Abstract
Many genetic risk factors for major mental disorders have key roles in brain development. Thus, exploring the roles for these genetic factors for brain development at the molecular, cellular, and neuronal circuit level is crucial for discovering how genetic disturbances affect high brain functions which ultimately lead to disease pathologies. However, it is a tremendously difficult task, given that most mental disorders have genetic complexities in which many genetic risk factors have multiple roles in different cell types and brain regions over a time-course dependent manner. Furthermore, some genetic risk factors are likely to act epistatically in common molecular pathways. For this reason, a technique for spatial and temporal manipulation of multiple genes is necessary for understanding how genetic disturbances contribute to disease etiology. Here we will review the said technique, in utero electroporation, which investigates the molecular disease pathways in rodent models for major mental disorders. This technique also is useful to examine the effect of genetic risks at the behavioral level. Furthermore, we will discuss the recent progress of this technology, such as inducible and cell type-specific targeting as well as nonepisomal genetic manipulation, which provide us further availability of this technique for research on major mental disorders.
Keywords: In utero electroporation, brain development, genetic risk factors, genetic manipulation, major mental disorder
Introduction
Identification of genetic susceptibility factors for psychiatric disorders, such as schizophrenia, bipolar disorder, and autism spectrum disorders has made it possible to conduct etiological, evidence-based molecular approaches to examine these devastating conditions (Bill and Geschwind 2009; Harrison and Weinberger 2005; McClellan and others 2007; O’Donovan and others 2009). Given that many genetic factors play roles at the pre-, peri-, and postnatal developmental periods, genetic vulnerability may affect proper brain development and increase susceptibility to mental disorders of neurodevelopmental origin (Harrison and Weinberger 2005; Jaaro-Peled and others 2009; Rapoport and others 2005). In order to understand how genetic insults result in impairment of higher brain function that ultimately leads to psychiatric disorders, the use of animal models is extremely important. It is quite challenging, however, because of the considerable limitations of the current knowledge concerning biological mechanisms of psychiatric disorders due to their etiological complexities, including genetic heterogeneities (Chen and others 2006; Nestler and Hyman 2010). In contrast to neurodegenerative disorders, such as Huntington’s disease and Parkinson’s disease, there have been no genes identified that harbor clearly causative mutations for any psychiatric disorders. Nonetheless, classical linkage and association studies, as well as cytogenetic approaches, have identified several candidate genes that may be involved in risk of major mental disorders (Harrison and Weinberger 2005; Owen and others 2005). Furthermore, recent genome-wide association studies (GWAS) have identified hundreds of new common variants and rare mutations with potential strong biological impact, which confer vulnerability to psychiatric conditions (Owen and others 2010; Sebat and others 2009). These mutations may be ideal entry points to explore how genetic insults affect brain functions, although most mutations do not fulfill unique roles for a single mental disorder, as defined by the current diagnostic criteria.
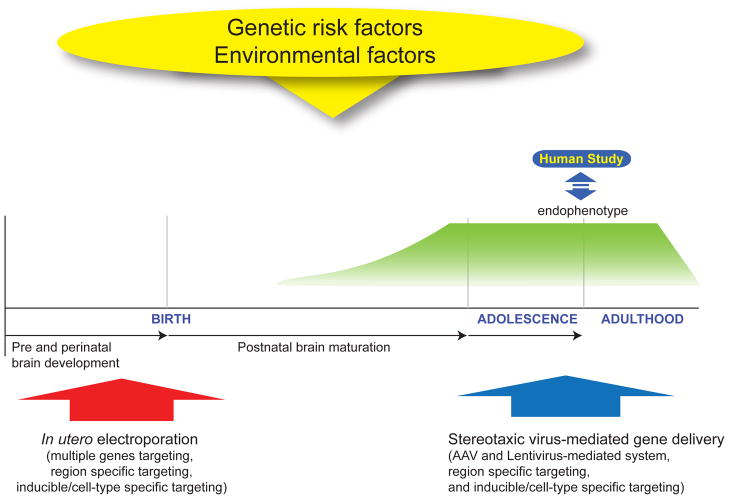
Many genetic risk factors play various roles for distinct molecular process in the region, cell type, and developmental stage-dependent manner. Some of them are likely to function in common molecular pathways (Harrison and Weinberger 2005; Jaaro-Peled and others 2009). Thus, to understand molecular mechanisms that directly affect the development of disease pathologies, it is important to segregate their roles in a specific molecular/cellular context in distinct brain regions during their developmental trajectory. Furthermore, the functional consequences of genetic manipulation must be observed not only at the molecular and cellular levels, but also neuronal circuit and behavioral levels. To this end, a technique is required to manipulate genes in vivo. One such method is in utero electroporation, a technique that can manipulate more than one target gene in a specific brain region during the embryonic and peri-natal periods (Fukuchi-Shimogori and Grove 2001; Saito and Nakatsuji 2001; Tabata and Nakajima 2001). In utero electroporation can be used complementarily with stereotaxic virus-mediated gene delivery, which can manipulate gene expression after birth (Figure 1). These techniques are valuable methods as alternatives to genetically engineered animals, since the genetic liabilities for psychiatric disorders are difficult to test in traditional knockout and transgenic animals due to genetic variability and complexity of major mental disorders. Additionally, these techniques have the benefit of avoiding compensation machinery in the classic genetically engineered mice by acute introduction of short hairpin RNA (shRNA) or expression constructs. For instance, although the genetic deletion of doublecortin (DCX), a X-linked gene for neuronal migration, does not display apparent migration defects in the cerebral cortex (Corbo and others 2002), knockdown of DCX via in utero electroporation leads to subcortical band heterotopias, an anatomical phenotype, which mimics malformation of the neocortex in the patients with DCX mutations (Bai and others 2003).
