Abstract
microRNAs (miRNAs), endogenous posttranscriptional repressors by base-pairing of their cognate mRNAs in plants and animals, have mostly been thought lost in the kingdom of fungi. Here, we report the identification of miRNAs from the fungus Cryptococcus neoformans. With bioinformatics and Northern blotting approaches, we found that these miRNAs and their hairpin precursors were present in this fungus. The size of miR1 and miR2 is 22 nt and 18 nt, respectively. The precursors are about ∼70 nt in length that is close to mammalian pre-miRNAs. Characteristic features of miRNAs are also found in miR1/2. We demonstrated that the identified miRNAs, miR1 and miR2, caused transgene silencing via the canonical RNAi pathway. Bioinformantics analysis helps to reveal a number of identical sequences of the miR1/2 in transposable elements (TEs) and pseudogenes, prompting us to think that fungal miRNAs might be involved in the regulation of the activity of transposons and the expression of pseudogenes. This study identified functional miRNAs in C. neoformans, and sheds light on the diversity and evolutionary origin of eukaryotic miRNAs.
Introduction
Small non-coding RNAs (sRNAs) ranging from 20 to 30 nucleotides (nt) in length are widely found in the major phyla of eukaryotes [1]–[3]. Many of them have been shown to function as regulatory agents in RNA interference (RNAi) networks [4], [5]. By precursor structure, biogenesis origin, and mode of action, sRNAs are generally categorized into three classes, i.e. microRNAs (miRNAs), small interfering RNAs (siRNAs), and piwi-interacting RNAs (piRNAs) [4], [6], [7]. The class miRNA is featured by its endogenous origin and its flexibility in base-pairing to cause degradation of target mRNA [2], [8], [9]. miRNAs are derived from larger stem-looped hairpin precursors that are transcribed from miRNA genes and are processed by Dicer or Dicer-like proteins [7], [8], [10]. Usually, they act as posttranscriptional repressors of the target gene to cause silencing via binding partially or perfectly to the complementary sequences located in the 3′untranslated regions (3′-UTRs) or the other part of target mRNAs [2], [3], [11], [12].
Gene silencing by RNAi is also conserved many fungi. Diverse small RNAs (sRNAs) and RNAi pathways have been described from a number of fungi [13], [14]. As a matter of fact, most fungal sRNAs are siRNAs, for instance, in the yeast Schizosaccharomyces pombe [15], budding yeast Saccharomyces castellii, Candida albicans and Cryptococcus neoformans [16]–[19], as well as in the filamentous fungus Neurospora crassa [13], [14]. In N. crassa, miRNA-like sRNAs (milRNAs) were lately reported [14]. Twenty five milRNAs were predicted based on non-coding precursor RNA genes that contain inverted RNA sequences to form hairpin structure by bioinformatics analysis. Some milRNAs are formed from different pathways other than the conventional pathways that generate miRNAs in animals and plants [14]. However, a typical miRNA in fungi has been largely elusive by far.
C. neoformans is an opportunistic human pathogen for cryptococcosis in immuno-compromised patients, e.g. AIDS-afflicted people [20]. The fungus possesses the core components for RNAi process, including Argonaute proteins (encoded by AGO1 and AGO2, mainly the AGO1), Dicers (DCR1 and DCR2, mainly DCR2) and RNA-dependent RNA polymerase (RDP1) [19], [21], [22]. The RNAi machinery in C. neoformans is functional as transgenic dsRNA has been used to knock down the target genes [18]. A sex-induced silencing of transgenes (multiple copies in tandem) was observed via RNAi during the sexual stage in this fungus [19]. Deep sequencing of siRNAs in C. neoformans revealed a portion of siRNAs were derived from putative repetitive transposon-like sequences, and the biogenesis of these siRNAs depended on the function of RNAi machinery [19]. Here, we report the identification of microRNA in C. neoformans. After sequencing approximately 200 of small RNAs of ∼20–25 nt, two of them, designated as miR1 and miR2, were proven to have the characteristic features of miRNAs. miR1/2 was likely derived from 70-nt RNA precursors that were revealed by bioinformatics analysis and by Northern blotting. When miR1/2 was fused to reporter genes, URA5 and CLC1, at the 3′ untranslated region, URA5 and CLC1 were silenced at the posttranscriptional level, which was RNAi machinery-dependent. Both miR1 and miR2 were mapped to the transposable elements (TEs) and pseudogenes.
Results
Cloning and analyses of small RNAs in C. neoformans
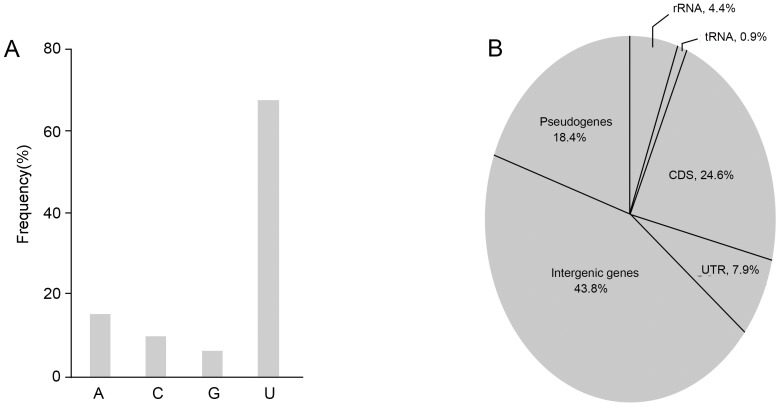
To comprehensively search for miRNAs, a library for small RNAs (20–25 nt) was constructed for a two-day-old culture of C. neoformans, containing approximately 20000 clones. Two hundred clones were randomly selected for sequencing. The size of the inserts ranged from 14 to 25 nt. The majority of them (67.5% of the total) had a preference of U at the 5′ end (Figure 1A), a characteristic hallmark of small RNAs from animals, plants and other fungi [2], [14], [19]. To find out the distribution pattern of these sRNAs in the genome, we made blasting alignment against GenBank databases. As shown in Figure 1B, a small fraction (5.3%) of the cloned sRNAs perfectly matched the sequences of rRNAs (4.4%) and tRNAs (0.9%). And 32.5% of them hit transcriptional products, covering both coding sequences (CDS, 24.6%) and 3′-untranslated region (UTR, 7.9%). The rest (62.2%) of the sRNAs matched pseudogenes and intergenic regions. This distribution pattern is remarkably close to the data reported by other laboratories from different organisms [14], [19]. That sRNAs match to multiple loci indicates the diverse origination of sRNAs at this range of size. Obviously, the function of these sRNAs remains a challenging issue to address for researchers.
