Abstract
Zinc supplementation decreases the morbidity of lower respiratory tract infection in pediatric patients in the developing world. We sought to determine if zinc mediates a specific inhibitory effect against the major cause of pediatric lower respiratory tract disease, respiratory syncytial virus (RSV). We determined the in vitro inhibitory effect of three zinc salts (zinc acetate, lactate, and sulfate) on the replication of RSV at various concentrations of 10 and 1 mM and 100 and 10 μM. The degree of inhibition of RSV replication was examined in the presence of zinc during preincubation, adsorption, or penetration and was compared with that caused by salts of other divalent cations. Complete inhibition of RSV plaque formation was observed at 1 and 10 mM, representing reductions that were ≥106-fold. At the lowest concentration tested, 10 μM, we observed ≥1,000-fold reductions in RSV yield when zinc was present during preincubation, adsorption, penetration, or egress of virus. The therapeutic indices, determined as ratios of 50% toxicity concentration to 50% inhibitory concentration, were 100, 150, and 120 for zinc acetate, zinc lactate, and zinc sulfate, respectively. The inhibitory effect of zinc salts on RSV was concentration dependent and was not observed with other salts containing divalent cations such as calcium, magnesium, and manganese. RSV plaque formation was prevented by pretreatment of HEp-2 cell monolayer cultures with zinc or by addition of zinc to methylcellulose overlay media after infection. The results of this study suggest that zinc mediates antiviral activity on RSV by altering the ability of the cell to support RSV replication.
Respiratory syncytial virus (RSV) is the most important viral cause of acute respiratory tract infection (ARI) in infancy and early childhood, with its greatest morbidity in infants (6, 8, 9). Throughout the world, RSV contributes significantly to annual hospital admissions due to ARI, especially during the epidemic season (18, 23, 27). In developing countries, malnutrition and deficiencies of micronutrients are associated with increased incidence and severity of ARI (25). Improvement in nutritional status might be a practical strategy for prevention of severe RSV disease in developing countries, if efficacious. At this time, however, we have a poor understanding of the role of micronutrients such as zinc in susceptibility to severe disease caused by RSV infection.
Zinc supplementation in children in developing countries was demonstrated to cause a significant reduction in the prevalence of pneumonia (3, 20). Zinc has been shown to mediate antiviral effects against certain viruses. Clinical studies showed that zinc significantly shortens the duration of symptoms during rhinovirus infection (17, 19, 28). Topical application of zinc sulfate also was found in one study to be effective in the treatment of herpes simplex virus (HSV) infection (26). The specific mechanism by which zinc mediated these clinical effects is unknown in most cases. Zinc also enhances the host response to many infections and plays an important role in the homeostasis of the immune system (12, 21, 24). Zinc deficiency, often seen in the context of malnutrition, is associated with an impaired cellular immune response (2, 4).
Several studies have examined the direct inhibitory effect of zinc on viruses that infect the human respiratory tract, including the enveloped virus HSV and the nonenveloped virus rhinovirus (7, 13, 14). The mechanism of inhibition of rhinovirus likely involves direct binding of zinc to virus particles (13). Zinc also inhibits the replication of human immunodeficiency virus type 1 (HIV-1) (10), vaccinia virus (11), and polioviruses (15) in vitro. Although zinc supplementation reduces the incidence of severe clinical pneumonia in the developing world, the effect of zinc on replication of the primary agents of viral pneumonia in infants such as RSV is unknown. We determined the in vitro inhibitory effect of three zinc salts (acetate, lactate, and sulfate) on RSV in virus yield or virus plaque reduction assays by treatment of wild-type RSV strain A2 at various concentrations of 10 and 1 mM and 100 and 10 μM. Because of the dramatic effect of reduction of RSV when zinc was present throughout the virus replication cycle, we also sought to determine the stage of virus replication that was inhibited. The degree of virus inhibition was examined in the presence of zinc during preincubation with the cell culture monolayer or during viral adsorption, penetration, or budding and was compared with that mediated by salts of other cations at similar concentrations. The experiments suggest that zinc inhibits RSV by altering the ability of cells to support RSV replication rather than by a direct effect on the virus.
MATERIALS AND METHODS
Cells and virus
HEp-2 cell monolayer cultures were maintained in Opti-MEM I medium (Life Technologies, Gaithersburg, Md.) supplemented with glutamine, amphotericin B, gentamicin, and 5% fetal calf serum. Opti-MEM I medium, which contains less than 3.4 nM zinc in the form of zinc sulfate, was used as mock treatment for the experiments. Working stocks of the virus were prepared by propagation in HEp-2 cell monolayer cultures at 37°C after infection at a multiplicity of infection (MOI) of 0.1 PFU/cell. When a significant cytopathic effect was detected, the supernatant was collected, clarified, aliquoted, and stored in cryovials at −70°C until used. The endpoint plaque titer of the virus stock used was 5.3 log10 PFU/ml.