Figure 1. Animals models via Genetic manipulation in the developmental trajectory.
Genetic manipulation techniques, such as in utero electroporation and stereotaxic virus-mediated gene delivery, are useful for examining the role for genetic risk factors for brain development and resultant higher brain function. The typical onset of psychiatric symptoms in most major mental disorders, such as schizophrenia and bipolar disorder, occurs during adolescence and young adulthood, whereas some psychiatric conditions, such as autism spectrum disorder, develop in childhood. Thus, it is important to understand the role for genetic risk factors in the developmental trajectory by segregating their functions at specific developmental stages in order to address these mechanisms precisely.
The feasibility of animal models via in utero electroporation for behavioral examinations has been recently reported (Niwa and others 2010). However, an important question is, how do we evaluate human mental disorders in the context of animal phenotypes? There are no specific biological markers for major mental conditions at the present and current diagnosis is defined by the syndromal categorization (DSM-IV; Diagnostic and Statistical Manual of Mental Disorders. 4th edn, text revision, 2000). However, findings from neuropathological and pharmacological examinations, as well as brain imaging and neuopsychological studies, imply some hallmarks for biological and behavioral assessments of psychiatric conditions. For example, deficits in working memory and sensory motor gating, as well as enlargement of the lateral ventricles have been repeatedly reported in the patients with schizophrenia (Gottesman and Gould 2003; McCarley and others 1999).
In this article, we provide an overview of the current progress of methods for in vivo genetic manipulation. In particular, in utero electroporation will be discussed, along with its feasibility to address the role of genetic risk factors and their pathophysiological processes in major mental disorders. The evaluation of phenotypic changes relevant to disease pathology in animal models via in utero electroporation at neuronal circuit and behavioral levels will also be discussed.
Genetic manipulation of specific cell types in the developmental trajectory
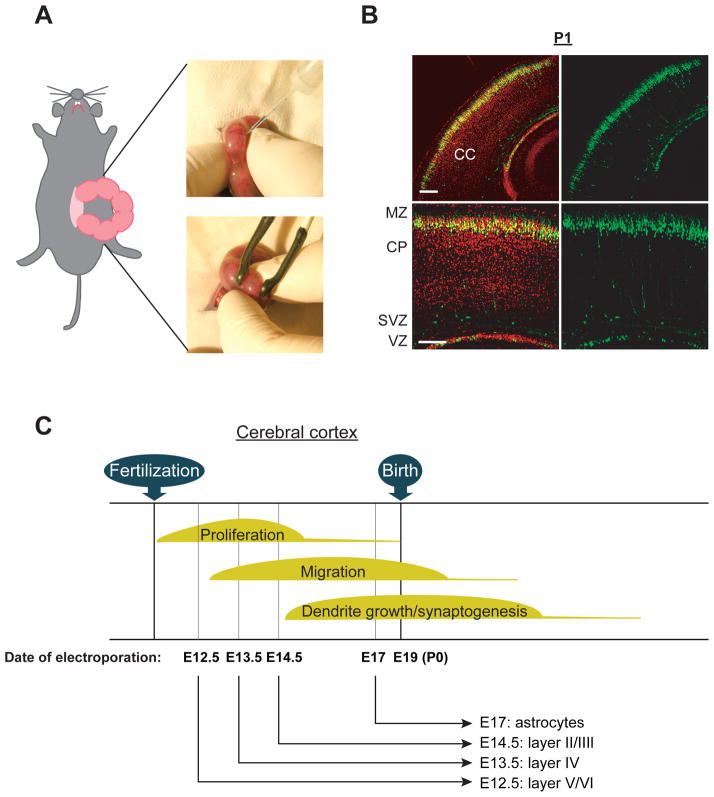
In utero electroporation is a useful technique for segregating the roles for genetic risk factors in specific cell types. This technique requires the injection of overexpression and/or shRNA expression constructs into the lateral ventricles of the embryonic rodent brain and electroporation into the cells lining the lateral ventricle (Bai and others 2003; Kamiya and others 2005; Kamiya and others 2008; Manent and others 2009; Niwa and others 2010; Saito 2006; Tabata and Nakajima 2001; Young-Pearse and others 2007; Young-Pearse and others 2010) (Figure 2). Electroporated embryos can be characterized at any developmental stage and the adult stage (Manent and others 2009; Niwa and others 2010; Young-Pearse and others 2007). Effects of overexpression and/or knockdown of the target gene can be quickly tested in specific cell populations. For example, in the developing mouse cerebral cortex, targeting progenitor cells in the ventricular zone at embryonic day 12.5 (E12.5), E13.5, or E14.5 results in the targeting of cells that mainly differentiate into pyramidal neurons at cortical layers V/VI, IV, and II/III, respectively (Kamiya 2009; Kubo and others 2010a; LoTurco and others 2009; Rice and others 2010) (Figure 2). This pattern of targeting at increasing developmental age reflects the “inside-out” pattern of cortical development, whereby early born neurons migrate to deep layers of the cortex, and later born neurons migrate to more superficial layers. Progenitor cells electroporated at E18 rat embryo are differentiated into astrocytes (LoTurco and others 2009), which is consistent with the notion that progenitors in the subventricular zone from late embryonic stages to early postnatal stages (E17 to postnatal 14 (P14)) produce astrocytes (Sauvageot and Stiles 2002). Furthermore, progenitor cells in the ganglionic eminence, a major source of cortical interneurons, can be exclusively electroporated, indicating that interneuron-specific gene targeting also is possible (Borrell and others 2005). Taken together, in utero electroporation allows us to manipulate the expression of target gene in a specific cell population, depending on the timing of electroporation and the region targeted during the prenatal stages.
