Abstract
The present study examined the phototoxicities of a series of 7-(3-aminopyrrolidinyl) quinolones containing various substituents at position 1 (in which the substituent at R8 is a hydrogen or a halogen) by use of a mouse model. For the 7-(3-aminopyrrolidinyl) quinolones with a halogen atom at position 8, well-known substituent groups such as a cyclopropyl, an ethyl, or a difluorophenyl at position 1 were found to be responsible for severe phototoxicity. However, when an aminodifluorophenyl or an isoxazolyl group was placed at position 1, even 8-halogeno quinolones were found to be mildly phototoxic. This is the first report of 8-halogeno quinolones that are not severely phototoxic. Two structurally similar 8-chloro quinolones (the 1-aminodifluorophenyl 8-chloro quinolone and the 1-difluorophenyl 8-chloro quinolone) were investigated further. The former was mildly phototoxic; the latter was severely phototoxic. We demonstrate that these two 8-chloro quinolones have practically the same areas under the concentration-time curves from 0 to 4 h in auricular tissue, suggesting that the mild phototoxicity is not due to pharmacokinetic instability. The rates of UV photodegradation of these compounds were also measured. We found that these two quinolones photodegrade at similar rates, suggesting that the mild phototoxicity is not attained through increased photostability. In conclusion, the phototoxic potentials of fluoroquinolones are influenced not only by the substituent at position 8 but also by that at position 1 (a new finding from this study). We also discovered a mildly phototoxic 8-chloro quinolone which did not have increased photostability.
Fluoroquinolones (FQs) are very effective for the treatment of various bacterial infections. The results of studies of the structure-activity relationships of FQs have been reported previously (5, 19). It has also been demonstrated that a cyclopropyl group at position 1, in combination with a 3-aminopyrrolidinyl group at position 7 and a halogen atom such as fluorine or chlorine at position 8, confers the most potent antibacterial activity on the FQ molecule. In particular, the introduction of a halogen atom at position 8 provides an FQ molecule with potent antibacterial activity in vivo via expansion of the antibacterial spectrum and improvements in oral bioavailability.
Clinical and experimental studies of most FQs developed in the past have reported that they cause phototoxicity with various degrees of severity (2, 3, 5, 9, 10, 18, 19). Derivatives of this class of drugs containing a halogen atom at position 8 were found to have the greatest phototoxic properties. Lomefloxacin, sparfloxacin, fleroxacin, and clinafloxacin are included in this group of FQs. On the other hand, a hydrogen group at position 8 provides an FQ molecule with only a mildly phototoxic potential (5, 8, 9, 19, 20). UV irradiation of 8-halogeno quinolones has been reported to cause photolability (14, 16, 17). FQ derivatives with a methoxy group at position 8, such as gatifloxacin and moxifloxacin, have been demonstrated to be photostable and the least phototoxic (12, 13, 15). The introduction of a methoxy group at position 8 made the molecule more photostable and less phototoxic than other FQs. Therefore, use of a methoxy group is thought to be useful for the avoidance phototoxicity. This has led to the recent development of many FQs with a methoxy group at position 8. Furthermore, it has generally been thought that FQs that are less photostable have more severe phototoxicities. However, the structure-phototoxicity and photostability-phototoxicity relationships described above may not be generalized because the majority of FQs examined for phototoxicity in the past have been confined to derivatives with a cyclopropyl or an ethyl group at position 1.
