Abstract
Background and Purpose
The signals that initiate the post-stroke inflammatory response are unknown. High mobility group box (HMGB) 1 protein is a nuclear protein that is passively released from necrotic tissue and is able to activate leukocytes, which in turn secrete HMGB1. HMGB1 is also able to activate antigen presenting cells and therefore stands at the crossroads of innate and adaptive immunity.
Methods
Plasma HMGB1 concentrations were determined at multiple time points after ischemic stroke (N=110) and correlated to stroke severity and biomarkers of inflammation. The relationships between HMGB1, stroke outcome and autoimmune responses to brain antigens were also assessed.
Results
Stroke resulted in an increase in HMGB1 that persisted for 30 days. Plasma HMGB1 was correlated with the number of circulating leukocytes but not predictive of stroke outcome nor the development of autoimmune responses to brain antigens. Patients with a TH1(+) response to myelin basic protein at 90 days after stroke, however, had higher plasma HMGB1.
Conclusions
HMGB1 appears to be involved in the post-ischemic inflammatory response, but it remains unclear whether HMGB1 initiates this response or merely reflects activation of leukocytes by another signal.
Keywords: stroke, HMGB1, alarmin, DAMP, inflammation, monocytes
Introduction
Stroke induces complex changes in the immune response, leading to systemic inflammation as well as impaired host defense.1–4 Both the degree of inflammation and the degree of host response impairment are related to stroke severity/infarct volume.1,2,4 The dysfunction in host defense is mediated by the sympathetic nervous system; the signals that initiate systemic inflammation are unknown.3
The post-stroke systemic inflammatory response is not directed in an antigen specific fashion. Systemic infections that activate the innate immune response, however, increase the likelihood of TH1 type immune responses to brain antigens in patients with stroke.5 The link between infection and the development of central nervous system (CNS) autoimmunity may be mediated by danger associated molecular patterns (DAMPs), which are derived from pathogens or released from host cells.6 High mobility group box (HMGB) 1 is a ubiquitous nuclear protein released from necrotic cells and secreted by activated leukocytes.7 Once released, HMGB1 functions as a DAMP by activating antigen presenting cells (APCs) through toll like receptors (TLRs) and the receptor for advanced glycation end products (RAGE).6
Neutralizing HMGB1 improves outcome in experimental stroke.8,9 The relationship between plasma HMGB1 and clinical stroke outcome is unknown. In this study we investigate whether plasma HMGB1 concentrations in patients 1) reflect infarct size, 2) promote TH1(+) response to brain antigens, or 3) are predictive of outcome.
Materials and Methods
Research Subjects
The patient population for this study is described elsewhere.5 Patients with acute ischemic stroke were enrolled as soon as possible after stroke onset. Blood was drawn at 24 hours (±6 hours; N=38), 72 hours (±12 hours; N=98), 7 days (±1 day; N=94), 30 days (±5 days; N=89), 90 days (±5 days; N=72), 180 days (±5 days; N=70) and 365 days (±5 days; N=24) after stroke. The study was approved by the Institutional Review Board. Patients or their surrogates provided informed consent.
Clinical Data
Stroke severity was determined by the National Institutes of Health Stroke Scale (NIHSS) and outcome by the modified Rankin Scale (mRS). Infarct volume on initial diffusion weighted MRI imaging was calculated by the ABC/2 method.10
Laboratory Studies
Leukocyte counts and concentrations of C reactive protein (CRP) were determined by hospital clinical laboratories. Additional plasma was immediately frozen at −80° and HMGB1 concentrations determined by enzyme linked immunoassay (IBL International); the sensitivity of the assay was 0.20 ng/mL. Isolated lymphocytes were isolated and frozen in liquid nitrogen until use. TH1(+) responses to lymphocytes were determined as described elsewhere.5
Statistics
Descriptive data are presented as median and interquartile range (IQR); group comparisons were performed using the Kruskal-Wallis H test or the Mann-Whitney U test. Correlations are presented as either Pearson’s r or Spearman’s rho (ρ). Logistic regression was used to assess the contribution of HMGB1, CRP and leukocyte subsets to poor outcome (mRS>3) at 90 days after stroke and to the risk of developing a TH1(+) response to MBP. Significance was set at P<0.05.