Figure 2. Genetic manipulation via in utero electroporation in selective cell population in the cerebral cortex.
(A) Images displaying injection of DNA solution into lateral ventricle followed by gene delivery into the ventricular zone (VZ) via electroporation by holding the embryos with forceps-type electrodes.
(B) Representative images of GFP-positive cells distributed at the cortical plate (CP) in the cerebral cortex at postnatal day 1 (P1); GFP expression constructs were introduced at embryonic day 15 (E15). CC, cerebral cortex; MZ, marginal zone; SVZ, subventricular zone; VZ, ventricular zone. Red, Nucleus. Scale bars, 100 μm. Adapted from the Kamiya (Kamiya 2009).
(C) Schematic representation of the genetic manipulation in the selective cell population in the cerebral cortex. Electroporation directed into progenitor cells in the neocortical ventricular zone at E12.5, E13.5, E14.5, and E17 allows for the modulation of gene expression in a lineage of pyramidal neurons in the layer V/VI, layer IV, layer II/III, as well as astrocytes, respectively.
Within the same cell population, many genetic risks factors may play multiple distinct roles depending on the developmental cellular processes, such as cell proliferation, neuronal migration, and axon/dendrite growth, and spine formation. Isolating the roles of genes at different moments in brain development can be an extremely ambitious and challenging task. In this regard, the use of in utero electroporation with inducible and cell type specific gene targeting systems (Manent and others 2009; Matsuda and Cepko 2007) is useful for dissecting the temporal requirement, such that multifunctional molecules can be studied in specific cellular processes. In this system, a stop codon flanked by two LoxP sites downstream from the CAG promoter is contained in an overexpression construct and microRNA30-based shRNA expression vector. In a separate plasmid, Cre recombinase fused to an altered form of the estrogen receptor is expressed under the control of the CAG promoter (in CAG-ERT2CreERT2). This Cre is ubiquitously expressed under this promoter, but is only active in response to injection of 4-hydroxytamoxifen (4-OHT). In the presence of active Cre recombinase, the target protein or microRNA30-based shRNA is expressed under the control of the CAG promoter. By using this system, the timing of gene targeting can be controlled, which allows for the analysis of the specific cellular effect of the target gene. For instance, we can segregate the effect of the suppression of target gene expression on dendrite development independent of the secondary effects on dendrites caused by affected cell proliferation and/or migration.
In addition to the inducible system that addresses the temporal requirement in studies of multifunctional genetic factors, a second approach, cell type-specific promoter-mediated genetic manipulation, can further dissect the mechanisms in targeted cell types. Given that expression constructs with diverse promoters, such as Nestin and DCX promoters, become activated within specific cell types, such as progenitor cells or post-mitotic migrating neurons (Miyagi and others 2004; Wang and others 2007), cell type-specific roles of genetic risk factors can be investigated. While conventional shRNA is driven exclusively by polymerase III promoters, recent advances using the microRNA30-based shRNA system allows for expression of a given shRNA under polymerase II promoters, such as the CAG promoter (Matsuda and Cepko 2007). The feasibility of this was demonstrated by Matsuda and Cepko through layer specific gene suppression in the retina with rhodopsin promoter-driven Cre recombinase exprsssion constructs (Matsuda and Cepko 2007). The possibility of the inducible overexpression of a target gene in a specific cell type was also confirmed in the rat cerebral cortex at P20 (Matsuda and Cepko 2007). This indicates that the temporal regulation of gene expression is possible even at the postnatal stages of the animals that were electroporated with inducible plasmids during embryonic stages. Taken together, the combination of Cre/LoxP recombination-mediated inducible shRNA system with cell type-specific promoters can be useful for gain and loss-of-function experiments in specific cell types during the developmental trajectory.