The present study examined the phototoxic reactions of a series of FQs with various substituents at position 1 in a mouse model (in which 40 mg of an FQ/kg of body weight was administered intravenously) to investigate the structure-phototoxicity relationships systematically. The series of FQs have a 3-aminopyrrolidinyl group at position 7 and a hydrogen or a halogen atom at position 8. Three novel substituents at position 1, such as an aminodifluorophenyl group (N. Hayashi, H. Amano, Y Ohshita, Y. Niino, J. Yoshida, and A. Yazaki, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F051, 1996), an isoxazolyl group (Y. Ohshita, Y. Hirao, N. Hayashi, H. Amano, S. Maruyama, S. Noda, Y. Kuramoto, and A. Yazaki, Abstr. 34th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F032, 1994), and an oxetanyl group (T. Nishino, M. Otsuki, N. Hayashi, and T. Yatsunami, Abstr. 31st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F1448, 1991), in addition to the known difluorophenyl, cyclopropyl, and ethyl groups, were used. We have reported that these novel substituent groups offer a broad antibacterial spectrum and excellent antibacterial activity against gram-positive and gram-negative pathogens. We established the protective effect of a 1-aminodifluorophenyl group against phototoxicity using structurally similar pairs of compounds with practically the same areas under the concentration-time curves from 0 to 4 h (AUC0-4s) in tissue. We also investigated the effect of this N-1 substituent on photodegradation, since observation of the photodegradation process would give us clues to help us understand the initial stage of phototoxicity.
MATERIALS AND METHODS
Compounds
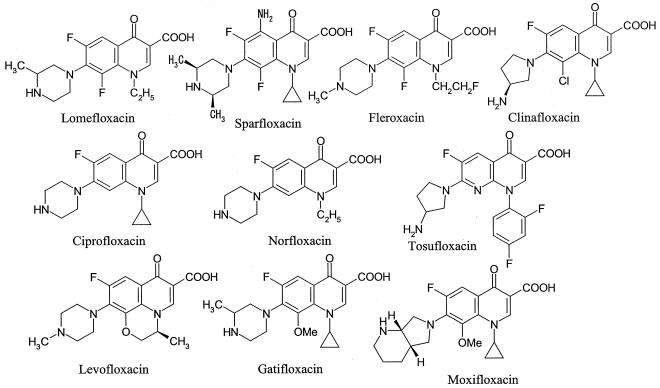
The reference FQs (Fig. 1) and various derivatives (see Table 3) used in this study were synthesized in our laboratory.
FIG. 1.
Chemical structures of reference quinolones.
TABLE 3.
Phototoxicity of a series of 7-(3-aminopyrrolidinyl) quinolones in mice receiving the drugs intravenouslya
Each compound was administered at a dose of 40 mg/kg.
The vehicle was 0.1 N NaOH.
The scores for ear redness were as follows: 0, normal; 1, mild erythema; 2, moderate erythema; 3, severe erythema and edema.
Animals
Female ICR strain mice (age, 5 to 6 weeks; weight, 23 to 32 g; Charles River Japan Inc., Hino, Japan) were used in this study. They were housed in plastic cages in groups of six and were maintained in an air-conditioned room (temperature, 23 ± 2°C; relative humidity, 55% ± 12%) with free access to commercial laboratory chow (CE-2; CLEA Japan Inc., Tokyo, Japan) and tap water.
UVA irradiation system
A UV type A (UVA) irradiation system was used to provide irradiation, as described by Marutani et al. (15). Briefly, the source of UVA light consisted of a bank of 10 black-light fluorescent bulbs (FL20SBLB; Toshiba, Tokyo, Japan) that emit radiation over a wavelength range of 300 to 400 nm. A 3-mm-thick glass plate was placed over a multichamber box (in which partitions divide the box into two by six chambers) to absorb light at wavelengths below 320 nm (UVB). The radiation dose was delivered at a distance of 15 cm from the light and was 1.8 mW/s · cm2 in the irradiated region, as measured with a digital illuminometer with interchangeable sensors (DRC-100X; Spectronics Corporation, Westbury, N.Y.). An electric fan was placed 50 cm away from the light source to prevent an increase in temperature in the irradiated area.
Phototoxicity test
Phototoxicity tests were performed by the methods described by Marutani et al. (15) and Wagai et al. (21, 22), with some modifications. Briefly, ICR mice received intravenous doses of FQs (40 mg/10 ml/kg) and were then placed into the chambers of the multichamber box and exposed to UVA light for 4 h (26 J/cm2), with a 15-cm distance between the lights and the ears of the mice. The FQs were dissolved in 0.1 N NaOH. Control mice that received 0.1 N NaOH were also irradiated. The redness of the ears of the mice was used as a phototoxicity index and was scored as follows at 0, 24, 48, 72, and 168 h after the irradiation: 0, normal; 1, mild erythema; 2, moderate erythema; 3, severe erythema and edema formation.
