHC8 loss results in reduced spine density and simpler dendrites and its replacement into Df(16)A+/− neurons rescued spine and dendrite deficiency51. Dgcr8 is involved in miRNA processing and its loss results in smaller spines and simpler dendrites50.
Postmortem expression studies revealed changes in molecules that regulate spine morphology in schizophrenia subjects. In parallel, neurobiological studies have uncovered interactions of schizophrenia-associated molecules with spine regulators. The initial link of the disrupted in schizophrenia 1 (DISC1) gene to schizophrenia was identified in a Scottish pedigree with a disruption of the DISC1 open reading frame52. Polymorphisms and frame shift mutations of DISC1 have been linked to schizophrenia in other lineages53. Long-term DISC1 knockdown in cortical neurons reduces spine area54. Although DISC1 mRNA levels seem unaffected in individuals with schizophrenia55, the expression of DISC1-interacting proteins was reduced in individuals carrying high-risk DISC1 SNPs55, suggesting that DISC1 function might be affected in schizophrenia. Disruption of DISC1’s ability to scaffold proteins in spines would be expected to have deleterious consequences on spine morphogenesis.
DISC1 is known to interact with several well-established regulators of spine morphogenesis, most prominently the RacGEF kalirin-7 (ref. 54). Recently, kalirin-7, via activation of its downstream effector Rac1, was found to directly regulate the effects of DISC1 on spine morphology54. Notably, the expression of KALRN (kalirin) mRNA was reduced in the DLPFC of individuals with schizophrenia, irrespective of antipsychotic treatment56. Loss of kalirin strongly correlates with spine loss in layer 3 prefrontal cortex neurons56. Recently, several missense mutations in the KALRN gene were identified in schizophrenia. These mutations occurred in evolutionary conserved gene regions and are predicted to have functional consequences57.
Scaffolding proteins function as organizing molecules in spines and provide a structural link between surface receptors, including glutamatergic receptors, and intracellular signaling networks. ErbB4 and DISC1 interact with PSD-95 in spines54,58. Notably, PSD-95 protein levels are reduced in the schizophrenia cortex59. A loss of scaffolding proteins in spines could alter glutamatergic receptor signaling, disruption of which is theorized to contribute to the etiology of schizophrenia60.
Although genetic findings have substantially directed Alzheimer’s disease research, the contributions of specific genes to Alzheimer’s disease are complex and incompletely understood. Familial Alzheimer’s disease, which has an autosomal dominant form of inheritance and early onset, has been associated with mutations in APP, PSEN1 and PSEN2, three genes that are critical for beta amyloid (Aβ) production61. Mutations found in familial Alzheimer’s disease are well known to increase Aβ production and cellular studies provide compelling evidence that soluble Aβ oligomers disrupt synaptic signaling (reviewed in ref. 62). Furthermore, Aβ oligomers have been clearly shown to target spines, induce spine dysgenesis, and reduce spine density63,64.
The vast majority of Alzheimer’s disease cases, however, develop after age 65, referred to as late-onset Alzheimer’s disease (LOAD). Although hundreds of genes have been proposed as LOAD risk factors, the gene encoding apolipoprotein E (APOE) is widely accepted as the most important risk factor65. Specifically, the ε4 (APOE ε4) allele is associated with greater risk of developing Alzheimer’s disease, whereas APOE ε2 is considered to be neuroprotective. Studies with transgenic mice recently revealed that ApoE isoforms differentially influence dendrite and dendritic spine morphology. Mice expressing human APOE ε4 display reduced spine density in the dentate gyrus when compared with wild-type mice and mice expressing human APOE ε3 (ref. 66). The authors also found an inverse correlation between APOE ε4 dose and dentate gyrus spine density in human brain. In another study, human APOE ε4 was found to reduce dendritic length and branching in the mouse cortex and hippocampus67. It has also been reported that expression of APOE ε2 in a mouse model of Alzheimer’s disease can restore spine density to control levels68. It is fascinating that the major genetic risk factor for LOAD affects dendrite and spine morphology, but the underlying mechanisms remain unknown and require further investigation.