Results
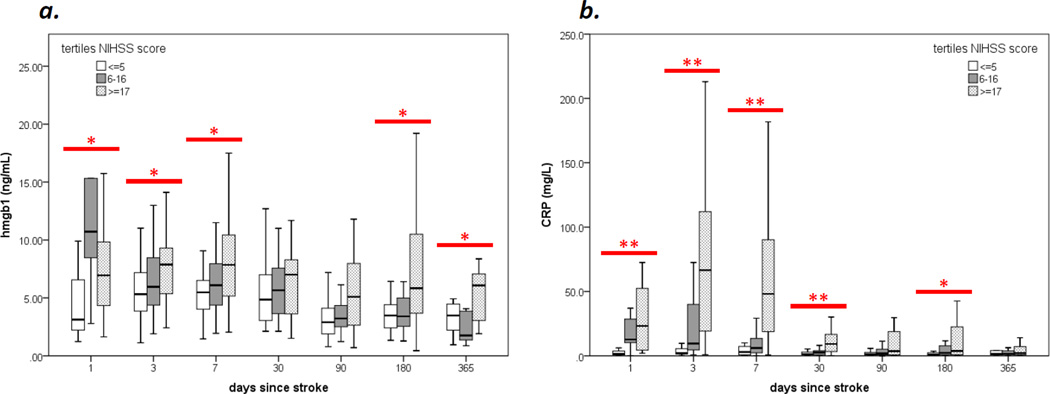
A total of 114 patients were enrolled in the parent study; baseline characteristics are described elsewhere.5 Plasma HMGB1 was available for 110 of these patients who are the subject of this report. At day 3 after stroke, there were weak correlations between HMGB1, infarct volume (r=0.217, P=0.024) and stroke severity (ρ=0.230, P=0.015). Plasma HMGB1 and CRP were highest in patients with severe strokes (NIHSS≥17) and remained elevated for months (Figure).
Figure. Changes in plasma HMGB1 and hsCRP over time after stroke.
Both plasma HMGB1 (a) and CRP (b) are higher among patients with severe stroke; these differences persist for months after stroke onset. Tertiles of stroke severity differ from each other at *P<0.05 or *P<0.01 level (Kruskal-Wallis H test).
Neither the numbers of leukocytes nor the plasma concentrations of HMGB1 early after stroke were independently predictive of stroke outcome at 90 days (Table 1). Higher concentrations of CRP early after stroke, however, were associated with worse 90 day outcomes. The number of leukocytes was highly correlated (independent of infarct volume) to plasma HMGB1 throughout the study period: r=0.415, P=0.415 at day 1, r=0.312, P=0.002 at day 3, r=0.0297, P=0.004 at week 1, r=0.374, P<0.001 at month 1, r=0.475, P<0.001 at month 3 and r=0.539, P=0.010 at year 1. The relationship between CRP and HMGB1 was more variable.
Table 1.
Predictive value of early (day 3) markers of inflammation on poor outcome (mRS>3) at 90 days after stroke.
| unadjusted | adjusted for NIHSS |
adjusted for NIHSS and age |
|||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
| 90 days (N=102) | leukocytes (per thou/microL) |
1.183 (1.047–1.336) | 0.007 | 1.035 (0.884–1.212) | NS | 1.033 (0.879–1.213) | NS |
| neutrophils (per thou/microL) |
1.367 (1.138–1.641) | 0.001 | 1.120 (0.884–1.418) | NS | 1.133 (0.893–1.438) | NS | |
| lymphocytes* (per thou/microL) |
0.181 (0.052–0.638) | 0.008 | 0.518 (0.132–2.031) | NS | 0.575 (0.144–2.294) | NS | |
| monocytes (per thou/microL) |
41.40 (5.481–312.8) | <0.001 | 7.490 (0.527–106.5) | 0.137 | 6.832 (0.427–109.3) | 0.174 | |
| HMGB1 (per ng) |
0.998 (0.939–1.061) | NS | 0.960 (0.882–1.044) | NS | 0.974 (0.896–1.059) | NS | |
| CRP (per 10 mg/L) |
1.311 (1.160–1.482) | <0.001 | 1.166 (1.026–1.325) | 0.019 | 1.197 (1.038–1.380) | 0.013 | |
mRS=modified Rankin Scale, NIHSS=National Institutes of Health Stroke Scale, thou/microL=thousand per microliter, HMGB=high mobility group box, CRP = C reactive protein, OR=odds ratio. CI=confidence interval. NS=not significant.
All values represent the highest recorded value within the first 3 days except for lymphocytes, where the lowest recorded value was used.
Among patients with a TH1(+) response to MBP at 90 days5, plasma HMGB1 and CRP were also elevated at that time point (Table 2). There was, however, no relationship between HMGB1 concentrations early after stroke onset and the propensity to develop a TH1(+) response to MBP at 90 days.
Table 2.