Region-specific genetic manipulation
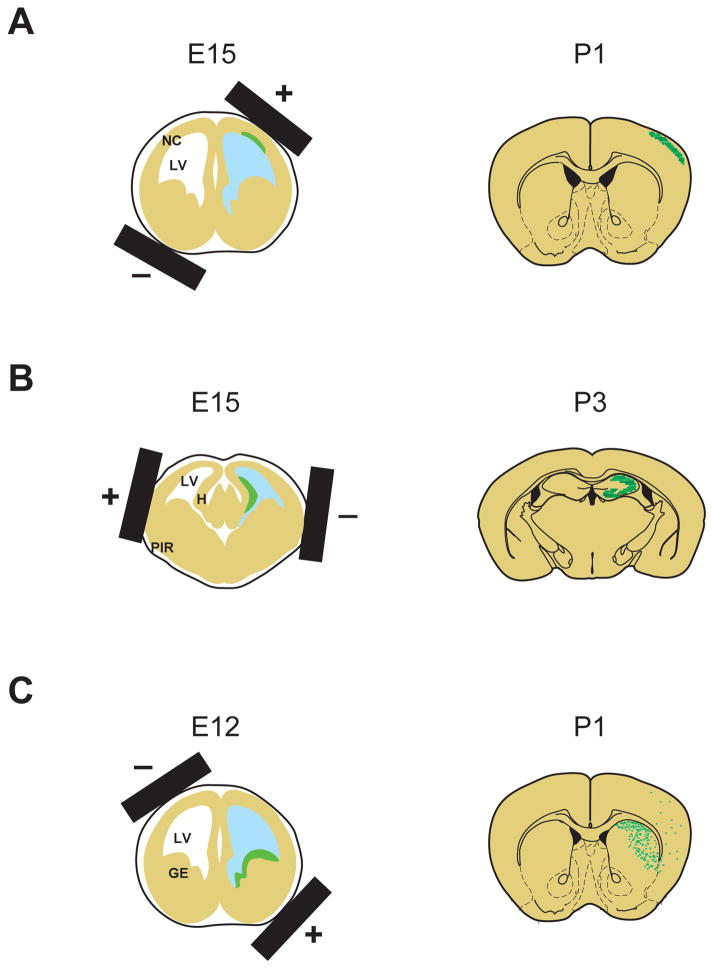
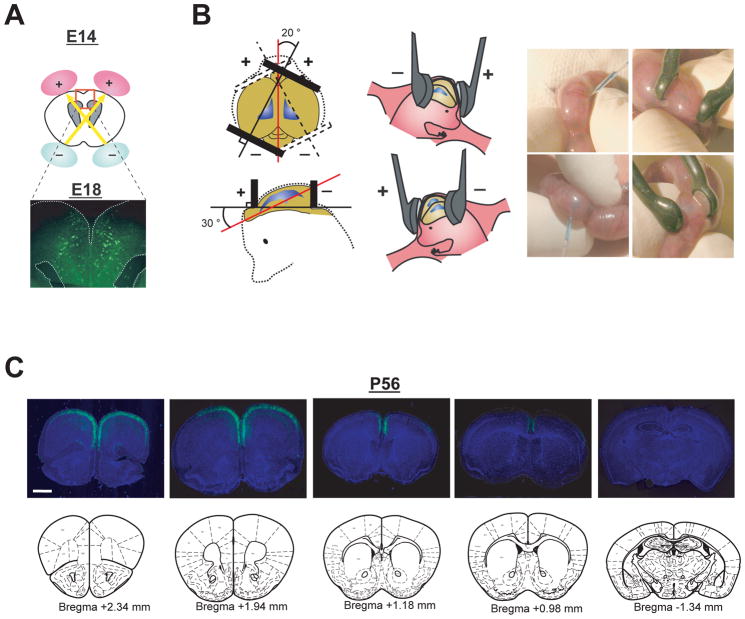
Evidence from postmortem brain examinations and imaging studies of major mental disorders, especially schizophrenia, suggest that there are structural and functional disturbances in diverse brain regions (Lewis and Sweet 2009; McCarley and others 1999). Developmental maturation of proper neuronal circuit connections are required for higher brain functions, such as cognition, memory, and emotion (Harrison and Weinberger 2005; Rapoport and others 2005). Although current genetic engineering techniques can produce a variety of cell type-specific inducible and conditional animals, including many types of Cre-driver and reporter lines (Madisen and others 2010), region-specific genetically-engineered animals have not yet been well established. However, in utero electroporation can target genes in distinct cellular sources that are developing in specific regions of the brain by simply altering the position of the electrodes. By altering the position of the electrodes, we can manipulate gene expression in the progenitor cells in diverse regions, such as the neocortical neuroepithelium, ammonic neuroepithelium, and lateroventral pallial neuroepithelium. These regions develop into the cerebral cortex, hippocampus, as well as piriform cortex and amygdala, respectively (Bai and others 2008; Nakahira and Yuasa 2005; Remedios and others 2007; Tabata and Nakajima 2001) (Figure 3). Notably, electroporation into the ganglionic eminence allows selective genetic manipulation of interneurons, including multiple subtypes of interneurons in the cerebral cortex, as described above (Borrell and others 2005). Furthermore, we have recently reported the use of in utero electoporation for selective gene targeting in the prefrontal cortex (Niwa and others 2010), a critical brain region associated with cognitive function, of which deficits have been frequently reported in schizophrenia (Tan and others 2009) (Figure 4).
Figure 3. Region-specific gene targeting by in utero electroporation.
Schematic representation of in utero electroporation for gene targeting into specific regions depending on the direction of electroporation.
(A) For manipulating the cells in the neocortex, the neocortical neuroepithelium is targeted by placing the positive electrode on the dorsal lateral side. LV, lateral ventricle; NC, neocortex.
(B) For manipulating the cells in the hippocampus, ammonic neuroepithelium is targeted via dorsal lateral placement of the positive electrode on the opposite side of their orientation for targeting the neocortex. PIR, piriform cortex; H, hippocampus.
(C) For manipulating interneurons, ganglionic eminence, main sources of GABAergic interneurons, including cortical interneurons, are targeted by means of ventral lateral placement of the positive electrode at an approximate 30 degree outward angle from the horizontal plane. GE, ganglionic eminence.
Figure 4. Selective targeting to pyramidal neurons in the prefrontal cortex.
(A) Schematic representation of bilateral in utero injection of constructs followed by their incorporation by electroporation into progenitor cells in the ventricular zone at E14. Migrating cells with GFP are visualized at E18 after injection of a GFP expression construct.
(B) The embryo’s head in the uterus was held with a forceps-type electrode, consisting of two disc electrodes. The electrodes were oriented at approximately a 20 degree outward angle from the midline and a rough 30 degree angle downward from an imaginary line from the olfactory bulbs to the caudal side of cortical hemisphere.
(C) Representative rostral-caudal image series of coronal sections of brains with GFP expression at P56 (+2.34 mm, +1.94 mm, +1.18 mm, +0.98 mm, and −1.34 mm from Bregma). Blue, nucleus. Scale bar, 1mm. Adapted from the Niwa et al (Niwa and others 2010).