Zinc solutions
USP-grade zinc acetate (AMEND Chemical, Irvington, N.J.), or zinc lactate or sulfate (Sigma Chemical, St. Louis, Mo.), was used in all experiments. Calcium acetate, magnesium sulfate, and manganese sulfate (Sigma Chemical) were used as divalent cation control salts. Stock solutions of 250 mM salts were prepared in deionized water and were sterilized by passage through a 0.22-μm-pore-size syringe tip filter.
Zinc cytotoxicity
The cytotoxic effect of zinc salts on HEp-2 cell monolayer cultures was assessed by addition of salts in cell culture media to 90% confluent cell culture monolayers in a 24-well plate and by incubation at 37°C for 96 h. At the end of the incubation period, cells were trypsinized and were gently resuspended in medium and cell viability was assessed with trypan blue exclusion.
Treatment with zinc salts
An equal volume of virus was combined with various concentrations of zinc salt or control salts in 50 mM morpholinepropanesulfonic acid (MOPS)-buffered Opti-MEM I medium, pH 7.2, in a total volume of 250 μl, and the mixture was incubated at 37°C for 2 h. The MOPS buffer was used to prevent alteration or significant variation of pH due to various zinc concentrations, which otherwise would be a confounding variable. Control wells without zinc were treated similarly with MOPS-buffered Opti-MEM I medium. At the end of the incubation period, the zinc-virus mixture was diluted in medium with or without zinc according to the experimental design, and the plaque assay was performed as described below.
Plaque reduction assay
Serial 10-fold dilutions of zinc-virus mixtures were performed. One hundred microliters of suspension was transferred to 90% confluent HEp-2 cell monolayer cultures in 24-well plates (Costar, Cambridge, Mass.). Virus was allowed to adsorb for 2 h at 37°C, overlaid with a semisolid medium containing 0.75% methylcellulose (Sigma Chemical), and incubated at 37°C. At 96 h postincubation, the cells were fixed in 80% methanol at 4°C for 1 h. RSV plaques were stained by an immunoperoxidase staining method with murine RSV F glycoprotein-specific monoclonal antibodies (clones 1269, 1243, and 1129), goat anti-mouse immunoglobulin G-horseradish peroxidase conjugate, and an appropriate precipitating substrate (4CN) (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.). In plaque assays, the virus was diluted to give approximately 90 PFU per well in the absence of inhibitory salts. In addition, some assays were performed during which HEp-2 cell monolayer cultures were pretreated with zinc or control salt for 2 h at 37°C prior to infection with RSV. Experiments were also performed in which zinc salts were present in the experiment only in the semisolid overlay media that was added to the culture after virus absorption and penetration.
Effect of zinc on viral yield
HEp-2 cell monolayer cultures were infected with RSV at an MOI of 0.1 PFU/cell and were allowed to adsorb for 2 h at 37°C. Nonadsorbed virus was removed by washing with phosphate-buffered saline (PBS) three times, and the medium was replaced with medium containing the desired concentration of zinc or control salt. After 96 h, virus was harvested by collecting supernatants from the cultures and was frozen in cryovials and stored at −70°C pending determination of viral titer.
Effect of zinc on viral penetration
To determine the effect of zinc on viral penetration, HEp-2 cell monolayer cultures were prewashed with ice-cold PBS, infected with RSV at an MOI of 0.1 PFU/cell, and then incubated at 4°C for 2 h. Nonabsorbed virus was removed by washing three times with cold PBS. Medium containing a desired concentration of zinc or control salt was added to the monolayer cultures and was further incubated at 37°C for 4 h. Excess zinc or control salt was removed by washing with PBS and was replaced with complete Opti-MEM I medium without added salts. After a 96-h period of incubation, supernatants containing virus were harvested from the cultures and were frozen in cryovials at −70°C pending determination of viral titer.
Statistics
Data analysis was performed with SPSS for Windows version 10.0 statistical software (Chicago, Ill.). Percent cell viability was determined by comparing the number and proportion of cells viable in zinc-treated cell culture monolayers to those in mock-treated monolayers. Percent of RSV plaque counts in the presence of zinc or control salts were determined by normalizing the plaque counts for the mock-treated wells to 100%. Mean values were compared by the analysis of variance and Student t test where appropriate. A statistically significant difference was defined as a P of <0.05.