Figure 1. Statistic diagram of small RNAs in C. neoformans B4500.
(A) Frequency of the nucleotides at the 5′ end of the sequenced small RNAs. (B) Genomic distribution of sRNAs in C. neoformans.
Identification of miRNA in C. neoformans
To screen for cryptococcal miRNA in the sequenced sRNA library, the following criteria were taken into account. First, with bioinformatics tools such as software Vmir (http://books.elsevier.com/companions/ 978012379179), we examined the loci of sRNAs in the genome for precursor transcripts with hairpin structures that harbor a sRNA on the stem [8]. Secondly, these sRNAs that had more than three homologous loci in the genome were chosen except for those from the rRNA and tRNA loci. And most importantly, the presence of the sRNAs could be detected by Northern blotting. By these standards, two miRNA candidates, miR1 (22 nt) and miR2 (18 nt), were acquired. Both miR1 and miR2 begins with a U at the 5′ end and are located on the arm of putative hairpin-form RNA precursors (Figure 1 and 2) [14]. The miRs are distributed on several highly complementary loci in the genome. For instance, five loci have identical sequence to miR1, while nine loci perfectly match miR2. Most of these loci encode transposable elements (TEs) and pseudogenes (Table 1). A few others are protein-encoding or intergenic regions. The possible precursors (pre-miRNA) were also found by computation with Vmir and other software that predicts hairpin structures (Figure 2) (carried out by commercial service).
Figure 2. Structure of the predicted pre-miRNAs of miR1 and miR2.
The sequences of miR1 and miR2 are highlighted by red letters.
Table 1. Chromosomal distributions of miR1 and miR2.
| Name/Length | Chr. | Loci | Identities (%) | Coding |
| miR1/22 nt5′-TCCTGAACTTGATCACCATTGA | Chr12 | 132350–132329 | 100 | Protein AAW 46453.1 |
| 166582–166603 | 100 | Transposase_21 (AAW 46457.1) | ||
| Chr4 | 55830–55851 | 100 | Protein XM 570676.1 | |
| Chr9 | 331418–331439 | 100 | Intergenic gene | |
| 370991–370970 | 100 | pseudogene | ||
| Chr1 | 1365317–1365335 | 86.4 | Intergenic gene | |
| 2111331–2111318 | 63.6 | Protein AAW41297.1 | ||
| miR2/18 nt5′-TCGACACCTTCATCGTAA | Chr3 | 215067–215049 | 100 | MULE (AAW42067.1)/pseudogene |
| 1816706–1816723 | 100 | Protein AAW42575.1 | ||
| Chr13 | 647216–647234 | 100 | Protein AAW46987.1/AAW46986.1 | |
| Chr9 | 397836–397818 | 100 | MULE (AAW45547.1) | |
| 1139994–1139982 | 72.2 | Intergenic gene | ||
| Chr8 | 1114372–1114354 | 100 | MULE (AAW44911.1) | |
| Chr6 | 797890–797908 | 100 | MULE (AAW44276.1) | |
| 81752–81739 | 77.8 | Protein AAW44202.1 | ||
| Chr4 | 16424–16406 | 100 | MULE (AAW43368.1)/pseudogene | |
| 1656773–1656760 | 77.8 | Chromatin Remodeling Complex (AAW43182.1) | ||
| Chr1 | 1482167–1482184 | 100 | Protein AAW41058.1 | |
| 1484501–1484484 | 100 | MULE (AAW41060.1) | ||
| 788546–788534 | 72.2 | Intergenic gene |
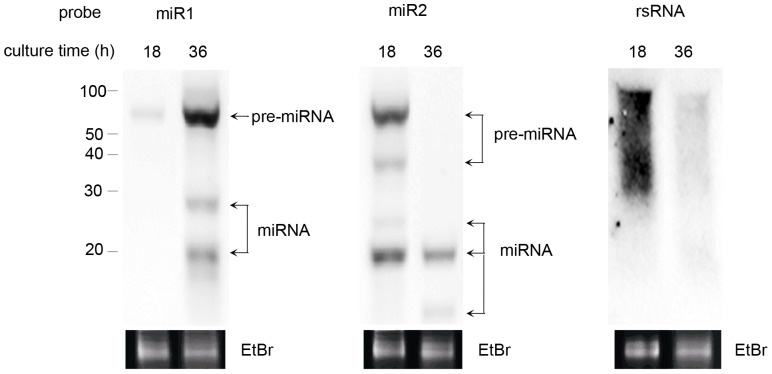
Distinct from other sRNAs, miRNAs are processed from larger precursors of ∼70-nt in animals, and from 47 to 698 nt in plants [23], [24]. It is critical to demonstrate the existence of miR1/2 and their precursors in C. neoformans. Therefore, we carried out Northern blotting to detect miR1/miR2 and their precursors (Figure 3). Probes for the blots were miR1, miR2, respectively. A third sRNA randomly picked in the library (rsRNA) served as control (see Materials and Methods). RNA samples were prepared from yeast cells grown for 18 and 36 hours. As anticipated, miR1, miR2 and their precursors were detected in the blots (two left panels, Figure 3). Pre-miR1 was approximately 70 nt in size which was close to the length of mammalian miRNAs [14]. A couple bands between the 20-nt and 70-nt bands were seen, suggesting that intermediate steps may be required for the maturation of miR1 (left panel, Figure 3). Interestingly, expression of miR1 and miR2 seemed to be related to culture time. At 18 hr, miR1 was hardly detectable and its precursor pre-miR1 started to be transcribed. At 36 hr, both pre-miR1 and miR1 were seen at a high level. For miRNA2, a higher expression level was observed at 18 hr than at 36 hr (the central panel, Figure 3). A similar precursor was confirmed for miR2 at ∼70 nt. Two more intermediate bands were also seen for miR2. It is noteworthy that only smeared signal was obtained in the blot for the control sRNA that did not has interfering effect (Right panel, Figure 3).
Figure 3. Detection of miRNAs by Northern blotting.
Positive bands for miR1 and miR2 (the left two panels) and their precursors were detected. On the right, rsRNA, a randomly picked small RNA only formed a smear band signal. Probes were the synthesized DNA sequence corresponding to miR1, miR2 or rsRNA. Total RNA was prepared from cultures collected at 18 hr and 36 hr as indicated. EtBr stands for ethium bromide-stained gel showing the 22-nt miRNA bands. Approximately 10 µg of total RNA was equally loaded. The size of the RNA markers is shown on left.