The experiments described above target specific cell populations via altering electrode placement following injection of DNA into the lateral ventricle. However, one also can affect which region is targeted by altering the location of the injection. Since only the cells immediately adjacent to the location of the DNA will be electroporated, more cells will be targeted if DNA injection is into a space that allows some diffusion of the DNA (like the ventricles). For example, the developing retina can be targeted very early (E9.5) via injection into the optic vesicle using ultrasound technology (Punzo and Cepko 2008). Thus, three variables can be altered to target precise targeting of specific cell populations: the location of the injection of the DNA, the developmental stage of injection, and the placement of the electrodes.
Disease pathways: multiple genes targeting and the use of rescue components
Given the genetic heterogeneity in psychiatric disorders, it is critical to explore the common molecular pathways underlying disease associated phenotypes, where multiple genetic risk factors play roles. To this end, in utero electroporation is a good tool to modulate expression of more than one gene simultaneously. We have previously reported that multiple genetic risk factors, including Disrupted-in-Schizophrenia 1 (DISC1), Pericentriolar material 1 (PCM1), and BBS4, a causative gene for Bardet-Biedl syndrome (BBS) that is frequently characterized by psychosis and cognitive deficits, interact with each other for centrosome function in cortical development (Kamiya and others 2008). This study has shown that knockdown of PCM1 by in utero electroporation leads to neuronal migration defects, which is phenocopied by the suppression of DISC1 or BBS4, and concomitant knockdown of both DISC1 and BBS4 exacerbates migration defect (Kamiya and others 2008).
Another important aspect of genetic risks in major mental disorders is that there is no single gene that is a smoking gun or that shows obvious loss- or gain-of function leading to specific disease pathologies. Instead, there may be subtle genetic alterations such as single amino acid changes or genetic heterogeneity in a non-coding region that may increase risk. Thus, a combination of knockdown by RNA interference (RNAi) approaches and rescue experiments with co-electroporation with multiple constructs that include disease-associated mutants, are suitable for addressing molecular components associated with disease pathways. Young-Pearse et al (Young-Pearse and others 2010) reported the protein interaction of DISC1 and Amyloid Precursor Protein (APP), a well known protein that is cleaved to produce Aβ, the primary component of amyloid plaques in the brains of patients with Alzheimer’s disease. In this study, in utero electroporation was used to show that this interaction was important, for cortical development, and examined which splice variants and protein interaction sites of DISC1 are critical for neuronal migration in this context. Loturco and colleagues (Manent and others 2009) reported another elegant study using Cre/LoxP recombination-mediated overexpression system with rescue components. Conditional re-expression at P0 of DCX which plays a role for neuronal migration, normalized migration deficits elicited by the conventional knockdown of DCX at E14. These results suggest that in utero electroporation combined with inducible gene expression systems can be utilized for the identification of critical periods for specific molecular function for distinct cellular events.
The use of this methodology is capable of addressing cellular signaling in vivo. As such, rescue experiments which introduce shRNA together with overexpression constructs, including mutated ones, allows for the examination of not only protein-protein interaction, but also the effect of translational modifications and transcriptional regulation (Langevin and others 2007). Indeed, we have recently reported that phosphorylation of DISC1 at Serine 710 is a critical molecular switch signaling from progenitor cell proliferation to neuronal radial migration in the developing neocortex (Ishizuka and others 2011). By utilizing in utero electroporation, this study has shown that a phosphor-dead mutant DISC1 can rescue only the proliferation defect elicited by DISC1 knockdown, whereas a phosphor-mimic mutant of DISC1 can exclusively recover impaired migration. Taken together, the functional interaction between target molecules and cellular signaling can be assessed by co-electroporation of multiple constructs.
The use of in utero electropolation at neuronal circuit and behavioral levels
The feasibility of the animal models manipulated by in utero electroporation has recently been reported at the neuronal circuit and behavioral levels. By using the aforementioned technique for selective gene targeting in the prefrontal cortex (Figure 4), Niwa et al (Niwa and others 2010) have reported that selective knockdown of DISC1 in the developing prefrontal cortex leads to abnormal dendritic development of pyramidal neurons and disturbances in neuronal circuitry, involving mesocortical dopaminergic neurons, pyramidal neurons, and interneurons, as well as associated behavioral changes after puberty, including those in information processing and cognition. A critical question that must be further addressed is how the impairment of dendritic development caused by the knockdown of DISC1 leads to disturbance of overall neuronal circuits associated with the prefrontal cortex. Another example is the study by Manent et al (Manent and others 2009), reporting that re-expression of DCX ameliorated the susceptibility to epileptic seizures in DCX knockdown animal. These results suggest how in utero electroporation can have an impact not only at the molecular and cellular level, but also at circuit and behavioral levels even after developmental periods.
Although there are no behavioral abnormalities relevant to specific diagnostic criteria, some hallmarks of mental disorders at behavioral levels that are translatable between human and rodent models have been reported (Nestler and Hyman 2010). For instance, patients with major mental disorders, such as schizophrenia, have been frequently reported to have deficits in working memory and attention, as well as impaired somatosensory function evaluated via prepulse inhibition test. (Gottesman and Gould 2003). While these cognitive domains are not unique characteristics in the specific current diagnostic criteria, they could be characterized in animal models and provide valuable insights into the behavioral consequences of genetic manipulation.