RESULTS
Zinc toxicity
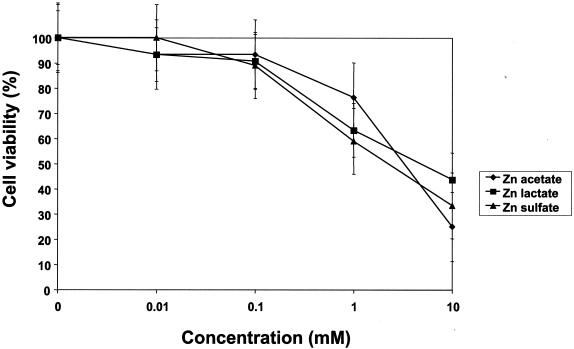
Figure 1 shows the cytotoxicity of zinc salts on HEp-2 cell monolayer cultures. The toxicity concentration (TC50) for HEp-2 cells was 3, 5, or 7.5 mM for zinc sulfate, acetate, or lactate, respectively. Zinc concentrations between 10 and 100 μM did not mediate a cytotoxic effect for HEp-2 cell monolayer cultures that could be detected by visual inspection with light microscopy. Cytotoxicity as determined by cell viability with the trypan blue dye exclusion test was observed at 1 and 10 mM, although more than 50% of the cell monolayer was intact at those concentrations.
FIG. 1.
Cytotoxicity of zinc salts for HEp-2 cell monolayer cultures. Cytotoxicity was determined by the addition of zinc salts to 90% confluent cell monolayers in a 24-well plate and by incubation at 37°C for 96 h. At the end of the incubation period, cells were trypsinized and resuspended in medium and cell viability was assessed with trypan blue exclusion. Cell viability was determined by comparing zinc to mock-treated cell monolayers. Values represent mean and SEM results from six independent experiments.
Zinc inhibits RSV in plaque reduction assay. (i) Effect of zinc on RSV when present throughout adsorption, penetration, and egress
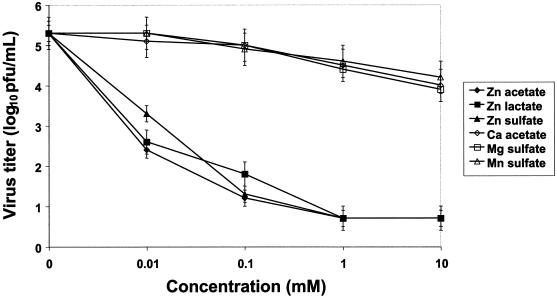
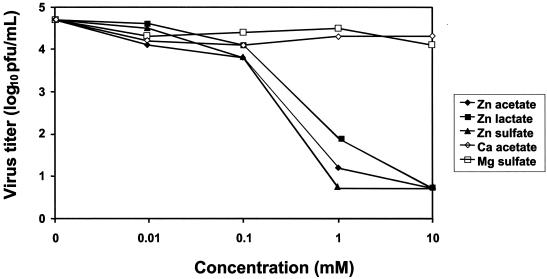
(a) Virus yield. When zinc was present during adsorption, penetration, and egress, there was significant reduction in virus yield, even at the lowest concentration of 10 μM, when comparisons were made to mock-treated or control salt-treated wells (Fig. 2). The mean virus titer in the mock-treated wells was 5.3 log10 PFU/ml (standard error of the mean [SEM] = 0.7 log10 PFU/ml) compared to 2.8 log10 PFU/ml (SEM = 0.7 log10 PFU/ml) for any of the zinc salts (P < 0.01). A reduction in viral yield of approximately 10,000-fold or more was observed in the presence of any of the zinc salts at a concentration of 100 μM. In contrast, calcium acetate, magnesium sulfate, and manganese sulfate did not show a similar inhibition of RSV even at the highest concentration of 10 mM (Fig. 2). When zinc was not preincubated with the virus, complete inhibition by zinc salt was observed only with 1 and 10 mM zinc salts, and these reductions were significant compared to those found with the control salts (P < 0.01). Zinc salts at concentrations of 10 and 100 μM did not exert a significant effect on RSV when there was no preincubation.
FIG. 2.
Effect of zinc salts on RSV yield when present during adsorption, penetration, and egress. Virus was combined with various concentrations of zinc salt or control salts in 50 mM MOPS-buffered Opti-MEM I medium, pH 7.2, in a total volume of 250 μl, and the mixture was incubated at 37°C for 2 h. Control wells without zinc (0 mM) were treated similarly. At the end of the incubation period, the zinc-virus mixtures were inoculated onto HEp-2 cell monolayer cultures. After 96 h supernatants were collected and the viral titer was determined by standard 4-day plaque assay on HEp-2 cell monolayer cultures. Virus titer values indicate the mean values and SEM of three independent experiments.