A recent independent GWAS, in addition to reaffirming APOE as the primary Alzheimer’s disease genetic risk factor, identified new LOAD susceptibility genes65. One example is the gene encoding for clusterin (CLU), also known as ApoJ, which has many similarities to ApoE, including the ability to bind Aβ. It will be interesting to learn whether clusterin, similar to ApoE, modulates expression of dendrites and dendritic spines. Notably, PICALM, another susceptibility gene identified by GWAS, has been associated with inducing dendritic dystrophy and disrupting vesicle transport when underexpressed in embryonic hippocampal neurons65. As new genetic risk factors are identified and manipulated experimentally, it will be important to assess dendrite and dendritic spine phenotypes.
An abundance of genetic data suggests that Aβ is vital for Alzheimer’s disease pathogenesis and molecular studies indicate that Aβ acts on synapses to mediate its toxic effects. Individuals with Alzheimer’s disease demonstrate altered expression of many synaptic proteins21. Presynaptic proteins such as synaptophysin are reduced in individuals with MCI or Alzheimer’s disease7 and several postsynaptic signaling molecules are also affected in Alzheimer’s disease. Although the precise mechanisms that cause spine degeneration in Alzheimer’s disease remain unclear, recent findings suggest that signaling pathways regulating synaptic plasticity may be crucial (Fig. 4).
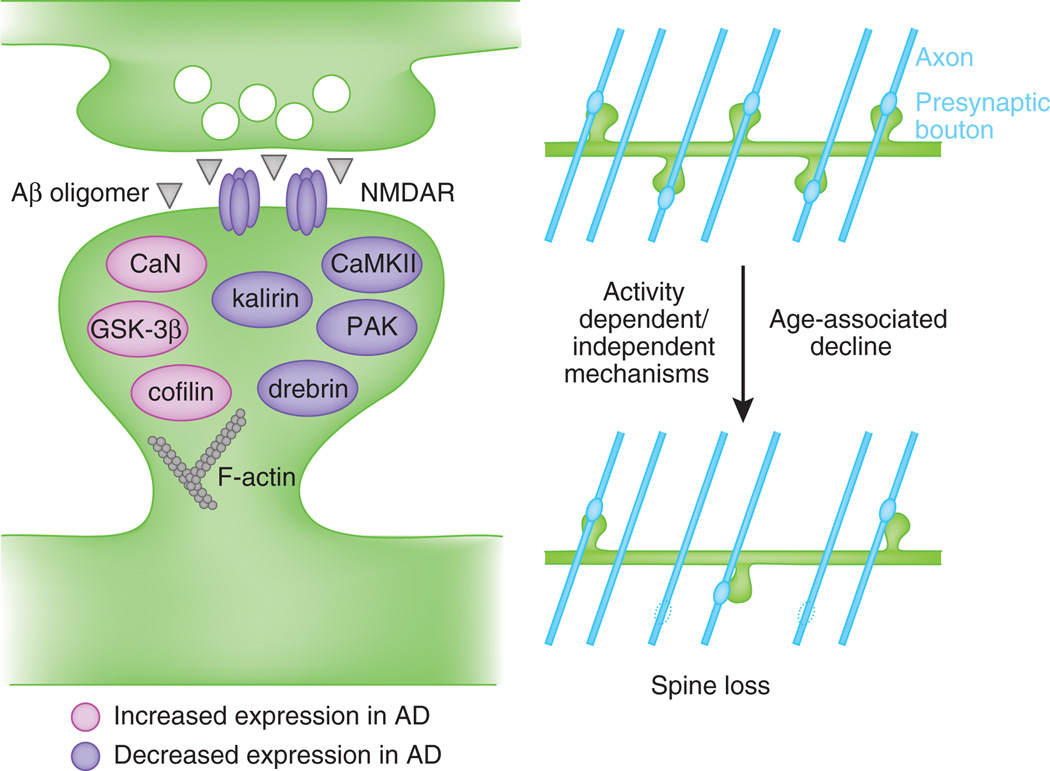
Figure 4.
Model of molecular mechanisms involved in spine pathology in Alzheimer’s disease. Aβ oligomers disrupt synaptic plasticity mechanisms and induce spine dysgenesis, likely by interfering with NMDAR-dependent regulation of the spine cytoskeleton, causing synapse loss and decreased connectivity with nearby axons (blue lines) later in life.