Differences in inflammatory markers between patients with a Th1(+) response to MBP at 90 days and those without.
| Th1(+) response to MBP | |||
|---|---|---|---|
| 90 days: | yes N=19 |
no N=47 |
P |
| leukocytes (thou/microL) | 7.73 (5.94, 9.41) | 6.94 (5.39, 7.99) | 0.111 |
| neutrophils (thou/microL) | 4.44 (3.36, 6.51) | 4.01 (3.09, 5.29) | NS |
| lymphocytes (thou/microL) | 1.75 (1.37, 2.14) | 1.73 (1.33, 2.14) | NS |
| monocytes (thou/microL) | 0.57 (0.44, 0.66) | 0.50 (0.41, 0.66) | NS |
| HMGB1 (ng/mL) | 5.70 (2.51, 8.11) | 3.27 (2.12, 4.49) | 0.030 |
| CRP (mg/L) | 8.10 (0.80, 21.1) | 1.90 (0.60, 3.92) | 0.036 |
MBP=myelin basic protein, HMGB=high mobility group box, CRP=C reactive protein, NS=not significant. Th1(+) response to MBP is a response greater than that seen in 75% of the control population.5
Discussion
A systemic inflammatory response is common after stroke. Alarmins like HMGB1 are candidate molecules that could initiate the innate immune response following tissue damage.11,12 Given that HMGB1 is released from necrotic cells, we hypothesized that HMGB1 concentrations would reflect the degree of tissue injury. Similar to a previous study, however, plasma HMGB1 was only weakly associated with infarct volume.13 Activated leukocytes are also a source of HMGB7, and the robust association between HMGB1 and the leukocyte numbers suggest that immune cells might be the primary source of plasma HMGB1 following stroke.
Given its ability to promote inflammation and activate APCs through TLRs and RAGE, we expected that high concentrations of plasma HMGB1 early after stroke onset would be predictive of poor outcome and predispose to autoimmune responses to brain antigens, yet plasma HMGB1 was predictive of neither. The lack of an association between HMGB1, infarct size and autoimmune responses to brain antigens suggest that HMGB1 not the single factor initiating inflammation or activating APCs after stroke. At day 90 after stroke, however, those patients with a TH1(+) response to MBP had increased plasma HMGB1; the source of this HMGB1 is unknown.
In summary, plasma HMGB1 is elevated following ischemic stroke; patients with severe stroke have higher HMGB1 and these elevations last for months. The correlation between plasma HMGB1 and leukocyte numbers is more robust than that between plasma HMGB1 and infarct volume, suggesting that plasma HMGB1 reflects secretion by leukocytes. Finally, HMGB1 did not predict outcome or development of autoimmune responses to MBP. Further studies are needed to define the role of HMGB1 in post-stroke inflammation.
Acknowledgments
Sources of Funding
This work was funded by National Institute of Neurological Disorders and Stroke R01NS049197.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflicts of Interest
None.
References
- 1.Buck BH, Liebeskind DS, Saver JL, Bang OY, Yun SW, Starkman S, et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke. 2008;39:355–360. doi: 10.1161/STROKEAHA.107.490128. [DOI] [PubMed] [Google Scholar]
- 2.Audebert HJ, Rott MM, Eck T, Haberl RL. Systemic inflammatory response depends on initial stroke severity but is attenuated by successful thrombolysis. Stroke. 2004;35:2128–2133. doi: 10.1161/01.STR.0000137607.61697.77. [DOI] [PubMed] [Google Scholar]
- 3.Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke t helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hug A, Dalpke A, Wieczorek N, Giese T, Lorenz A, Auffarth G, et al. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke. 2009;40:3226–3232. doi: 10.1161/STROKEAHA.109.557967. [DOI] [PubMed] [Google Scholar]
- 5.Becker KJ, Kalil AJ, Tanzi P, Zierath DK, Savos AV, Gee JM, et al. Autoimmune responses to the brain after stroke are associated with worse outcome. Stroke. 2011;42:2763–2769. doi: 10.1161/STROKEAHA.111.619593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oppenheim JJ, Tewary P, de la Rosa G, Yang D. Alarmins initiate host defense. Adv Exp Med Biol. 2007;601:185–194. doi: 10.1007/978-0-387-72005-0_19. [DOI] [PubMed] [Google Scholar]
- 7.Lotze MT, Tracey KJ. High-mobility group box 1 protein (hmgb1): Nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 8.Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, Kanke T, et al. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J. 2007;21:3904–3916. doi: 10.1096/fj.07-8770com. [DOI] [PubMed] [Google Scholar]
- 9.Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, et al. The hmgb1 receptor rage mediates ischemic brain damage. J Neurosci. 2008;28:12023–12031. doi: 10.1523/JNEUROSCI.2435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, et al. Abc/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–2110. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, et al. Early release of hmgb-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28:927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- 12.Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, et al. Hmgb1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006;26:6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogelgesang A, May VE, Grunwald U, Bakkeboe M, Langner S, Wallaschofski H, et al. Functional status of peripheral blood t-cells in ischemic stroke patients. PLoS ONE. 2010;5:e8718. doi: 10.1371/journal.pone.0008718. [DOI] [PMC free article] [PubMed] [Google Scholar]