Combination of in utero electroporation with other technologies
Many other technologies for in vivo genetic modulation and regulation of neuronal function are now available. To identify sensitive periods of specific cellular processes during brain development for the manifestation of disease-related phenotypes, the aforementioned combination of in utero electroporation with inducible and cell type-specific expression systems is used. Another useful method is the combination of conditional genetically-engineered mice (with target gene flanked with LoxP sites) with in utero electroporation of Cre-driver. These methods are capable of providing the genetic contribution of psychiatric conditions during specific developmental periods, ranging from molecular and cellular processes to behaviors. This may further enable us to identify their specific functional domains responsible for disease pathology.
One of the drawbacks of in utero electroporation is that non-viral vectors are likely to be maintained episomally in the proliferating cells in the developing stages, making it unclear how long transgene expression continues. However, transposon-mediated gene expression system, including piggyBac and Tol2, may overcome this particular issue by integrating transgenes into the chromosomal DNA of the targeted cells. (Ding and others 2005; Nakanishi and others 2010). The effect of Tol2 transposon was recently tested via in utero electroporation (Yoshida and others 2010). By using Tol2 transposon with glial promoters, such as GFAP and S100β, transgenes are stably expressed in astrocyte and oligodendrocytes (Yoshida and others 2010). Accumulating evidence from clinical studies suggests that immunological processes in central nervous system may play a role in major mental disorders (Khairova and others 2009; Potvin and others 2008; van Berckel and others 2008). Many groups have used stereotaxic virus-mediated gene delivery to manipulate target gene expression (Cetin and others 2006). However, this technique is not appropriate for addressing the role for genetic risk factors in immune system, because the injection of recombinant viruses at postnatal stages may induce inflammatory response and microglia activation. On the other hand, since in utero electroporation can deliver the transgenes at embryonic stages when the immune system is immature (minimal immune response in the brain during surgical intervention), this method allows for characterization of target genes for immune signaling in the glial cells which might trigger abnormal brain maturation relevant to the disease pathology.
Optogenetic tools have developed recently to control and monitor biological cellular processes at the cells and neuronal circuit levels (Zhang and others 2010). The expression of opsin genes, such as the Chlamydomonas channelrhodopsin-2 (ChR2) and Natronomonas halorhodopsin (NpHR), through the use of virus-mediated gene delivery is frequently used to modulate neuronal activity in the specific brain regions (Knopfel and others 2010; Zhang and others 2010). Although cell-type specific expression with recombinant promoters derived from genes expressed only in the targeted cell types (e.g. pyramidal neuron-specific expression using CaMKII promoter) is applicable, there is a limitation of packaging capacity of viral vectors which hampers the use of large sized cell type specific promoters for virus production (Davidson and Breakefield 2003). In this regard, in utero electroporation allows the transfection of plasmid DNA with a large size of promoters for targeting specific type of cells.
Concluding remarks
Many genetic risk factors for major mental disorders have multiple functions, depending on the cell type, region, and developmental stages. In utero electroporation is an advantageous method for investigating their etiological roles in brain development and higher brain function as described in this review (Table 1). It is also important to examine whether animal models via in utero electroporation is useful for the study on the effect of gene-environment interaction. However, there is limited knowledge of the genetic basis for mental disorders due to both the etiological complexities and difficulties in translating from human brain function to rodent behaviors and vice versa and the lack of biomarkers. Nonetheless, it is worth investigating animal models with in vivo genetic manipulation, and validating such models at the molecular to behavioral range, based on the evidence from epidemiological, pathological, and pharmacological examinations. Consequently, to understand the pathophysiologies for major mental disorders, focusing on the underlying molecular mechanisms for the possible endophenotype (i.e., specific neurobehavioral domains), rather than diagnostic characteristics, could prove to be a better option.
Table 1. The advantage of in utero electroporation.
There are many advantages of in utero electroporation for addressing the effect of genetic risk factors for major mental disorders as listed in this table. Furthermore, this method is relatively simple and quick (the entire process of surgery with electroporation for one pregnant rodent can be done within 30 min), and less labor intensive approach relative to the generation of genetically engineered mice.
| Advantages | Details | Selected references |
|---|---|---|
| Region specific gene targeting | Prefrontal cortex, piriform cortex, somatosensory cortex, hippocampus, amygdala, and retina | (Bai and others 2008; Kubo and others 2010a; Nakahira and Yuasa 2005; Niwa and others 2010; Punzo and Cepko 2008; Remedios and others 2007; Tabata and Nakajima 2001) |
| Inducible gene targeting | Study specific cellular processes in the developmental trajectory | (Manent and others 2009; Matsuda and Cepko 2007) |
| Cell type specific gene targeting | Pyramidal neurons in layer II/III, IV, and V/VI, interneurons, astrocytes, and oligodendrocytes, as well as progenitor cells and post-mitotic migrating neurons | (Borrell and others 2005; Kamiya 2009; LoTurco and others 2009; Miyagi and others 2004; Rice and others 2010; Wang and others 2007; Yoshida and others 2010) |
| Multiple gene targeting | Synergistic and epistatic effect of genetic risks; More than one gene can be modulated simultaneously | (Kamiya and others 2008; Young-Pearse and others 2007) |
| Rescue experiment | Explore disease pathways (protein interaction and cellular signaling, including translational modification and transcriptional regulation) The effect of disease-associated mutations | (Ishizuka and others 2011; Kubo and others 2010b; Langevin and others 2007; Manent and others 2009; Young-Pearse and others 2007; Young-Pearse and others 2010) |
| Acute suppression by RNAi | Avoiding compensation machinery by acute introduction of shRNA | (Bai and others 2003) |
| Behavior and circuit | Region and neuronal circuit dependent behaviors and functional outcome can be tested | (Manent and others 2009; Niwa and others 2010) |
Acknowledgments
We thank Dr. Ken-ichiro Kubo for valuable discussion and Mr. Peter Yoon for critical reading of the manuscript. We thank Ms. Yukiko Lema for preparation of the figures.