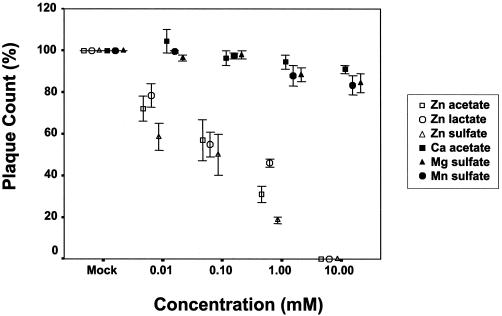
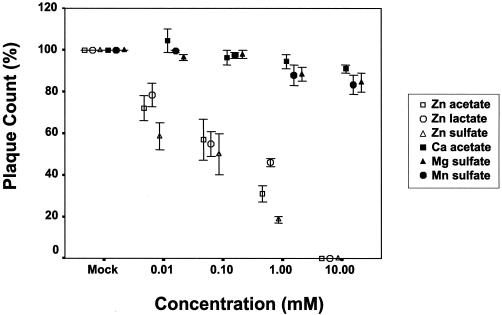
(b) Plaque formation. To further determine the effect of treating RSV with zinc salts, we examined the effect of zinc on RSV plaque formation under a semisolid overlay, which requires cell-to-cell spread in culture. Figure 3 shows the plaque counts after treatment of RSV with zinc or control salts in a plaque reduction assay with approximately 90 PFU of RSV per well. The inhibitory effect of zinc salts on RSV was concentration dependent and was significantly greater than that during treatment with calcium, magnesium, or manganese salts (P < 0.001). Complete inhibition of RSV plaque formation was observed in the presence of a 10 mM concentration of any zinc salt, while 88% inhibition (SEM, 4%; P < 0.001) was seen at 1 mM. Calcium acetate and manganese sulfate salts at concentrations of 10 mM exhibited a minimal inhibitory effect in the plaque reduction assay. At this concentration, calcium acetate caused a mean percent inhibition of 15% (SEM, 9%; P = 0.03), while manganese sulfate caused an inhibition of 21% (SEM = 9%; P = 0.04). The mean percent inhibition of RSV by a 10 or 100 μM concentration of any zinc salt was 40% (SEM, 2.7%) or 64% (SEM, 2.6%) respectively (Fig. 3) (P < 0.001). The therapeutic index, determined as the ratio of TC50/50% inhibitory concentration, was 100, 150, or 120 for zinc acetate, zinc lactate, or zinc sulfate, respectively.
FIG. 3.
Effect of zinc salts on RSV plaque formation when present during adsorption, penetration, and egress. Plaque counts in the presence of indicated salts in a plaque reduction assay with approximately 90 PFU of wild-type RSV strain A2. RSV was incubated with various concentrations of zinc or control salts for 2 h before infection of HEp-2 cell monolayer cultures. The indicated salts were present during adsorption and were added to the overlay placed after adsorption. Percent plaque count was determined by adjusting the plaque count for the mock-treated cultures to 100. Values indicate the mean values and SEM of three independent experiments. There was a significant difference in inhibition of RSV in zinc treatment experiments compared to those including treatment with calcium, magnesium, or manganese salts (P < 0.001). There was not a significant difference in percent inhibition between the zinc salts.
(ii) Effect of zinc on viral penetration
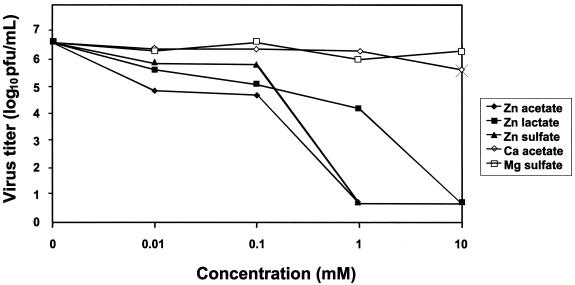
The inhibitory effect of zinc salts on RSV penetration of cells was evaluated (Fig. 4) by separating the process of adsorption from penetration by using temperature shifts, ensuring that zinc salts were added only during viral penetration. Figure 4 shows the effect of zinc and control salts on RSV penetration. A significant inhibitory effect by zinc salts on viral penetration was observed at 100 μM, 1 mM, or 10 mM when compared to the effect caused by control salts. A statistically significant effect was not observed with a 10 μM concentration of any of the zinc salts. The mean virus titer in the mock-treated cultures was 6.6 log 10 PFU/ml (SEM, 0.2 log10 PFU/ml).
FIG. 4.
Effect of zinc salts on RSV yield when present only during virus penetration. HEp-2 cell monolayer cultures were prewashed with ice-cold PBS, infected with RSV at an MOI of 0.1 PFU/ml, and incubated at 4°C for 2 h. Nonabsorbed virus was removed by repeated washing three times with cold PBS; medium containing zinc or control salt was added, and further incubation at 37°C for 4 h took place. Excess salt was removed and replaced with Opti-MEM medium. After 96 h of incubation at 37°C, virus was harvested and titers were determined. Virus titers represent means for three independent experiments.