Demonstration of URA5-miRs silencing
Since miRNAs regulate target mRNAs through complementary base-pairing [25], we constructed a fusion gene, URA5-miR1 and URA5-miR2, as reporter to examine the interfering activity of miR1 and miR2 (see the section Materials and Methods, Figure 4). miR1 or miR2 was inserted by a PCR method at the 3′ UTR of URA5 which encodes an enzyme in the biosynthesis of uracil [26] (Figure 4). In principle, silencing Ura5 would yield auxotrophic phenotype for uracil and resistance to the toxin 5-fluoro-orotic acid (5-FOA). Plasmids carrying respectively URA5-miR1 and URA5-miR2, were introduced into a recipient strain B4500FOA (ura5-) by electroporation. For control, half of the sequence (50% of the nucleotides) of miR1 and miR2 were randomly mutated to generate miR1-m and miR2-m that were likewise fused to URA5 (Materials and Methods). All transformants were selected on appropriate plates containing 100 µg/ml hygromycin (the plasmids contained hygromycin B resistant marker).
Figure 4. Schematic diagram of the construction of reporters in silencing assay.
The upper panel shows the construction of URA5-miRs or URA5-miR-ms. Two pair of primers, URA5-XhoI-S/URA5-miRs-BamHI and URA5-BamHI-S/URA5-XbaI-A, were used to PCR amplify the ORF and the terminator regions of URA5, respectively. Among the primers, URA5-miRs-BamHI harbored the sequence of miRs or miR-ms through primer design. The two PCR fragments were then ligated after digested by BamH I. Similarly, the construction of CLC-miR1/2 and CLC-miR1-m/miR2-m was made (the bottom part). The position of miRs or miR-ms is indicated by the boxes. Restriction sites are in small letters. Arrows mark the position and direction of the primers. Start codons and stop codons of URA5 and CLC1 are underlined. For detailed description, see the section of Materials and Methods.
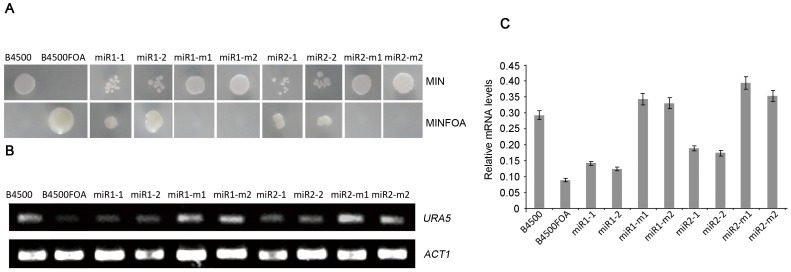
Transformants were tested for uracil prototrophy on selective medium MIN (minimal medium without uracil). Two transformants of URA5-miR1 (miR1-1 and miR1-2, Figure 5A) and URA5-miR2 (miR2-1 and miR2-2, Figure 5A) grew significantly slower than the wild type B4500 (and the negative control B4500FOA failed to grow). In contrast, transformants of miR1-m and miR2-m (miR1-m1/miR1-m2, and miR2-m1/miR2-m2, respectively) exhibited a similar growth rate to the wild type B4500 (the upper panels of Figure 5A). In a concomitance, transformants of URA5-miR1 and URA5-miR2 survived in the presence of 5-FOA, whereas transformants carrying mutated miR1/miR2 (miR1-m1/miR1-m2 and miR2-m1/miR2-m2 in Figure 5A), failed to grow, as well as the wild type B4500 (the bottom panels in Figure 5A). These results clearly demonstrated that miR1 and miR2 had a silencing effect on the expression of URA5. When miR1 and miR2 were mutated, the silencing did not occur to the reporter URA5.
Figure 5. Silencing of the reporter gene URA5-miRs.
(A) The upper panels show a slower growth rate of the transformants of URA5-miR1 (two transformants was picked in each case, namely, miR1-1 and miR1-2), and URA5-miR2 (miR2-1 and miR2-2), than the wild type B4500, and the transformants of miR1-m (i.e. miR1-m1 and m2) and miR2-m, on MIN agar supplemented with 100 µg/ml hygromycin B, no hygromycin for B4500 and B4500FOA. The negative control B4500FOA (ura5) did not grow on MIN. On MINFOA (the bottom panels), the wild type strains and transformants of miR1-m and miR2-m were killed by FOA. Transformants containing miR1 and miR2, as well as ura5 − strain B4500FOA grew properly. MIN: minimal medium, and MINFOA: minimal medium with 5-FOA and 50 mg/l uracil. For selection of transformants, 100 µg/ml hygromycin B was added to MIN or MINFOA when needed. (B) Reverse transcription-PCR confirmed the decreased mRNA level of URA5 in URA5-miR transformants. URA5 mRNA levels in the wild type and in the transformants of miR-ms were close to each other. In each assay, two transformants were picked for double examination. (C) A semi-quantification of URA5 mRNA to ACT1 mRNA in the strains in (B).
The relative transcriptional level of URA5 was determined by semi-quantitative reverse transcription-PCR (with the software Quantity One, BioRad, CA, USA). The result was shown in Figure 5B. The level of URA5 mRNA in the transformants of URA5-miR1 and URA5-miR2 was significantly lower than that in the wild type B4500 and the non-silencing controls (miR1-m1/miR1-m2, or miR2-m1/miR2-m2) (Figure 5C). When the relative abundance of URA5 mRNA in each sample was calculated against the internal standard ACT1 mRNA, the ratio of the transcripts in the miR1-1, 2 and miR2-1, 2 was 0.13±0.013 and 0.18±0.009, significantly lower than the value for the wild type B4500 (0.29±0.01), and the controls, miR1-m1/2 (0.34±0.0098) and miR2-m1/2 (0.37±0.028), confirming that URA5 expression was knocked down by miRs.
Still more, quantitative real time PCR (qRT-PCR) was adopted for further verification of URA5-miRs silencing in the transformants. Ratio of URA5 to the internal standard ACT1 mRNA was calculated in each strain. As shown in Table 2, URA5 mRNA decreased by 6 and 4 fold respectively in the miR1 and miR2 transformants compared to the wild type. In a striking contrast, in transformants of miR1-m1, 2 and miR1-m1,2, expression level of URA5 was close to the wild type, which was significantly higher than in the knock-down transformants. Putting together, by phenotypic assays, reverse transcription PCR and qRT-PCR, we have confirmed that miRNAs, miR1 and miR2, have interfering activity in C. neoformans.