Cofilin and drebrin are actin-binding proteins that exert opposite effects on actin dynamics, but both are affected in Alzheimer’s disease. Active cofilin causes actin destabilization and much evidence supports a role for cofilin in neurodegeneration, including Alzheimer’s disease20. Drebrin, a postsynaptic protein that binds and stabilizes actin in spines, is reduced in the brains of individuals with Alzheimer’s disease and in transgenic animal models of the disease20.
A critical regulator of actin assembly in spines is p21-activated kinase (PAK), a downstream effector molecule of Rac69,70. In the hippocampus of individuals with Alzheimer’s disease and in animal models of Alzheimer’s disease, PAK activation is markedly reduced and mislocalized69. Furthermore, pharmacological inhibition of PAK in mice was sufficient to cause memory impairment, cofilin pathology and drebrin loss. Notably, mRNA and protein levels of kalirin-7, a key regulator of spine morphogenesis and an upstream activator of PAK in spines, were found to be substantially diminished in the hippocampus of individuals with Alzheimer’s disease71,72, suggesting a role for the kalirin-7–Rac1–PAK pathway in Alzheimer’s disease–associated spine pathology.
Most proteins implicated in Alzheimer’s disease pathology Experience downregulation in the disease; however, calcineurin over-activation has been reported in individuals with Alzheimer’s disease and animal models73. Calcineurin (CaN or PP2B) is a calcium-sensitive phosphatase involved in synaptic plasticity whose activation leads to synaptic weakening and Alzheimer’s disease–related pathology has been shown to increase activation of GSK-3β, a downstream effector molecule of calcineurin74,75. Long-term depression induced by Aβ oligomers in hippocampal CA1 requires calcineurin activity, evidence that aberrant molecular changes in Alzheimer’s disease also give rise to functional deficits74. Thus, over-activation of a NMDAR–calcineurin–GSK-3β pathway may indicate a mechanism by which synapses degenerate in Alzheimer’s disease. Notably, Aβ oligomer–induced spine loss and dendritic dystrophies can be prevented by calcineurin inhibition76.
Many of the signaling molecules identified as having a potential role in Alzheimer’s disease pathogenesis lend support to the emerging hypothesis that, in Alzheimer’s disease, Aβ oligomers promote synaptic degeneration by acting on spines to create an imbalance of synaptic plasticity mechanisms62,77 (Fig. 4). Aberrant NMDAR activation can induce calcium signaling perturbations and, indeed, most of the signaling molecules discussed above are known to be downstream of NMDARs. The putative role of NMDARs in mediating Alzheimer’s disease pathology gains further support from studies showing that Aβ reduces surface expression of NMDARs and AMPARs77,78 and others demonstrate synaptotoxic effects of Aβ can be prevented by NMDAR antagonists64. Moreover, in vitro and in vivo models of Alzheimer’s disease, Aβ oligomers produce aberrant long-term potentiation expression and impair memory62.
Although much progress has been made, precise signaling cascades underlying synapse loss and cognitive decline need to be further elucidated. Determining the molecular processes underlying synapse degeneration in Alzheimer’s disease is critical for understanding the disease and for developing effective therapeutics.
Animal models
Animal models, particularly transgenic mouse lines, have proved to be invaluable for understanding the biological processes behind human diseases. Their diversity and the development of new experimental manipulations, such as in vivo imaging and advanced behavioral characterization, have led to an improvement in their applications.
Mouse models of ASD, with deficits in up to three core behavioral domains, have begun to approximate ASDs in newly developed behavioral tasks, such as social approach, ultrasonic vocalizations and measurements of repetitive motor behaviors79. Surprisingly, however, very little is known about spine morphology in ASD animal models. NLGN3-R451C transgenic mice display impaired social interaction behavior, but show enhanced performance in the Morris water maze, as well as enhanced inhibitory synaptic strength80. In contrast, independently generated NLGN3-R451C transgenic mice were observed to have delayed developmental phenotypes, such as decreased ultrasonic vocalizations and slower righting reflexes, but no changes were seen in social interaction or spatial learning as measured by Morris water maze81. As suggested by the authors81, discrepancies between experimental designs of the adult social approach tests, statistical analyses and genetic background of the two mouse models may be the source of these contradictory results. Notably, the synaptic structural differences that might underlie these phenotypes, such as whether a compensatory increase in excitatory synapse number occurs in these mice, have not been explored in either of these transgenic models. NLGN4−/− knockout mice exhibit social interaction deficits and reduced ultrasonic vocalizations in response to a novel female82, but, again, dendritic spine morphology has not been analyzed in this model. Further work is necessary to determine the dendritic spine phenotypes of these animal models and to correlate them with human pathological findings, potentially from individuals with the analogous genetic variant.