(iii) Effect of zinc on viral yield after absorption
In order to determine the effect of zinc salts on viral replication (Fig. 5), virus yields were determined in the presence of zinc salts added only after viral adsorption. A significant reduction in viral yield was observed at 1 mM in the presence of zinc salts, with a mean titer of 1.6 log10 PFU/ml (SEM, 0.2 log10 PFU/ml) for any zinc salt compared to 4.7 log 10 PFU/ml (SEM, 0.3 log10 PFU/ml) in mock-treated or control salt-treated cultures (P < 0.01). Although some cytotoxicity was observed with 1 mM zinc salts, cells were still able to maintain viral replication. There was a 10-fold reduction in viral yield in the presence of 100 μM zinc salts, a difference that was not significant when compared to yield associated with mock-treated or control salt-treated wells (P = 0.97). Zinc salts at 10 μM concentration did not exert a significant inhibitory effect on viral yield. The mean viral yield in the supernatant was 4.7 log 10 PFU/ml (SEM, 0.2 log10 PFU/ml) with any zinc salt at a concentration of 10 μM, which was similar to the yield in mock-treated or control salt-treated cultures.
FIG. 5.
Effect of zinc salts on RSV yield when added only after absorption and/or penetration. HEp-2 cell monolayers were infected with RSV at an MOI of 0.1 PFU/cell and were then allowed to adsorb for 1 h at 37°C. Nonadsorbed virus was removed by washing with PBS three times, and the medium was replaced with medium containing the desired concentration of zinc or control salt. After 96 h, virus was harvested from the cultures and frozen at −70°C pending determination of viral titer. Viral titers represent mean values from three independent experiments. The mean viral yield for the mock-treated culture was 4.7 log 10 PFU/ml (SEM, 0.4 log 10 PFU/ml). The effect of zinc salt on viral yield was concentration dependent and was significantly different from that found with mock-treated and the control salts. A statistically significant difference in viral yield was not observed between the zinc salts.
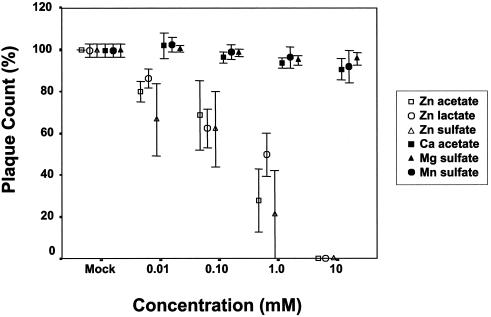
(iv) Effect of zinc on plaque formation when applied only before or only after absorption
Addition of zinc salt to the semisolid overlay media after infection significantly prevented RSV plaque formation by preventing cell-to-cell spread (Fig. 6). When HEp-2 cell monolayer cultures were pretreated with zinc prior to infection of the monolayer with 90 PFU of virus/well, a concentration-dependent inhibitory effect was observed with zinc salt compared to control salts. The difference was significant even at the lowest zinc concentration tested, 10 μM (P = 0.03) (Fig. 7).
FIG. 6.
Effect of zinc salts on RSV plaque formation when added only after adsorption and/or penetration. RSV plaque counts in the presence of the indicated salts in a plaque reduction assay with approximately 90 PFU of wild-type RSV strain A2. HEp-2 cell monolayers were infected with virus, incubated at 37°C for 1 h, and then overlaid with methylcellulose containing various concentrations of zinc or control salts. Percent plaque counts were determined by adjusting the plaque count percentage for the mock-treated cultures to 100. Values are means and SEM of two independent experiments.
FIG. 7.
Inhibitory effect of zinc salts on RSV plaque formation when the cell culture monolayer was pretreated with zinc salts. RSV plaque counts in the cell cultures pretreated with indicated salts in a plaque reduction assay with approximately 90 PFU of wild-type RSV strain A2. Percent plaque count was determined by adjusting the plaque count percentage for the mock-treated cultures to 100. Values are means and SEM of three independent experiments.