Table 2. Expression of URA5-miRs determined by qRT-PCR in different transformants.
| strains | ΔCT | ΔΔCT | Normalized |
| (Avg.URA5CT-Avg.ACT1CT) | (Avg.ΔCT-Avg.ΔCT, B4500) | URA5 amount relative to B4500 2−ΔΔC T | |
| B4500 | 2.42±0.19 | 0.00±0.19 | 0.88–1.14 |
| miR1 | 4.48±0.12 | 2.07±0.13 | 0.22–0.26 |
| miR2 | 4.97±0.05 | 2.55±0.15 | 0.15–0.19 |
| miR1-m | 2.54±0.17 | 0.12±0.03 | 0.90–0.94 |
| miR2-m | 2.07±0.14 | −0.35±0.10 | 1.2–1.4 |
The silencing of reporter CLC-miRs
Using another gene CLC1 as reporter, we here present one more example to show gene silencing caused by miR1 and miR2. CLC1 encodes a chloride channel that is essential for pigment formation in the presence of polyphenolic substrate in C. neoformans [27]–[29]. CLC1 disruption results in albino yeast colonies on nor-epinephrine (NE)-containing agar. The reporter construct CLC-miRs were made in a similar way for URA5-miRs (Figure 4). The recipient strain was a disruption mutant of CLC1, Δclc1 (melanin-deficiency, see Materials and Methods) [28].
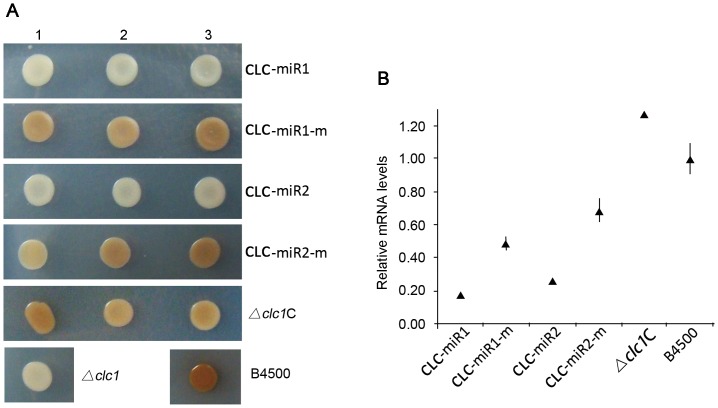
As anticipated, transformants of CLC-miRs (# 1,2 and 3) formed light colonies on NE plates, a phenotype similar to Δclc1 strain, suggesting a silencing outcome of CLC-miR1/2 in the transformants (the first and the third rows from the top, Figure 6A). The transformants of CLC1- miR1-m1 or miR2-m restored to produce pigment on NE plates, a phenotype similar to the wild type B4500 and the complement strain Δclc1C (Figure 6A). The ratio of CLC1 mRNA to ACT1 mRNA by reverse transcription PCR confirmed the knockdown of CLC1 expression in the transformant of miR1 and miR2 (Figure 6B). In addition, remarkable down-regulation of CLC1 mRNA was confirmed by qRT-PCR in transformants of miR1 and miR2 (Table 3), i.e. approximately 2.8- and 2.6-fold lower than that in the wild type.
Figure 6. Silencing of the reporter CLC-miRs.
A similar silencing assay was carried out for the reporter CLC-miRs. (A) Melanin-deficient phenotype of the transformants of CLC-miRs (#1 to #3) was observed as expected, suggesting knockdown expression of CLC1. When miRs were mutated, CLC-miR-ms restored melanin production (Second and forth rows from the top). Cells were placed on low-glucose (0.1%) Asn agar with NE and 100 µg/ml hygromycin except for the wild types. (B) Decreased mRNA level of CLC1 by miRs silencing determined by reverse transcription-PCR for the strains in (A). Relative abundance of CLC1 mRNA verse actin-encoding gene ACT1 mRNA was considered. PCR reaction was performed in triplicate.
Table 3. Expression of CLC1-miRs determined by qRT-PCR in different strains.
| strains | ΔCT | ΔΔCT | Normalized |
| (Avg.CLCCT-Avg.ACT1CT) | (Avg.ΔCT-Avg.ΔCT, B4500) | CLC amount relative to B4500 2−ΔΔC T | |
| B4500 | 4.30±0.13 | 0.00±0.13 | 0.91–1.09 |
| ΔCLC1C | 3.95±0.02 | −0.35±0.02 | 1.26–1.29 |
| CLC-miR1 | 6.81±0.02 | 2.51±0.02 | 0.17–0.18 |
| CLC-miR2 | 6.24±0.07 | 1.94±0.07 | 0.25–0.27 |
| CLC-miR1-m | 5.34±0.12 | 1.04±0.12 | 0.45–0.53 |
| CLC-miR2-m | 4.85±0.15 | 0.55±0.15 | 0.62–0.76 |
In summary, through silencing assays for reporter URA5 and CLC1, we demonstrated that miRNA-mediated gene silencing occurs in C. neoformans.
miRNA-mediated gene silencing via canonical RNAi machinery in C. neoformans
We further found that miRNA-mediated gene silencing involves the RNAi machinery in this fungus. The reporter silencing assay was carried out in several mutant strains in which components required for the RNAi have been deleted. These strains include NE465 (ago1Δ::NAT-STM, ura5), NE493 (rdp1Δ::NAT-STM ura5), NE473 (dcr1Δ::NEO, ura5), and NE475 (dcr21Δ::HYG-STM, ura5) [30]. Likewise, plasmids carrying URA5-miR1 and URA5-miR2, and their corresponding mutated forms (as control), were introduced into the mutants, separately. URA5-miR1 and URA5-miR2 restored the auxotrophic phenotype of all the strains (ura5−) (Figure 7A), suggesting that gene silencing was abolished in the RNAi-deficient mutants. In contrast, transformants with the mutated form of miR1 and miR2 were able to grow on minimal media (without uracil). All the transformants failed to grow in the presence of 5-FOA, reinforcing that URA5-miRs was appropriately expressed in RNAi-defective mutants (Figure 7A). Still more, qRT-PCR ratified that the reporter URA5-miRs was properly expressed in the mutants of ago1, rdp1, and dcr1, dcr2 (Figure 7B). Taking together, the above results suggest that miRNA-mediated gene silencing occurs via RNAi pathway in C. neoformans.
Figure 7. miR-mediated gene silencing requires RNAi machinery in C. neoformans.