Funding
The authors disclosed receipt of the following financial support for the research and/or publication of this article. This work was supported by grants from NIMH: MH-091230 (A.K.), MH-85004 (T.L.Y-P), MH-069853 (A.S.), MH-088753 (A.S.), and Silvio Conte Center grants MH-084018 (A.S.), and foundation grants from NARSAD (A.K., A.S.), and S-R (A.K., A.S.).
Footnotes
Declaration of Conflicting Interests
The authors disclosed no conflicts of interests with respect to the authorship and/or publication of this article.
References
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;6(12):1277–83. doi: 10.1038/nn1153. [DOI] [PubMed] [Google Scholar]
- Bai J, Ramos RL, Paramasivam M, Siddiqi F, Ackman JB, LoTurco JJ. The role of DCX and LIS1 in migration through the lateral cortical stream of developing forebrain. Dev Neurosci. 2008;30(1–3):144–56. doi: 10.1159/000109859. [DOI] [PubMed] [Google Scholar]
- Bill BR, Geschwind DH. Genetic advances in autism: heterogeneity and convergence on shared pathways. Curr Opin Genet Dev. 2009;19(3):271–8. doi: 10.1016/j.gde.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell V, Yoshimura Y, Callaway EM. Targeted gene delivery to telencephalic inhibitory neurons by directional in utero electroporation. J Neurosci Methods. 2005;143(2):151–8. doi: 10.1016/j.jneumeth.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Cetin A, Komai S, Eliava M, Seeburg PH, Osten P. Stereotaxic gene delivery in the rodent brain. Nat Protoc. 2006;1(6):3166–73. doi: 10.1038/nprot.2006.450. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Weinberger DR. Genetic mouse models of schizophrenia: from hypothesis-based to susceptibility gene-based models. Biol Psychiatry. 2006;59(12):1180–8. doi: 10.1016/j.biopsych.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Corbo JC, Deuel TA, Long JM, LaPorte P, Tsai E, Wynshaw-Boris A, et al. Doublecortin is required in mice for lamination of the hippocampus but not the neocortex. J Neurosci. 2002;22(17):7548–57. doi: 10.1523/JNEUROSCI.22-17-07548.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson BL, Breakefield XO. Viral vectors for gene delivery to the nervous system. Nat Rev Neurosci. 2003;4(5):353–64. doi: 10.1038/nrn1104. [DOI] [PubMed] [Google Scholar]
- Ding S, Wu X, Li G, Han M, Zhuang Y, Xu T. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122(3):473–83. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294(5544):1071–4. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10(1):40–68. doi: 10.1038/sj.mp.4001558. image 5. [DOI] [PubMed] [Google Scholar]
- Ishizuka K, Kamiya A, Oh CE, Kanki H, Seshadri S, Robinson FJ, et al. A DISC1-dependent switch from neuronal proliferation to migration in the developing cortex. Nature. 2011 doi: 10.1038/nature09859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32(9):485–95. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A. Animal models for schizophrenia via in utero gene transfer: understanding roles for genetic susceptibility factors in brain development. Prog Brain Res. 2009;179:9–15. doi: 10.1016/S0079-6123(09)17902-5. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7(12):1167–78. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Tan PL, Kubo K, Engelhard C, Ishizuka K, Kubo A, et al. Recruitment of PCM1 to the centrosome by the cooperative action of DISC1 and BBS4: a candidate for psychiatric illnesses. Arch Gen Psychiatry. 2008;65(9):996–1006. doi: 10.1001/archpsyc.65.9.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khairova RA, Machado-Vieira R, Du J, Manji HK. A potential role for pro-inflammatory cytokines in regulating synaptic plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2009;12(4):561–78. doi: 10.1017/S1461145709009924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopfel T, Lin MZ, Levskaya A, Tian L, Lin JY, Boyden ES. Toward the second generation of optogenetic tools. J Neurosci. 2010;30(45):14998–5004. doi: 10.1523/JNEUROSCI.4190-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo K, Honda T, Tomita K, Sekine K, Ishii K, Uto A, et al. Ectopic Reelin induces neuronal aggregation with a normal birthdate-dependent “inside-out” alignment in the developing neocortex. J Neurosci. 2010a;30(33):10953–66. doi: 10.1523/JNEUROSCI.0486-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo K, Tomita K, Uto A, Kuroda K, Seshadri S, Cohen J, et al. Migration defects by DISC1 knockdown in C57BL/6, 129X1/SvJ, and ICR strains via in utero gene transfer and virus-mediated RNAi. Biochem Biophys Res Commun. 2010b;400(4):631–7. doi: 10.1016/j.bbrc.2010.08.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin LM, Mattar P, Scardigli R, Roussigne M, Logan C, Blader P, et al. Validating in utero electroporation for the rapid analysis of gene regulatory elements in the murine telencephalon. Dev Dyn. 2007;236(5):1273–86. doi: 10.1002/dvdy.21126. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Sweet RA. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest. 2009;119(4):706–16. doi: 10.1172/JCI37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoTurco J, Manent JB, Sidiqi F. New and improved tools for in utero electroporation studies of developing cerebral cortex. Cereb Cortex. 2009;19(Suppl 1):i120–5. doi: 10.1093/cercor/bhp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–40. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manent JB, Wang Y, Chang Y, Paramasivam M, LoTurco JJ. Dcx reexpression reduces subcortical band heterotopia and seizure threshold in an animal model of neuronal migration disorder. Nat Med. 2009;15(1):84–90. doi: 10.1038/nm.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Cepko CL. Controlled expression of transgenes introduced by in vivo electroporation. Proc Natl Acad Sci U S A. 2007;104(3):1027–32. doi: 10.1073/pnas.0610155104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, et al. MRI anatomy of schizophrenia. Biol Psychiatry. 1999;45(9):1099–119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan JM, Susser E, King MC. Schizophrenia: a common disease caused by multiple rare alleles. Br J Psychiatry. 2007;190:194–9. doi: 10.1192/bjp.bp.106.025585. [DOI] [PubMed] [Google Scholar]