DISCUSSION
The results of our study demonstrate that zinc salts inhibit RSV in vitro. The inhibition is concentration dependent and specific. The salts of other divalent cations, such as calcium, magnesium, and manganese, do not mediate a similar inhibition. At the lowest concentration of 10 μM, we were able to observe a 800-fold reduction in virus when zinc was present during preincubation, adsorption, penetration, and egress. Similar to the behavior that we observed, zinc ions inhibited in vitro replication of viruses such as rhinovirus (13), HSV (1), HIV (10), and vaccinia virus (11). Both laboratory-adapted and clinical isolates strains of HSV were inhibited by 100 μM zinc sulfate (1, 14). The degree of inhibition of these viruses was influenced by the duration of treatment with zinc salt. However, complete inhibition was observed at a concentration 50 to 5,000 times higher than observed in our study. Zinc compounds at 100 μg/ml inhibited HIV infection in vitro (10), though an effect on HIV syncytium formation was not observed at this concentration. This finding is in contrast to our observation, i.e., that zinc not only inhibited RSV replication but also prevented plaque formation, even when added in the semisolid overlay media only after virus infection of the cell monolayer. We observed a difference in the magnitude of the inhibition of plaque formation and that of virus yield, with virus yield more significantly inhibited. Previous studies have demonstrated that the mechanisms of viral budding and viral attachment and fusion with cells differ from the mechanisms involved in cell-cell fusion. Also, the yield experiments were performed under conditions in which multicycle growth curves were studied, which may have accentuated the magnitude of virus inhibition. The inhibitory effect that we observed was not due simply to cytotoxic effect on the culture monolayer, since more than 50% of the area of the cell culture monolayer was intact during the plaque assay.
Zinc salts also prevented RSV plaque formation when present on the cell monolayer only prior to viral infection. The significant effect of zinc salts on viral yield and penetration observed in this study suggests that the inhibitory effect of zinc salt is mainly due to an alteration of the ability of cells to support viral replication. Previous electron microscopy studies with HSV demonstrated massive deposition of zinc onto HSV virions, which interfered with viral glycoprotein-mediated membrane fusion (15). In contrast, the inhibitory effect of zinc salts on RSV that we observed in this study is likely due to deposition of zinc on or in the cell monolayer, which prevented viral adsorption and subsequent plaque formation. This idea is supported by the observation of reduction in plaque formation when only the HEp-2 monolayer cells were pretreated with zinc prior to infection with RSV. It has been previously demonstrated that there is minimal zinc uptake into cultured cells treated in vitro (16). We also observed that addition of zinc to semisolid overlay media containing methylcellulose inhibited efficient RSV infection by preventing cell-to-cell spread in culture. This observation further suggests a principal effect on cells, rather than on virions in solution. These data do not completely rule out a contribution of direct binding of zinc to virion particles, since in some of the assays both cells and virion particles were exposed to zinc. Nevertheless, the data as a whole show that the dominant effect is that on the cell substrate.
The molecular mechanism of antiviral effect of zinc is not clear in these studies. It has been proposed that zinc may act in several ways to inhibit viruses (15). Zinc may block the binding of rhinovirus virions to the cell surface by fitting into the canyons on the surface of the virus, thereby preventing attachment to the cell. Alternatively, zinc may block the protease activity in rhinovirus, thereby preventing the breakdown of the virus polypeptide necessary to generate individual functional proteins. Zinc compounds owe their anti-HIV-1 effects to inhibition of HIV-1 DNA-to-RNA transcription, rather than inhibition of the adsorption or penetration, contrary to our observation on RSV in this study (10).
Our study indicates that HEp-2 cell monolayer cultures, a cell line derived from a human laryngeal carcinoma, appear to be more tolerant of high concentrations of zinc than are many other cell lines that have been examined in vitro. The TC50 for zinc salts was between 3 and 7.5 mM in our study. This finding is in contrast to the previous observation that zinc concentrations more than 200 μM were toxic to the monkey cell line CV-1, human neonatal kidney cells, or the human diploid lung fibroblast cell line MRC-5 (16). Zinc salts at a concentration of 1 mM or 300 μM caused extensive cellular toxicity with destruction of uninfected MRC-5 fibroblast monolayer cell cultures after 1 day of exposure (16). The TC50 of zinc compounds on human T-cell lines was between 8 and 550 μM; severe toxicity was observed at concentration of 1 mM (16). The tolerance of the HEp-2 cell line for zinc enabled us to study the inhibitory effect of zinc salts on RSV at a broader range of concentrations than did previous studies of zinc inhibition of rhinovirus, HSV, or HIV.