(A) URA5-miR1/2 restored the growth of B4500FOA (ura5) on MIN agar in the RNAi-deficient mutant strains, NE465, NE493, NE473 and NE475, i.e. gene silencing of URA5-miRs that was observed in Fig. 4 did not occur in these mutants. And these transformants failed to grow on plates containing 5-FOA (the panels of MINFOA agar). MIN or MINFOA agar was supplemented with 100 µg/ml hygromycin B for the selection of all transformants of the reporters. The drug was left out for B4500 and B4500FOA. The transformants of miR1-m and miR2-m, together with the wild type B4500 and B4500FOA, served as control. (B) qRT-PCR confirmation of restored expression of URA5 in RNAi-deficient mutants, NE465, NE493, NE473 and NE475, which are originally ura5 defective strains.
Discussion
In this work, we identified two miRNAs, miR1 (22 nt) and miR2 (18 nt), from the basidiomycetous yeast C. neoformans. They carry structural hallmarks of conventional miRNAs defined by studies in other eukaryotic organisms, for instance, a U at the 5′ end of the small RNAs [14]. We found that miR1 and miR2 are putatively derived from stem-loop RNA precursors by bioinformatics approach (Figure 2). Besides, precursor RNAs, together with miR1 and miR2, were detected by Northern blotting (Figure 3). Notably, the pre-miR1 and pre-miR2 are approximately 70 nt in size and is close to the length of mammalian miRNAs [14]. Northern blots also showed that expression of miR1 and miR2 depended on culture time, suggesting that action of cryptococcal miRs is under regulation. We used different approaches to show that miR1 and miR2 have interfering activity of small RNAs. We designated two reporters, URA5-miRs and CLC1-miRs, with miR1 and miR2 inserted at the 3′ UTR of URA5 and CLC1, accordingly, in the rational that miRNAs cause gene silencing via base-paring of the homologous sequence on the target mRNA [3], [12]. These reporters were indeed silenced when introduced in the fungus (Figure 5 and 6). Molecular data generated by qRT-PCR or reverse transcription PCR made a further confirmation of the dramatic decrease of the reporter genes, URA5 and CLC by miR1 and miR2 (Figure 5, Figure 6 and Figure 7).
Further more, we showed that the silencing caused by miR1 and miR2 was actually realized through RNAi pathway in C. neoformans. We took advantage of four available mutant strains NE465 (ago1Δ::NAT-STM, ura5), NE493 (rdp1Δ::NAT-STM ura5), NE473 (dcr1Δ::NEO, ura5), and NE475 (dcr21Δ::HYG-STM, ura5), in which RNAi components, AGO1 for Argonaute, RDP1 for RNA-dependent RNA polymerase and DCR1 and DCR2 for two Dicer proteins, were separately disrupted [30]. As shown in Panel A of Figure 7, disruption of the genes in any of the mutants demonlished URA5-miRs-caused gene silencing. As a result, transformants were unable to grow on 5-FOA-containing plates (the ura5 − controls grew on 5-FOA). Also, qRT-PCR confirmed that there was no discernable knock-down effect for the reporter gene URA5-miR1 or URA5-miR2 in the transformants (Panel B, Figure 7). These results clearly demonstrate that miRs-mediated gene silencing is realized via RNAi machinery in C. neoformans.
Distribution of miRNAs across the genome may represent the targets or pre-RNA genes of the miRNAs. Via bioinformatics analysis, identical sequences of miR1 and miR2 are revealed on four and seven chromosomes, respectively (Table 1). Seven homologs of miR1 were found in the genome, five of them share 100% identity. Four of them are putatively located within a hairpin RNA structure (Figure 2). Whereas, thirteen homologous loci of miR2 were located over the genome, nine of them harbor identical miR2. Homologous loci of miR1 are located in pseudogenes, intergenic genes and transposable elements (TEs) (Table 1). Loci of miR2 can be divided into two groups: the long TEs and unknown protein family, including AAW46987.1, AAW41058.1, AAW42575.1 and AAW46986.1. Putative conserved domains of TEs loci were detected in the sequences surrounding both miRs [31]. In the loci of protein AAW 46457.1 that harbors miR1, peptide sequence from residues 245 to 406 shares a identity of 81.9% to the cryptococcal Tnp2-like transposase (Transposase_21) [32]. The ORFs containing miR2, AAW 42067.1, AAW41060.1, AAW45547.1, AAW44276.1 and AAW44911.1, putatively encode FHY3 (Far-red Elongated Hypocotyl 3) as well as MULE (Mutator-like Transposable Element) domains [32]. These TEs may be the target of miRNA-mediated silencing, or likely as the biogenesis source of the miRs. A previous study showed that RNAi pathway in C. neoformans serotype D controls the mobility of transposons [31]. In this respect, the biological significance of miRNA-mediated gene silencing may lie on the fact that miR1 and miR2 inhibit transposon activity. Experimental efforts have been made under the way to illustrate the possible targets of the miRs in this fungus. Based on our data, it appears safe to draw a conclusion that miR1 and miR2 at least plays a role in defending the genome integrity by interfering transgene expression in C. neoformans.
On the other hand, TEs likely serve as the genomic source for the biogenesis of miRNAs. This is very likely for miR1 in C. neoformans as four miR1-containing loci harbor a hairpin RNA structure (Figure 2). A similar case was reported in human, the family of miRNA genes, has-mir-548, are derived from Made1 transposable elements that are short miniature inverted-repeat transposable elements [33], [34]. In plants, miRNAs are also encoded by a number of individual TE insertions [35], [36]. More than 30% of miRNA genes in plants are associated with interspersed repeats [36]. It is believed that the dispersed repetitive nature of TE sequences provides multiple novel pre-miRNA genes as well as serves as target sites throughout the genome [33].
Our data indicate that pseudogenes may play a role in miRNA-associated gene silencing, despite that pseudogenes were thought for a long time as non-functional relics of evolution. A recent study revised this view by showing that transcripts produced from pseudogenes regulate the effects of miRNAs on their targets by competing for miRNA binding. Poliseno et al. showed that PTENP1, a pseudogene, can derepress their cognate genes, because they retain many of the miRNA binding sites and can compete for the binding of many miRNAs at once [37]. And the pseudogenes were reported as microRNA decoys lately [38]. It is intriguing to believe that cryptococcal pseudogenes may have an intrinsic biological role in miRNA regulatory networks.
It should be noted that miR1 and miR2 share little similarity to known miRNAs in plants and animals by sequence comparison (E-value>0.1), supporting an independent evolution view of miRNAs in fungi [14]. The discovery of functional miRNAs in C. neoformans would generate significant effect on gene regulation network in this fungi. And accurate investigation on the function of miR1 and miR2 is also critical for the pathogenesis studies of C. neoformans. However, open questions, such as the exact biogenesis of the miRNAs, the elusive targets of miR1 and miR2, and their relationship to pseudogenes, are still challenging.