Rett, fragile X and Angelman syndromes, which exhibit comorbidity with ASDs, are associated with well-established mouse models and share synaptic pathology. Rett syndrome can be effectively modeled using mice deficient in MeCP2, which exhibit decreases in the number of functional, excitatory synapses in these mice83, mirroring synapse loss seen in humans. Modeling fragile X syndrome using Fmr1 knockout mice has recapitulated the immature spine morphologies observed in the brains of humans with fragile X syndrome84. The Angelman syndrome mouse, which lacks the maternal UBE3A gene, displays motor deficits, impaired context-dependent learning, impaired plasticity and altered spine density on hippocampal and cortical pyramidal neurons45,85. Notably, pharmacological and genetic manipulations have successfully rescued both structural and behavioral phenotypes in PTEN86, FMR1 (ref. 87) and MECP2 (ref. 88) knockout mice. That behavioral recovery in each of these rescue strategies was paralleled by reversal of spine pathologies underscores the importance of these structural perturbations in the pathogenesis of ASDs and comorbid disorders and the potential for the use of animal models in the development of therapeutics.
Support for the contribution of spine loss to schizophrenia comes from animal models that are able to model schizophrenia-related behavioral phenotypes as well as model the forebrain spine loss in the disease. Animal models are used to determine whether genetic abnormalities found in schizophrenia are able to produce both forebrain spine loss as well as salient behavioral phenotypes (the gene-driven model) or if mice exhibiting schizophrenia-related phenotypes show spine loss (the phenotype-driven model). We will focus on gene-driven approaches.
Behaviors that have been deemed relevant for schizophrenia are plentiful, but not without controversy. Nevertheless, a few behavior assays are among the standard requisite for animal modeling and include sensory-motor gating (usually measured by pre-pulse inhibition), locomotor activity assessments, sociability and cognitive deficits (usually working memory).
Mice deficient in NRG1 type III show reductions in spine density in hippocampal neurons89. Mice lacking erbB2 and erbB4 in the CNS show reduced spine density in both the hippocampus and cortex48. In both of these mouse lines, spine morphological deficits co-occur with schizophrenia-related behavioral phenotypes. NRG1 and ERBB4 mutant mice show several schizophrenia-relevant behavioral phenotypes, of which locomotor hyperactivity has been a consistent phenotype and can often be rescued through an acute dosage of the antipsychotic clozapine47.
The effects of DISC1 mutations in mice on spine density reflect brain region and developmentally influenced effects. Namely, spine numbers in dentate gyrus granule cells are reduced in a mouse model of disease-associated chromosomal translocation90. Spine density in cortical pyramidal neurons was increased by prenatal expression of mutant DISC1, whereas combined prenatal and postnatal expression increased spine density in hippocampal granule cells91.
Because of kalirin’s important synaptic functions, its interactions with DISC1 and its reduced expression in schizophrenia, recent studies have examined how kalirin loss affects spines and behavior. Notably, Kalrn−/− mice show severe reductions in spine density and dendrite complexity in the frontal cortex, as well as schizophrenia-related impairments in working memory, sociability and prepulse inhibition92,93. Both spine loss and behavioral dysfunction emerged during adolescence92. This is interesting given the onset of schizophrenia symptoms in adolescence in humans and points to a tight association between the onset of spine loss and the onset of behavioral impairments in these animals. These findings highlight the need to chart the trajectory of spine structural and behavioral phenotypes across the presymptomatic and symptomatic stages of the disease.
Mice modeling the 22q11.2 microdeletion syndrome (Df(16)A+/−) show reduced hippocampal spine density and sizes51. Mice deficient in individual genes in this region (ZDHHC8 and DGCR8) show simplified dendritic trees and reduced spine density51 or smaller spines50, respectively. Moreover, mice with a hemizygous chromosomal deficiency modeling the human 22q11.2 microdeletion syndrome have schizophrenia-related behavioral phenotypes, including impaired working memory, hyperactivity, deficient sensory-motor gating and impaired fear learning50.