Zinc mediates a therapeutic effect in vivo in certain viral infections. While some clinical studies showed that zinc significantly shortened the duration of symptoms during rhinovirus infection (5, 17), other studies showed that zinc had no therapeutic effect (22, 28). Topical application of zinc sulfate has also been found effective in the treatment of HSV infection in one study (17). In vitro studies have also demonstrated inactivation of both HSV types 1 and 2 by zinc salts (1, 14). The therapeutic relevance of zinc in RSV infection is not clear. Our studies were not designed to examine therapy per se. The physiological level of zinc in plasma is 10 to 20 μM (24), and only about 10 to 20% of the ingested dose of zinc is absorbed. Although we observed reduction in infectivity with 10 μM zinc, complete inhibition was only seen at a concentration about 100-fold higher than the normal plasma zinc level. To achieve systemic concentrations at such levels with oral zinc therapy likely would be difficult without resulting local zinc toxicity such as mucosal ulceration. However, during supplementation and correction of deficiency in zinc-deficient states, the proportion of supplemental zinc that is absorbed increases (4). This mechanism may explain partially the decrease in incidence of ARI and pneumonia observed in children under 5 years of age with moderate-dosage zinc supplementation in certain developing countries (3). The reduction in incidence of ARI in those populations might also be due to other factors, such as an improvement in immune function.
Zinc enhances host resistance to infection and plays a critical role in homeostasis of the immune system. Zinc deficiency is associated with an impaired cellular immune response and may play a role in the reduced cellular immunity seen in malnutrition (4, 19, 21). An imbalance between Th1 and Th2 functions has been reported during zinc deficiency (2, 19). In human studies, zinc deficiency results in decreased production of interleukin 2 (IL-2) and gamma interferon (cytokines associated with Th1 cells), whereas the production of IL-4, -6, and -10 (cytokines associated with Th2 cells) is not affected (2).
In conclusion, zinc salts inhibited RSV in vitro when present during preincubation, adsorption, penetration or egress. In addition, zinc salts prevented efficient RSV infection by preventing cell-to-cell spread in culture when the HEp-2 cell monolayer cultures were pretreated with zinc only prior to infection with RSV or when zinc was added to semisolid overlay media only after infection. The inhibitory effect was concentration dependent and was not observed during treatment with salts of other divalent cations. The inhibitory effect appears to be due to an effect of zinc salts on epithelial cell ability to support virus replication rather than a direct effect on the virus itself. Studies that examine the role of zinc deficiency and infant zinc supplementation on RSV infection may be important, especially among populations with zinc deficiency. In the absence of zinc deficiency, however, zinc treatment of RSV infection likely would not be tolerated at the concentrations that were observed by us to inhibit RSV replication in this study.
REFERENCES
- 1.Arens, M., and S. Travis. 2000. Zinc salts inactivate clinical isolates of herpes simplex virus in vitro. J. Clin. Microbiol. 38:1758-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck, F. W. J., A. S. Prasad, J. Kaplan, J. T. Fitzgerald, and G. J. Brewe. 1997. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am. J. Physiol. 272:E1002-E1007. [DOI] [PubMed] [Google Scholar]
- 3.Bhutta, Z. A., R. E. Black, K. H. Brown, J. M. Gardner, S. Gore, et al. 1999. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized control clinical trials. J. Pediatr. 135:689-697. [DOI] [PubMed] [Google Scholar]
- 4.Castillo-Duran, C., G. Heresi, M. Fisberg, and R. Uauy. 1987. Controlled trial of zinc supplementation during recovery from malnutrition: effects on growth and immune function. Am. J. Clin. Nutr. 45:602-608. [DOI] [PubMed] [Google Scholar]
- 5.Eby, G. A., D. R. Davis, and W. W. Halcomb. 1984. Reduction in duration of common cold by zinc gluconate lozenges in a double-blind study. Antimicrob. Agents Chemother. 25:20-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher, R. G., W. C. Gruber, K. M. Edwards, G. W. Reed, S. J. Tollefson, J. M. Thompson, and P. F. Wright. 1997. Twenty years of outpatient respiratory syncytial virus infection: a framework for vaccine efficacy trials. Pediatrics 99:7-16. [DOI] [PubMed] [Google Scholar]