Materials and Methods
Strains and Growth Conditions
C. neoformans serotype D strain B4500 (also named as JEC21, ATCC MYA-565) was used as the wild type in this study. B4500FOA is a uracil auxotrophic mutant [39]. Δclc1 is CLC-type chloride channel mutant derived from B4500 [28]. Both were used as recipient strains for gene silencing assay. Other recipient strains included NE465 (ago1Δ::NAT-STM, ura5), NE493 (rdp1Δ::NAT-STM, ura5), NE473 (dcr1Δ::NEO, ura5), and NE475 (dcr2Δ::HYG-STM, ura5), in which RNAi components, AGO1 for Argonaute, RDP1 for RNA-dependent RNA polymerase and DCR1 and DCR2 for two Dicer proteins, were separately disrupted, in that order [30]. The ΔclcC strain was a reconstituted strain in which a wild-type copy of CLC1 was transformed into Δclc1 strain [28]. YPD medium (2% glucose, 2% bacto-peptone, 1% yeast extract) was used for routine growth of C. neoformans. For testing uracil auxotrophy, minimal medium (MIN; 1.7 g yeast nitrogen base without amino acids and ammonium sulfate, 5 g ammomium sulfate, 20 g glucose per 1 liter) were supplemented with 1 g/l 5-fluoroorotic acid (5-FOA) and 50 mg/l uracil for make MINFOA. Low-glucose (0.1%) asparagine salt medium (Asn, 0.1% asparagine, 0.3% KH2PO4; pH 5.2) was used for melanin production in the presence of the laccase substrate NE (100 mg/liter), at 28°C, for two days [40]. All transformants were selected on YPD or other indicated media such as Asn or MIN containing 100 µg/ml hygromycin (the plasmids contained hygromycin B resistant marker).
Cloning of small RNAs
Total RNA was isolated from 48-hr culture using traditional guanidinium thiocyanate-phenol protocal. Small RNAs were separated on 15% PAGE urea (7 M) gels with the Small RNA Gel Extraction Kit (TaKaRa Biotechnology, Dalian Co. Ltd, Dalian, China) by following the manufacturer's instruction. A RNA ladder ranging from 20 to 100 was used as the marker. The small RNA band at approximately (22 nt were cut from the PAGE gel and cloned with the Small RNA Cloning Kit (TaKaRa Biotechnology, Dalian Co. Ltd, Dalian, China). Briefly, the purified sRNAs were ligated with the 3′ and 5′ RNA adaptors supplemented with the Kit. cDNA was consequently synthesized and amplified with the supplemented oligo primers. The cDNA were cloned into T-vector pMD20-T supplemented with the Kit to generate sRNA library. The bacterial clones containing sRNAs were randomly picked for sequencing (BGI, Shenzhen, China).
Identification of miRNAs via bioinformatics analysis
All sequences were searched against the C. neoformans genome in NCBI, as well as SGTC, Stanford Genome Technology Center: Cryptococcus neoformans Genome Project (http://sequence-www.stanford.edu/group/C.neoformans/). To determine miRNA candidates, sRNA from tRNA or rRNA were first excluded. Bioinformatics analysis was conducted to predict miRNAs and their location in the genome (carried out by BGI, Shenzhen, China). Structural features of cloned small RNAs was defined by comparison to known plant or mammalian miRNAs. The flanking upstream and downstream regions of miRNA candidates were screened for potential hairpin structure by the software VMir (http://books.elsevier.com/companions/978012379179) (BGI, Shenzhen, China). The final miRNA candidates were further verified by Northern blotting (next part in this section). Targets of the miRNAs were predicted by homology to sequences on chromosomes using bioinformatics tools such as TargetScan and miRanda (finished by BGI, Shenchen, China), and checked by blasting against cryptococcal genome project on GenBank.
Northern blot analyses
To confirm the existence of miRNA, Northern blotting analysis was conducted. Approximately 10 µg of total RNAs were separated on 15% denaturing PAGE with 7 M urea and transferred onto a Nylon Hybridization Membrane N+ (Osmonics, USA) which had been pre-wetted in distilled water. Crosslinking of RNA to the membrane was mediated by carbodimide at 60°C for 70 min [41]. Synthesized DNA probes corresponding to miR1 or miR2 were labeled with γ-[32P] ATP using T4 polynucleotide kinase (TaKaRa Biotechnology, China). Hybridization was performed at 37°C in high-efficiency hybrid-buffer (Beijing Biodev-tech Scientific & Technical Co., Ltd. China).
Reporter constructs and Transformation
Constructs for gene silencing assay were designed as following (Figure 4): a candidate miRNA (miR1 or miR2 in this case) was inserted into the reporter genes, URA5 or CLC1, at their 3′ UTR, to create fusion genes URA5-miR1/2 or CLC-miR1/2. Briefly, for the construction of URA5-miRs fusion gene, two pair of primers, URA5-XhoI-S/URA5-miRs-BamHI and URA5-BamHI-S/URA5-XbaI-A, were used to PCR amplify the ORF and the terminator regions of the wild-type URA5, respectively. The primer URA5-miRs-BamHI harbored miR1 or miR2 (Figure 4). The PCR fragments were cut with Xho I, BamH I and Xba I, accordingly and were ligated onto the plasmid pBluescript-Hyg digested with Xho I and Xba I. The plasmid carries hygromycin B resistance cassette for selection. All the primers used for fragment amplification were collected in Table 4.