AUC measurements
ICR mice were given a single intravenous dose of one of the FQs. For each of the time points of 15 and 30 min and 1, 2, and 4 h after drug administration, four mice were anesthetized with ether and the blood and the ears were collected. The blood samples were centrifuged to obtain serum. The ears were added to 1 N KOH and were maintained at 60°C for 1 h, following neutralization with 0.8 M phosphoric acid. The concentrations of FQs in the serum and ears were quantified with a high-performance liquid chromatograph (LC-10A; Shimadzu, Tokyo, Japan) with a TSK gel ODS-80Ts column. A mixture of 20 mM sodium dodecyl sulfate-Pi and acetonitrile (57:33; vol/vol) was used as the mobile phase at a flow rate of 1.0 ml/min. The column eluent was monitored at 290 nm. The AUC0-4s of the FQs were calculated by the trapezoid method.
Photostability
The FQs were dissolved in 0.02 N NaOH to a concentration of 2 mg/ml and were then diluted with 0.1 M phosphate buffer (pH 7.0) to achieve a final concentration of 20 μg/ml. Each of these solutions was transferred to four wells of a 24-well plate (1 ml/well); and the plates were irradiated with UVA light (1.8 mW/s · cm2) for 2, 5, 10, and 20 min, respectively. The control solution was maintained in the dark. To examine the effect of excess chloride ions on the photodegradation of FQs, 0.1 M phosphate buffer (pH 7.0) containing 0.5 M NaCl was used. The residual amounts of FQs were quantified with a high-performance liquid chromatograph (LC-10AT, Shimadzu) with a TSK gel ODS-80Ts column. A mixture of 20 mM sodium dodecyl sulfate-Pi and acetonitrile (3:2) was used as the mobile phase at a flow rate of 1.2 ml/min. The column eluent was monitored at 254 nm.
RESULTS
Dose-response study of phototoxicities of reference quinolones in a mouse model
To determine the proper dose and observation intervals for screening of the phototoxic potentials of the FQs, the dose-response relationships and the time course of toxicity were examined with levofloxacin, tosufloxacin, and lomefloxacin, which are known from experimental and clinical studies (2, 4, 5, 10, 11, 19) to be mildly, moderately, and seriously phototoxic FQs, respectively. These FQs were administered to ICR mice as an intravenous bolus to avoid the bias caused by the diversity of murine intestinal absorption of FQs. Immediately after FQ administration, the mice were exposed to UVA light for 4 h. Ear erythema was observed and was classified by use of the phototoxicity index for up to 168 h after UVA irradiation.
As shown in Table 1, we could determine whether the ear erythema was aggravated or alleviated when we observed the ears 0 and 48 h after UV irradiation. We were able to evaluate the phototoxic potencies of all the quinolones when they were used at a dose of 40 mg/kg.
TABLE 1.
Incidence and scores of ear erythema in mice receiving intravenously lomefloxacin, tosufloxacin, and levofloxacin
| Compound and dose (mg/kg)a | No. of animals with the indicated scoreb at:
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h
|
24 h
|
48 h
|
72 h
|
168 h
|
||||||||||||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |
| Vehicle | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| Lomefloxacin | ||||||||||||||||||||
| 20 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 6 |
| 40 | 0 | 0 | 6 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 |
| 80 | 0 | 0 | 4 | 2 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 |
| Tosufloxacin | ||||||||||||||||||||
| 20 | 0 | 1 | 5 | 0 | 3 | 3 | 0 | 0 | 5 | 1 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| 40 | 0 | 0 | 6 | 0 | 2 | 4 | 0 | 0 | 4 | 2 | 0 | 0 | 5 | 1 | 0 | 0 | 6 | 0 | 0 | 0 |
| 80 | 0 | 0 | 6 | 0 | 0 | 1 | 5 | 0 | 0 | 5 | 1 | 0 | 4 | 1 | 1 | 0 | 5 | 1 | 0 | 0 |
| Levofloxacin | ||||||||||||||||||||
| 20 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| 40 | 5 | 1 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| 80 | 2 | 4 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
Six mice were included in each dosing group.