- 7.Geist, F. C., J. A. Bateman, and F. G. Hayden. 1987. In vitro activity of zinc salts against human rhinovirus. Antimicrob. Agents Chemother. 31:622-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glezen, W. P., A. Peredes, J. E. Allison, L. H. Taber, and A. L. Frank. 1981. Risk of respiratory syncytial virus infection for infants from low-income families in relation to age sex, ethnic group, and maternal antibody level. J. Pediatr. 98:708-715. [DOI] [PubMed] [Google Scholar]
- 9.Glezen, W. P., A. Peredes, J. E. Allison, L. H. Taber, and J. A. Kasel. 1986. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 40:543-546. [DOI] [PubMed] [Google Scholar]
- 10.Haraguchi, Y., H. Sakurai, S. Hussain, B. M. Anner, and H. Hoshino. 1999. Inhibition of HIV-1 infection by zinc group metal compounds. Antivir. Res. 43:123-133. [DOI] [PubMed] [Google Scholar]
- 11.Katz, E., and E. Margalith. 1981. Inhibition of vaccinia virus maturation by zinc chloride. Antimicrob. Agents Chemother. 19:213-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keen, C. L., and M. E. Gershwin. 1990. Zinc deficiency and immune function. Annu. Rev. Nutr. 10:415-431. [DOI] [PubMed] [Google Scholar]
- 13.Korant, B. D., and B. E. Butterworth. 1976. Inhibition by zinc of rhinovirus protein cleavage: interaction of zinc with capsid polypeptides. J. Virol. 18:298-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korant, B. D., J. C. Kauer, and B. E. Butterworth. 1974. Zinc ions inhibit replication of rhinoviruses. Nature (London) 248:588-590. [DOI] [PubMed] [Google Scholar]
- 15.Kumel, G., S., Schrader, H. Zentgraf, H. Daus, and M. Brendel. 1990. The mechanism of antiherpetic activity of zinc sulphate. J. Gen. Virol. 71:2989-2997. [DOI] [PubMed] [Google Scholar]
- 16.Meshitsuka, S., M. Ishizawa, and T. Nose. 1987. Uptake and toxic effects of heavy metal ions: interactions among cadmium, copper and zinc in cultured cells. Experientia 43:151-156. [DOI] [PubMed] [Google Scholar]
- 17.Mossad, S. B., M. L. Macknin, S. V. Medendorp, and P. Mason. 1996. Zinc gluconate lozenges for treating the common cold: a randomized, double blind, placebo-controlled study. Ann. Intern. Med. 125:81-88. [DOI] [PubMed] [Google Scholar]
- 18.Mulholland, E. K., O. O. Ogunlesi, R. A. Adegbola, M. Weber, B. E. Sam, A. Palmer, M. J. A. Manary, O. Secka, M. Aidoo, D. Hazlett, H. Whittle, and B. M. Greenwood. 1999. Etiology of serious infections in young Gambian infants. Pediatr. Infect. Dis. J. 18(10 Suppl.):S35-S41. [DOI] [PubMed] [Google Scholar]
- 19.Prasad, A. S., J. T. Fitzgerald, B. Bao, F. W. J. Beck, and H. Chandrasekar. 2000. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. Ann. Intern. Med. 133:245-252. [DOI] [PubMed] [Google Scholar]
- 20.Sazawal, S., R. E. Black, S. Jalla, S. Mazumdar, A. Sinha, and M. K. Bhan. 1998. Zinc supplementation reduces the incidence of acute lower respiratory infections in infants and preschool children: a double blind controlled trial. Pediatrics 102:1-5. [DOI] [PubMed] [Google Scholar]
- 21.Shankar, A. H., and A. S. Prasad. 1998. Zinc and immune function: the biological basis of altered resistance to infection. Am. J. Clin. Nutr. 68(2 Suppl.):447S-463S. [DOI] [PubMed] [Google Scholar]
- 22.Smith, D. S., E. C. Helzner, C. E. Nuttall, Jr., M. Collins, R. A. Rofman, D. Ginsberg, C. B. Goswick, and A. Magner. 1989. Failure of zinc gluconate in treatment of acute upper respiratory tract infections. Antimicrob. Agents Chemother. 33:646-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suara, R. O., and W. P. Glezen. 1995. Contribution of respiratory viruses to the worldwide problem of pneumonia in young children. Semin. Pediatr. Infect. Dis. 6:121-127. [Google Scholar]
- 24.Sugarma, B. 1983. Zinc and infection. Rev. Infect. Dis. 5:137-147. [DOI] [PubMed] [Google Scholar]
- 25.Victoria, C. G., B. R. Kirkwood, A. Ashworth, R. E. Black, S. Rogers, S. Sazawal, H. Campbell, and S. Gove. 1999. Potential interventions for the prevention of childhood pneumonia in developing countries: improving nutrition. Am. J. Clin. Nutr. 70:309-320. [DOI] [PubMed] [Google Scholar]
- 26.Wahba, A. 1980. Topical application of micro zinc-solutions: a new treatment for herpes simplex infections of the skin? Acta Dermatol. Venerol. (Stockholm) 60:175-177. [PubMed] [Google Scholar]
- 27.Weber, M. W., K. Mulholland, and B. M. Greenwood. 1998. Respiratory syncytial virus infection in tropical and developing countries. Trop. Med. Int. Health 125:268-280. [DOI] [PubMed] [Google Scholar]
- 28.Weisman, K., J. P. Jokobsen, J. E. Weissman, U. M. Hammer, S. M. Nyholm, B. Hansen, K. E. Lomholt, and K. Schmidt. 1990. Zinc gluconate lozenges for common cold: a double blind clinical trial. Dan. Med. Bull. 37:279-291. [PubMed] [Google Scholar]