Table 4. Primers used in this study.
| No. | Primers | Sequence |
| 1 | CLC–EcoRI-A | GGG TGA ATT CCG AAG GAT TGA GGC TGA GCA AG |
| 2 | CLC–XhoI-S | CCT TCT CGA GTG TTT CTC CAG TTC ACC ACC CTG |
| 3 | CLC-mi1 -HindIII | CCC GAA GCT TTC CTG AAC TTG ATC ACC ATT GAT CAT CCT TGT ATT CCT TCG TCC |
| 4 | CLC-mi1-mut-HindIII | CCC GAA GCT TTC CTG AAT CCA GCT GTT ATT GAT CAT CCT TGT ATT CCT TCG TCC |
| 5 | CLC-mi2 -HindIII | CCG CAA GCT TTT ATC GAC ACC TTC ATC GTA ATC ATC CTT GTA TTC CTT CGT CC |
| 6 | CLC-mi2-mut-HindIII | CCG CAA GCT TTT ATC GGT GTT CCT GCT GTA ATC ATC CTT GTA TTC CTT CGT CC |
| 7 | CLC-HindIII-S | CCC CGA AGC TTA AAT TTT GTT AGA CTG ACC AG |
| 8 | URA5-XhoI-S | GGG ACT CGA GGA TCT TGG GGA TGG TAT TGA AGA CG |
| 9 | URA5-XbaI-A | CTC GTC TAG AGA TCC CAG TAC TAC CCG CTC TT |
| 10 | URA5-mi1-BamHI | GCC CGG ATC CTC CTG AAC TTG ATC ACC ATT GAT TAA GAC CTC TGA ACA CCG TAC |
| 11 | URA5-mi1-mut-BamHI | GCC CGG ATC CTC CTG AAT CCA GCT GTT ATT GAT TAA GAC CTC TGA ACA CCG TAC |
| 12 | URA5-mi2-BamHI | GCC CGG ATC CTT ATC GAC ACC TTC ATC GTA ATT AAG ACC TCT GAA CAC CGT AC |
| 13 | URA5-mi2-mut-BamHI | GCC CGG ATC CTT ATC GGT GTT CCT GCT GTA ATT AAG ACC TCT GAA CAC CGT AC |
| 14 | URA5-BamHI-S | TGG GGA TCC GGG TTT TCT TCT TAA ATG CAC GGG |
| 15 | qURA5 | TAC AGG AGG TTG AGG AAG A(up) |
| TTA AGA CCT CTG AAC ACC G(down) | ||
| 16 | qCLC1 | AAT GAC TGT GAA TAC GGG CG(up) |
| CTT GGT CGG ACA CGA GAA TG(down) | ||
| 17 | qACT | ATG GTA TTG CCG ACC GTA TGC(up) |
| TTT CGG TGG ACG ATT GAG GG(down) |
As control, miR1 and 2 were mutated to created miR1-m or miR2-m. The sequence of miR1-m was 5′-TCCTGAATCCAGCTGTTTAACT. Nucleotides from 8 to 17 in miR1 were changed. The sequence of miR2-m was 5′-TCGGTGTTCCTGCTGATT. Nucleotides from 4 to 14 were changed. By the same strategy with PCR, miR1-m and miR2-m were fused to URA5 or CLC1 in the place of miR1 or miR2 (Figure 4). The construction of the reporters CLC-miR1 or 2 and CLC-miR1-m or miR2-m was carried out in the same way.
Recipient cells, e.g. B4500FOA or Δclc1 cells were transformed by electroporation as described [42] using 5 µg of linearized DNA (URA5-miR1/2 or CLC-miR1/2) per 3×108 cells. Transformants were first selected on YPD containing 100 µg/ml hygromycin B and purified by streaking. Two or three transformants were picked for phenotype analysis on MIN, MINFOA, or Asn agar supplemented with 100 µg/ml hygromycin in the assays. For verification of the transformed reporters in the transformants, PCR amplification of the reporter genes including the miRs was conducted. The primers, HYG-200:5′- AAGTGCCGATAAACATAACGA and & URA5-XbaI: 5′-CTCGTCTAGAGATCC CAGTACTACCCGCTCTT, were designed to amplify URA5-miRs or URA5-miR-ms. The resulted 2.7-kb fragment was subject to sequencing. The pair of primers, T7 & CLC-EcoRI:5′-GTAATACGACTCACTATAGGGC/5′-GGGTGAATTCCGAAGGATTGA GGCTGAGCAAG, were used to amplify the CLC1 reporter. A 4.2-kb fragment was generated and sequenced.
qRT-PCR assay
Quantitative real-time PCR (qRT-PCR) was carried out with Eppendorf Mastercycler Realplex Detection System. Briefly, total RNA was purified from two-day fungal culture as previously described [43], [44] and treated with DNase I (60–80 U/µl, TaKaRa). Approximately 1 µg DNase-treated RNA was reverse transcribed with M-MLV (RNase H−) (TaKaRa, Dalian, China) using oligo(dT)15 primer. cDNAs (∼50 ng) was added to 45 µl qRT-PCR reaction mixture containing 25 µl FastStart Universal SYBR Green Master (Roche China, Shanghai, China) and 300 nM of each primers. Each reaction was conducted in triplicate and non-reverse-transcribed RNA samples were used as control. Gene-specific primers (Table 4) were designed using Primer Premier 5 (Premier Biosoft, California, USA), and each pair was validated by cDNA template titration to ensure similar amplification kinetics and a single melting point of quantitative PCR products. To calculate the expression levels of the target gene, relative quantification 2−ΔΔCT method was employed [32]. The housekeeping gene for beta-actin, encoded by ACT1, was used as an internal control in this study.
Acknowledgments
We acknowledge the use of the C. neoformans Genome Project, Stanford Genome Technology Center (http://www-sequence.standford.edu), funded by the NIAID/NIH under cooperative agreement AI47087. We also acknowledge the Duke Center for Genome Technology (http://cneo.genetics.duke.edu) for providing gene sequences related to this work.