- Miyagi S, Saito T, Mizutani K, Masuyama N, Gotoh Y, Iwama A, et al. The Sox-2 regulatory regions display their activities in two distinct types of multipotent stem cells. Mol Cell Biol. 2004;24(10):4207–20. doi: 10.1128/MCB.24.10.4207-4220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira E, Yuasa S. Neuronal generation, migration, and differentiation in the mouse hippocampal primoridium as revealed by enhanced green fluorescent protein gene transfer by means of in utero electroporation. J Comp Neurol. 2005;483(3):329–40. doi: 10.1002/cne.20441. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Higuchi Y, Kawakami S, Yamashita F, Hashida M. piggyBac transposon-mediated long-term gene expression in mice. Mol Ther. 2010;18(4):707–14. doi: 10.1038/mt.2009.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13(10):1161–9. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K, et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 2010;65(4):480–9. doi: 10.1016/j.neuron.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan MC, Craddock NJ, Owen MJ. Genetics of psychosis; insights from views across the genome. Hum Genet. 2009;126(1):3–12. doi: 10.1007/s00439-009-0703-0. [DOI] [PubMed] [Google Scholar]
- Owen MJ, Craddock N, O’Donovan MC. Schizophrenia: genes at last? Trends Genet. 2005;21(9):518–25. doi: 10.1016/j.tig.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Owen MJ, Craddock N, O’Donovan MC. Suggestion of roles for both common and rare risk variants in genome-wide studies of schizophrenia. Arch Gen Psychiatry. 2010;67(7):667–73. doi: 10.1001/archgenpsychiatry.2010.69. [DOI] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801–8. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Punzo C, Cepko CL. Ultrasound-guided in utero injections allow studies of the development and function of the eye. Dev Dyn. 2008;237(4):1034–42. doi: 10.1002/dvdy.21500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10(5):434–49. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Remedios R, Huilgol D, Saha B, Hari P, Bhatnagar L, Kowalczyk T, et al. A stream of cells migrating from the caudal telencephalon reveals a link between the amygdala and neocortex. Nat Neurosci. 2007;10(9):1141–50. doi: 10.1038/nn1955. [DOI] [PubMed] [Google Scholar]
- Rice H, Suth S, Cavanaugh W, Bai J, Young-Pearse TL. In utero electroporation followed by primary neuronal culture for studying gene function in subset of cortical neurons. J Vis Exp. 2010;(44) doi: 10.3791/2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T. In vivo electroporation in the embryonic mouse central nervous system. Nat Protoc. 2006;1(3):1552–8. doi: 10.1038/nprot.2006.276. [DOI] [PubMed] [Google Scholar]
- Saito T, Nakatsuji N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev Biol. 2001;240(1):237–46. doi: 10.1006/dbio.2001.0439. [DOI] [PubMed] [Google Scholar]
- Sauvageot CM, Stiles CD. Molecular mechanisms controlling cortical gliogenesis. Curr Opin Neurobiol. 2002;12(3):244–9. doi: 10.1016/s0959-4388(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Sebat J, Levy DL, McCarthy SE. Rare structural variants in schizophrenia: one disorder, multiple mutations; one mutation, multiple disorders. Trends Genet. 2009;25(12):528–35. doi: 10.1016/j.tig.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata H, Nakajima K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 2001;103(4):865–72. doi: 10.1016/s0306-4522(01)00016-1. [DOI] [PubMed] [Google Scholar]
- Tan HY, Callicott JH, Weinberger DR. Prefrontal cognitive systems in schizophrenia: towards human genetic brain mechanisms. Cogn Neuropsychiatry. 2009;14(4–5):277–98. doi: 10.1080/13546800903091665. [DOI] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry. 2008;64(9):820–2. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Wang X, Qiu R, Tsark W, Lu Q. Rapid promoter analysis in developing mouse brain and genetic labeling of young neurons by doublecortin-DsRed-express. J Neurosci Res. 2007;85(16):3567–73. doi: 10.1002/jnr.21440. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Yamaguchi Y, Nonomura K, Kawakami K, Takahashi Y, Miura M. Simultaneous expression of different transgenes in neurons and glia by combining in utero electroporation with the Tol2 transposon-mediated gene transfer system. Genes Cells. 2010;15(5):501–12. doi: 10.1111/j.1365-2443.2010.01397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Pearse TL, Bai J, Chang R, Zheng JB, LoTurco JJ, Selkoe DJ. A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci. 2007;27(52):14459–69. doi: 10.1523/JNEUROSCI.4701-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Pearse TL, Suth S, Luth ES, Sawa A, Selkoe DJ. Biocemical and Functional Interaction of Disrupted-in-Schizophrenia 1 and Amyloid Precursor Protein Regulates Neuronal Migration during Mammalian Cortical Development. J Neurosci. 2010;30(31):10431–10440. doi: 10.1523/JNEUROSCI.1445-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc. 2010;5(3):439–56. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]