The scores for ear redness were as follows: 0, normal; 1, mild erythema; 2, moderate erythema; 3, severe erythema and edema.
Phototoxicities of reference quinolones
Next, the phototoxic potentials of the reference quinolones (Fig. 1) at a dose of 40 mg/kg were examined in the mouse model (Table 2). The reference quinolones were categorized into four classes: 8-halogeno quinolones (lomefloxacin, sparfloxacin, fleroxacin, clinafloxacin), 8-methoxy quinolones (gatifloxacin, moxifloxacin), a 1,8-naphthylidine (tosufloxacin), and the other quinolones (ciprofloxacin, norfloxacin, levofloxacin). Each class of quinolones showed different degrees of phototoxicity. The FQs with a halogen atom at position 8 produced severe (progressive and irreversible) phototoxicity. Moderate or severe erythema was observed on the ears at 0 h. The scores of ear erythema reached the maximal value of 3.0 at 48 h. In contrast, the 8-methoxy quinolones did not induce any phototoxicity (Table 2). Tosufloxacin (1,8-naphthylidine) showed moderate phototoxicity. Moderate erythema was observed at 0 h, and the erythema was alleviated and disappeared completely by 168 h (data not shown). Mild (transient and reversible) phototoxicity was observed in mice treated with the other quinolones. Mild erythema was transiently observed from 0 to 24 h and disappeared completely by 48 h. These observations agree well with the results of previous oral dosing studies (5, 15, 21, 22).
TABLE 2.
Phototoxicities of the reference FQs in mice receiving the drugs intravenously
| Compounda | No. of animals with the indicated scoreb at:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 h
|
48 h
|
|||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |
| Vehicle | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| Lomefloxacin | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 6 |
| Sparfloxacin | 0 | 0 | 4 | 2 | 0 | 0 | 0 | 6 |
| Fleroxacin | 0 | 0 | 4 | 2 | 0 | 0 | 0 | 6 |
| Clinafloxacin | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 |
| Tosufloxacin | 0 | 0 | 6 | 0 | 4 | 2 | 0 | 0 |
| Ciprofloxacin | 4 | 2 | 0 | 0 | 6 | 0 | 0 | 0 |
| Norfloxacin | 4 | 2 | 0 | 0 | 6 | 0 | 0 | 0 |
| Levofloxacin | 5 | 1 | 0 | 0 | 6 | 0 | 0 | 0 |
| Gatifloxacin | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| Moxifloxacin | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
Each compound was administered at a dose of 40 mg/kg to six mice.
The scores for ear redness were as follows: 0, normal; 1, mild erythema; 2, moderate erythema; 3, severe erythema and edema.
Effects of substitutions at positions 1 and 8 on phototoxicity
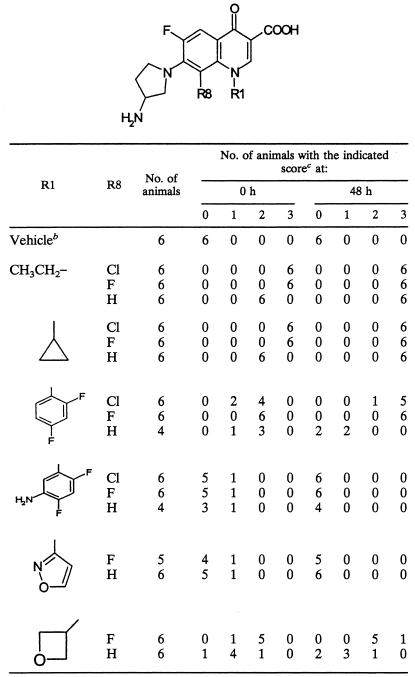
The phototoxic reactions to a series of 7-(3-aminopyrrolidinyl) quinolones with various substituents at position 1 (in which the substituent at R8 is hydrogen or halogen) (Table 3) were examined. A difluorophenyl, a cyclopropyl, or an ethyl group was used as the well-known substituent at position 1. Additionally, an aminodifluorophenyl, an isoxazolyl, or an oxetanyl group was used as novel substituents. Compounds with these groups offered broad-spectrum and excellent activity against gram-positive and gram-negative pathogens (data not shown).