Funding Statement
This work is supported, in part, by Natural Science Foundation of China (NSFC Grant #30770043), and a National Basic Research Program (“973” Program #2007CB707801). The authors also acknowledge use of the C. neoformans Genome Project, Stanford Genome Technology Center (http://www-sequence.standford.edu), funded by the National Institute of Allergy and Infectious Diseases/National Institutes of Health under cooperative agreement AI47087. No additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ambros V (2003) MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell 113: 673–676. [DOI] [PubMed] [Google Scholar]
- 2. Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carrington JC, Ambros V (2003) Role of microRNAs in plant and animal development. Science 301: 336–338. [DOI] [PubMed] [Google Scholar]
- 4. Ghildiyal M, Zamore PD (2009) Small silencing RNAs: an expanding universe. Nat Rev Genet 10: 94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang L, Wang MB, Tu JX, Helliwell CA, Waterhouse PM, et al. (2007) Cloning and characterization of microRNAs from Brassica napus . FEBS Letters 581: 3848–3856. [DOI] [PubMed] [Google Scholar]
- 6. Carthew RW, Sontheimer EJ (2009) Origins and mechanisms of miRNAs and siRNAs. Cell 136: 642–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halic M, Moazed D (2010) Dicer-independent primal RNAs trigger RNAi and heterochromatin formation. Cell 140: 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297. [DOI] [PubMed] [Google Scholar]
- 9. Benfey PN (2003) MicroRNA is here to stay. Science 425: 244–245. [DOI] [PubMed] [Google Scholar]
- 10. Grishok A, Pasquinelli AE, Conte D, Li N, Parrish S, et al. (2001) Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 106: 23–34. [DOI] [PubMed] [Google Scholar]
- 11. Hyun S, Lee JH, Jin H, Nam JW, Namkoong B, et al. (2009) Conserved microRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell 139: 1096–1108. [DOI] [PubMed] [Google Scholar]
- 12. Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I (2008) MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature 455: 1124–1128. [DOI] [PubMed] [Google Scholar]
- 13. Lee HC, Chang SS, Choudhary S, Aalto AP, Maiti M, et al. (2009) qiRNA is a new type of small interfering RNA induced by DNA damage. Nature 459: 274–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee HC, Li L, Gu W, Xue Z, Crosthwaite SK, et al. (2010) Diverse pathways generate microRNA-like RNAs and Dicer-independent small interfering RNAs in fungi. Mol Cell 38: 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buhler M, Moazed D (2007) Transcription and RNAi in heterochromatic gene silencing. Nat Struct Mol Biol 14: 1041–1048. [DOI] [PubMed] [Google Scholar]
- 16. Catalanotto C, Nolan T, Cogoni C (2006) Homology effects in Neurospora crassa . FEMS Microbiol Lett 254: 182–189. [DOI] [PubMed] [Google Scholar]
- 17. Drinnenberg IA, Weinberg DE, Xie KT, Mower JP, Wolfe KH, et al. (2009) RNAi in budding yeast. Science 326: 544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu H, Cottrell TR, Pierini LM, Goldman WE, Doering TL (2002) RNA interference in the pathogenic fungus Cryptococcus neoformans . Genetics 160: 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang X, Hsueh YP, Li W, Floyd A, Skalsky R, et al. (2010) Sex-induced silencing defends the genome of Cryptococcus neoformans via RNAi. Genes Dev 24: 2566–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Idnurm A, Bahn YS, Nielsen K, Lin X, Fraser JA, et al. (2005) Deciphering the model pathogenic fungus Cryptococcus neoformans . Nat Rev Microbiol 3: 753–764. [DOI] [PubMed] [Google Scholar]
- 21. Dang Y, Yang Q, Xue Z, Liu Y (2011) RNA interference in fungi: pathways, functions, and applications. Eukaryot Cell 10: 1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loftus BJ, Fung E, Roncaglia P, Rowley D, Amedeo P, et al. (2005) The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans . Science 307: 1321–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ (2004) Processing of primary microRNAs by the Microprocessor complex. Nature 432: 231–235. [DOI] [PubMed] [Google Scholar]
- 24. Jones-Rhoades MW, Bartel DP, Bartel B (2006) MicroRNAs and their regulatory roles in plants. Annu Rev Plant Biol 57: 19–53. [DOI] [PubMed] [Google Scholar]
- 25. Kim VN, Han J, Siomi MC (2009) Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10: 126–139. [DOI] [PubMed] [Google Scholar]
- 26. Edman JC, Kwon-Chung KJ (1990) Isolation of the URA5 gene from Cryptococcus neoformans var. neoformans and its use as a selective marker for transformation. Mol Cell Biol 10: 4538–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williamson PR, Wakamatsu K, ITO S (1998) Melanin Biosynthesis in Cryptococcus neoformans . J Bacteriol 180: 1570–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu X, Williamson PR (2003) A CLC-type chloride channel gene is required for laccase activity and virulence in Cryptococcus neoformans . Mol Microbiol 50: 1271–1281. [DOI] [PubMed] [Google Scholar]
- 29. Zhu X, Williamson PR (2004) Role of laccase in the biology and virulence of Cryptococcus neoformans . FEMS Yeast Res 5: 1–10. [DOI] [PubMed] [Google Scholar]
- 30. Janbon G, Maeng S, Yang DH, Ko YJ, Jung KW, et al. (2010) Characterizing the role of RNA silencing components in Cryptococcus neoformans . Fungal Genet Biol 47: 1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marchler-Bauer A, Lu S, Anderson SL, Chitsaz F, Derbyshire MK, et al. (2011) “CDD: a Conserved Domain Database for the functional annotation of proteins.”. Nucleic Acids Res 39: 225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 22DDCT method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 33. Piriyapongsa J, Jordan IK (2007) A family of human microRNA genes from miniature inverted-repeat transposable elements. PLoS ONE 2: e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Piriyapongsa J, Marino-Ramırez L, Jordan IK (2007) Origin and evolution of human microRNAs from transposable elements. Genetics 176: 1323–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Piriyapongsa J, Jordan IK (2008) Dual coding of siRNAs and miRNAs by plant transposable elements. RNA 14: 814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yu S, Li J, Luo L (2010) Complexity and Specificity of Precursor microRNAs Drivenby Transposable Elements in Rice. Plant Mol Biol Rep 28: 502–511. [Google Scholar]
- 37. Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, et al. (2010) A coding-independent function of gene and pseudogene mRNAs regulates tumor biology. Nature 465: 1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Swami M (2010) Pseudogenes act as microRNA decoys. Nat Rev Genet 11: 530–531. [DOI] [PubMed] [Google Scholar]
- 39. Drivinya A, Shimizu K, Takeo K (2004) Construction of a complete URA5 deletion strain of a human pathogenic yeast Cryptococcus neoformans . Nihon Ishinkin Gakkai Zasshi 45: 1–6. [DOI] [PubMed] [Google Scholar]
- 40. Williamson PR (1994) Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J Bacteriol 176: 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pall GS, Codony-Servat C, Byrne J, Ritchie L, Hamilton A (2007) Carbodiimide-mediated cross-linking of RNA to nylon membranes improves the detection of siRNA, miRNA and piRNA by northern blot. Nucleic Acids Res 35: e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wigkes BL, Edman JC (1994) Development of a transformation system for Cryptococcus neoformans. In Molecular biology of Pathogenic Fungi (Maresca, B. & Kobayashi, G.S., eds). New York: Telos Press. pp. 309–313.
- 43. Jiang N, Xiao D, Zhang D, Sun N, Yan B, et al. (2009) Negative roles of a novel nitrogen metabolite repression-related gene, TAR1, in laccase production and nitrate utilization by the basidiomycete Cryptococcus neoformans . Appl Environ Microbiol 75: 6777–6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jiang N, Sun N, Xiao D, Pan J, Wang Y, et al. (2009) A copper-responsive factor gene CUF1 is required for copper induction of laccase in Cryptococcus neoformans . FEMS Microbiol Lett 296: 84–90. [DOI] [PubMed] [Google Scholar]