Alzheimer’s disease can result from highly penetrant genetic mutations that faithfully give rise to the pathological hallmarks of the disease. Using the genetic factors identified in the human population, numerous transgenic mouse models of Alzheimer’s disease have been generated (reviewed in ref. 94). Given that memory loss is a defining characteristic of Alzheimer’s disease, mouse models are often assessed for behavioral deficits, especially in reference memory and working memory. In addition to cognitive deficits, animal models of Alzheimer’s disease often display dendritic and synaptic perturbations, similar to findings in human studies. The widely used Tg2576 mouse model expresses mutant human APP and displays decreased spine density in the CA1 and dentate gyrus, well before the development of amyloid plaques, further evidence that soluble Aβ oligomers initiate at least some Alzheimer’s disease–related pathology68,95. Notably, cognitive impairments arise in these mice at the time when spines become depleted, suggesting that synapse loss can drive cognitive decline. Mice expressing mutations in both APP and PS1 yield neurons with diminished frequency of large spines and dendritic abnormalities96. Specifically, dendrites near amyloid deposits are reported to experience shaft atrophy, neurite breakage and greater reductions in spine density97. Such Alzheimer’s disease animal models support the concept that synaptic degeneration represents a principal component of Alzheimer’s disease pathology that leads to memory impairment. However, at least some evidence suggests structural and functional alterations may be reversible pharmacologically, opening new therapeutic directions in Alzheimer’s disease98.
Animal models can provide valuable tools for establishing cause/effect relationships between genetic mutations, cellular alterations and specific disease endophenotypes, as well as tools for drug development. However, in modeling complex diseases, such as ASD, schizophrenia and Alzheimer’s disease, mouse models with a single transgene may fall short of the entire presentation of the disease as seen in humans. Whether the pitfalls of mouse models lie in the inherent differences in mouse and human behavior or in the need for multiple and perhaps subtle interactions between many genetic and environmental insults in one individual, insight gleaned from mouse models of complex disorders must be placed in the context of, and used as a reference for, the pathologies observed in affected humans.
Conclusions and future directions
Spine changes in disease have implications for both functional changes at the synapse and circuit-level connectivity, in the form of altered connectivity or changes in connection strength. As ASD seems to be associated with local hyperconnectivity and long-range hypoconnectivity, whereas schizophrenia is associated with reduced short- and long-range connectivity, it would be of interest to determine the effects of disease genes and mutations on the growth and maintenance of dendrites and axons to address changes in circuit organization in these disease states. Although measuring functional synaptic abnormalities in humans remains a daunting task, some insight into the functional aspects of these disorders is being gleaned. For example, the coincidence of epilepsy with ASD in a large percentage of individuals with ASD99 may be an expression of the excitatory/inhibitory synaptic imbalance observed in several ASD mouse models. Similarly, individuals with Alzheimer’s disease experience increased incidence of seizures compared with non-demented controls, suggesting that Alzheimer’s disease pathology may be involved in destabilizing broad neuronal networks100. Future functional studies in humans may help to guide basic research into structural changes in disease.
Changes in spine number or morphology may be the original insult that initiates symptom cascade, the secondary effects of neuronal changes or a compensatory response. Support for the idea that spine loss is an initial insult comes from model mice, such as Tg2576, and from genetic studies that directly implicate synaptic proteins in etiology. Spine alterations may be relevant no matter where in the pathological cascade of a disease they occur. Understanding where and when in the disease progression spine alterations occur, by carefully characterizing the time course of spine alterations and their relationships with other endophenotypes in affected individuals and animal models, may allow for the identification of new windows of opportunities for therapeutic intervention. Rescuing downstream pathophysiological changes that are closer to the clinical syndrome may provide effective treatments, even without addressing the underlying etiology.
Dendritic spines may serve as a common substrate for many neuropsychiatric disorders, particularly those that involve cognitive deficits. However, as spine modifications are associated with cognitive function, spine deficits may be more relevant for some cognitive symptoms or endophenotypes than others (for example, in schizophrenia, working memory deficits, but not hallucinations). Given the heterogeneity of these complex disorders, some individuals may exhibit more marked spine phenotypes, particularly those with more severe cognitive deficits. Further studies of human neuropathologies should strive to understand the degree of correlation between severity of cognitive deficits and dendritic spine dysmorphogenesis.
The inherent genetic heterogeneity of these disorders highlights the importance of determining common pathways of disease-associated genes. The molecular networks that control spines provide a framework for understanding how a large number of rare genetic perturbations can interact to disrupt synaptic function, neuronal circuit organization and behavioral output in a disease-specific manner. Thus, investigating the pathway can uncover future candidate genes and identify the best molecular candidates for therapeutic targeting. Indeed, many of the proteins in these pathways are enzymes that could be targeted with designer small molecules and drugs that target trophic and morphogenic signaling pathways may prove to be more effective, as they could alter cellular connectivity and induce fewer side effects. New drugs may be designed to prevent the emergence of symptoms in genetically susceptible individuals, delay the progression of symptoms in the early stages of the disease, or mitigate symptoms or promote functional recovery after the disease is fully manifested. Specifically, drugs that target dendritic spine regulation might aim to promote spine maturation and restore spine stability in ASD, to fortify existing synapses and restore spine plasticity in schizophrenia, or to prevent synapse loss in Alzheimer’s disease.
Acknowledgments
This work was supported by grants from the US National Institutes of Health (NIH) National Institute of Mental Health (MH071316, MH071533), National Alliance for Research on Schizophrenia and Depression and Alzheimer’s Association (to P.P.), NIH 1F31AG031621 (M.E.C.), NIH 1F31MH085362 (K.A.J.), NIH 1F31MH087043 (J.V.) and a predoctoral American Heart Association fellowship (K.M.W.).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–129. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 3.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol. Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 4.Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- 5.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch. Gen. Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 6.Tackenberg C, Ghori A, Brandt R. Thin, stubby or mushroom: spine pathology in Alzheimer’s disease. Curr. Alzheimer Res. 2009;6:261–268. doi: 10.2174/156720509788486554. [DOI] [PubMed] [Google Scholar]
- 7.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 8.Toro R, et al. Key role for gene dosage and synaptic homeostasis in autism spectrum disorders. Trends Genet. 2010;26:363–372. doi: 10.1016/j.tig.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb. Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- 10.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr. Opin. Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Irwin SA, et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am. J. Med. Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.Gogtay N. Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr. Bull. 2008;34:30–36. doi: 10.1093/schbul/sbm103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan HY, Callicott JH, Weinberger DR. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb. Cortex. 2007;17:i171–i181. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida T, et al. A prospective longitudinal volumetric MRI study of superior temporal gyrus gray matter and amygdala-hippocampal complex in chronic schizophrenia. Schizophr. Res. 2009;113:84–94. doi: 10.1016/j.schres.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br. J. Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 17.Kolomeets NS, Orlovskaya DD, Rachmanova VI, Uranova NA. Ultrastructural alterations in hippocampal mossy fiber synapses in schizophrenia: a postmortem morphometric study. Synapse. 2005;57:47–55. doi: 10.1002/syn.20153. [DOI] [PubMed] [Google Scholar]
- 18.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann. Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 19.Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Knobloch M, Mansuy IM. Dendritic spine loss and synaptic alterations in Alzheimer’s disease. Mol. Neurobiol. 2008;37:73–82. doi: 10.1007/s12035-008-8018-z. [DOI] [PubMed] [Google Scholar]
- 21.Arendt T. Synaptic degeneration in Alzheimer’s disease. Acta Neuropathol. 2009;118:167–179. doi: 10.1007/s00401-009-0536-x. [DOI] [PubMed] [Google Scholar]
- 22.Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res. Brain Res. Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- 23.Kolluri N, Sun Z, Sampson AR, Lewis DA. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am. J. Psychiatry. 2005;162:1200–1202. doi: 10.1176/appi.ajp.162.6.1200. [DOI] [PubMed] [Google Scholar]
- 24.Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb. Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 25.McClellan J, King MC. Genetic heterogeneity in human disease. Cell. 2010;141:210–217. doi: 10.1016/j.cell.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 26.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat. Rev. Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto D, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chih B, Afridi SK, Clark L, Scheiffele P. Disorder-associated mutations lead to functional inactivation of neuroligins. Hum. Mol. Genet. 2004;13:1471–1477. doi: 10.1093/hmg/ddh158. [DOI] [PubMed] [Google Scholar]
- 30.Dudanova I, Tabuchi K, Rohlmann A, Sudhof TC, Missler M. Deletion of alpha-neurexins does not cause a major impairment of axonal pathfinding or synapse formation. J. Comp. Neurol. 2007;502:261–274. doi: 10.1002/cne.21305. [DOI] [PubMed] [Google Scholar]
- 31.Durand CM, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat. Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berkel S, et al. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat. Genet. 2010;42:489–491. doi: 10.1038/ng.589. [DOI] [PubMed] [Google Scholar]
- 33.Roussignol G, et al. Shank expression is sufficient to induce functional dendritic spine synapses in aspiny neurons. J. Neurosci. 2005;25:3560–3570. doi: 10.1523/JNEUROSCI.4354-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steiner P, et al. Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron. 2008;60:788–802. doi: 10.1016/j.neuron.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bacchelli E, et al. Screening of nine candidate genes for autism on chromosome 2q reveals rare nonsynonymous variants in the cAMP-GEFII gene. Mol. Psychiatry. 2003;8:916–924. doi: 10.1038/sj.mp.4001340. [DOI] [PubMed] [Google Scholar]
- 36.Woolfrey KM, et al. Epac2 induces synapse remodeling and depression and its disease-associated forms alter spines. Nat. Neurosci. 2009;12:1275–1284. doi: 10.1038/nn.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourgeron T. A synaptic trek to autism. Curr. Opin. Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Butler MG, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J. Med. Genet. 2005;42:318–321. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tavazoie SF, Alvarez VA, Ridenour DA, Kwiatkowski DJ, Sabatini BL. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat. Neurosci. 2005;8:1727–1734. doi: 10.1038/nn1566. [DOI] [PubMed] [Google Scholar]
- 40.Fraser MM, Bayazitov IT, Zakharenko SS, Baker SJ. Phosphatase and tensin homolog, deleted on chromosome 10 deficiency in brain causes defects in synaptic structure, transmission and plasticity, and myelination abnormalities. Neuroscience. 2008;151:476–488. doi: 10.1016/j.neuroscience.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon CH, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat. Rev. Neurosci. 2005;6:376–387. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Z, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cook EH, Jr, et al. Autism or atypical autism in maternally but not paternally derived proximal 15q duplication. Am. J. Hum. Genet. 1997;60:928–934. [PMC free article] [PubMed] [Google Scholar]
- 45.Dindot SV, Antalffy BA, Bhattacharjee MB, Beaudet AL. The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum. Mol. Genet. 2008;17:111–118. doi: 10.1093/hmg/ddm288. [DOI] [PubMed] [Google Scholar]
- 46.Walsh T, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 47.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barros CS, et al. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc. Natl. Acad. Sci. USA. 2009;106:4507–4512. doi: 10.1073/pnas.0900355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stark KL, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat. Genet. 2008;40:751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- 51.Mukai J, et al. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat. Neurosci. 2008;11:1302–1310. doi: 10.1038/nn.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.St Clair D, et al. Association within a family of a balanced autosomal translocation with major mental illness. Lancet. 1990;336:13–16. doi: 10.1016/0140-6736(90)91520-k. [DOI] [PubMed] [Google Scholar]
- 53.Schumacher J, et al. The DISC locus and schizophrenia: evidence from an association study in a central European sample and from a meta-analysis across different European populations. Hum. Mol. Genet. 2009;18:2719–2727. doi: 10.1093/hmg/ddp204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayashi-Takagi A, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat. Neurosci. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lipska BK, et al. Expression of DISC1 binding partners is reduced in schizophrenia and associated with DISC1 SNPs. Hum. Mol. Genet. 2006;15:1245–1258. doi: 10.1093/hmg/ddl040. [DOI] [PubMed] [Google Scholar]
- 56.Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry. 2006;11:557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- 57.Kushima I, et al. Resequencing and association analysis of the KALRN and EPHB1 genes and their contribution to schizophrenia susceptibility. Schizophr. Bull. doi: 10.1093/schbul/sbq118. published online, (1 November 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc. Natl. Acad. Sci. USA. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kristiansen LV, Beneyto M, Haroutunian V, Meador-Woodruff JH. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol. Psychiatry. 2006;11:737–747. 705. doi: 10.1038/sj.mp.4001844. [DOI] [PubMed] [Google Scholar]
- 60.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell. Mol. Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bertram L, Tanzi RE. Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat. Rev. Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- 62.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav. Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lacor PN, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J. Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shankar GM, et al. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor–dependent signaling pathway. J. Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sleegers K, et al. The pursuit of susceptibility genes for Alzheimer’s disease: progress and prospects. Trends Genet. 2010;26:84–93. doi: 10.1016/j.tig.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Ji Y, et al. Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer’s disease patients. Neuroscience. 2003;122:305–315. doi: 10.1016/j.neuroscience.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 67.Dumanis SB, et al. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J. Neurosci. 2009;29:15317–15322. doi: 10.1523/JNEUROSCI.4026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lanz TA, Carter DB, Merchant KM. Dendritic spine loss in the hippocampus of young PDAPP and Tg2576 mice and its prevention by the ApoE2 genotype. Neurobiol. Dis. 2003;13:246–253. doi: 10.1016/s0969-9961(03)00079-2. [DOI] [PubMed] [Google Scholar]
- 69.Zhao L, et al. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat. Neurosci. 2006;9:234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
- 70.Penzes P, et al. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- 71.Xie Z, et al. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Youn H, et al. Kalirin is under-expressed in Alzheimer’s disease hippocampus. J. Alzheimers Dis. 2007;11:385–397. doi: 10.3233/jad-2007-11314. [DOI] [PubMed] [Google Scholar]
- 73.Norris CM, et al. Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer’s models. J. Neurosci. 2005;25:4649–4658. doi: 10.1523/JNEUROSCI.0365-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li S, et al. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tackenberg C, Brandt R. Divergent pathways mediate spine alterations and cell death induced by amyloid-beta, wild-type tau, and R406W tau. J. Neurosci. 2009;29:14439–14450. doi: 10.1523/JNEUROSCI.3590-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu HY, et al. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J. Neurosci. 2010;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsieh H, et al. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat. Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 79.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tabuchi K, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chadman KK, et al. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jamain S, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl. Acad. Sci. USA. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chao HT, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron. 2007;56:58–65. doi: 10.1016/j.neuron.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Irwin SA, et al. Dendritic spine and dendritic field characteristics of layer V pyramidal neurons in the visual cortex of fragile-X knockout mice. Am. J. Med. Genet. 2002;111:140–146. doi: 10.1002/ajmg.10500. [DOI] [PubMed] [Google Scholar]
- 85.Yashiro K, et al. Ube3a is required for experience-dependent maturation of the neocortex. Nat. Neurosci. 2009;12:777–783. doi: 10.1038/nn.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou J, et al. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J. Neurosci. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dölen G, et al. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen YJ, et al. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J. Neurosci. 2008;28:6872–6883. doi: 10.1523/JNEUROSCI.1815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kvajo M, et al. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc. Natl. Acad. Sci. USA. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ayhan Y, et al. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol. Psychiatry. doi: 10.1038/mp.2009.144. published online, (5 January 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cahill ME, et al. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc. Natl. Acad. Sci. USA. 2009;106:13058–13063. doi: 10.1073/pnas.0904636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xie Z, Cahill ME, Penzes P. Kalirin loss results in cortical morphological alterations. Mol. Cell. Neurosci. 2010;43:81–89. doi: 10.1016/j.mcn.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ashe KH, Zahs KR. Probing the biology of Alzheimer’s disease in mice. Neuron. 2010;66:631–645. doi: 10.1016/j.neuron.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jacobsen JS, et al. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Knafo S, et al. Widespread changes in dendritic spines in a model of Alzheimer’s disease. Cereb. Cortex. 2009;19:586–592. doi: 10.1093/cercor/bhn111. [DOI] [PubMed] [Google Scholar]
- 97.Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat. Neurosci. 2004;7:1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- 98.Smith DL, Pozueta J, Gong B, Arancio O, Shelanski M. Reversal of long-term dendritic spine alterations in Alzheimer disease models. Proc. Natl. Acad. Sci. USA. 2009;106:16877–16882. doi: 10.1073/pnas.0908706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spence SJ, Schneider MT. The role of epilepsy and epileptiform EEGs in autism spectrum disorders. Pediatr. Res. 2009;65:599–606. doi: 10.1203/01.pdr.0000352115.41382.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat. Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]