Unexpectedly, the phototoxicities of the 8-H quinolones with a 1-cyclopropyl or a 1-ethyl group were as severe as those of the reference 8-halogeno quinolones, such as lomefloxacin, sparfloxacin, fleroxacin, and clinafloxacin (Table 2). On the contrary, the 8-H quinolones with a 1-difluorophenyl or a 1-oxetanyl group were moderately phototoxic; 8-H quinolones with a 1-aminodifluorophenyl or a 1-isoxazilyl group were mildly phototoxic. Surprisingly, despite the substitution of a halogen atom at position 8, the quinolones with a 1-aminodifluorophenyl or a 1-isoxazolyl group caused mild phototoxicity. This is the first finding of mildly phototoxic 8-halogeno quinolones.
This mild phototoxicity might have been due to the pharmacokinetic instability of these quinolones. To examine this possibility, we measured the AUC0-4s of a pair of structurally similar quinolones in serum and auricular tissue: the 1-aminodifluorophenyl 8-chloro quinolone (which was mildly phototoxic) and the 1-difluorophenyl 8-chloro quinolone (which was severely phototoxic). The chemical structures of both quinolones were identical except for the amino group at position 1.
Distributions of 8-halogeno quinolones into the ears of mice
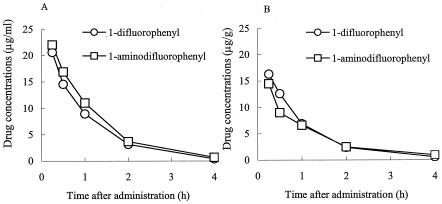
The concentrations of the quinolones with a 1-difluorophenyl or a 1-aminodifluorophenyl 8-chloro group in the sera and ear tissues of mice were determined after intravenous administration of the quinolones (40 mg/kg; n = 4). As shown in Fig. 2A and B, the pharmacokinetic profiles of both compounds were similar. The AUC0-4s of the 1-difluorophenyl quinolone and the 1-aminodifluorophenyl quinolone were 18.2 and 16.7 μg · h/g, respectively, in the ears and 22.5 and 26.3 μg · h/ml, respectively, in sera, indicating that the mildly phototoxic potential of the 1-aminodifluorophenyl quinolone was not due to its poor distribution into the ears.
FIG. 2.
Pharmacokinetics of the 1-difluorophenyl 8-chloro quinolone and the 1-aminodifluorophenyl 8-chloro quinolone in the sera (A) and ears (B) of mice to which the FQs were administered intravenously (40 mg/kg).
Photostabilities of 8-halogeno quinolones
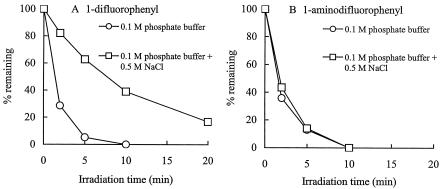
The photostability studies were carried out with the pair of 8-chloro quinolones mentioned above. Chloride ions inhibited the photodegradation of the 1-difluorophenyl 8-chloro quinolone (Fig. 3A), as reported previously for an 8-chloro quinolone (1). However, the effect of the chloride ions was not observed with the 1-aminodifluorophenyl 8-chloro quinolone (Fig. 3B).
FIG. 3.
Photodegradation of the 1-difluorophenyl 8-chloro quinolone (A) and the 1-aminodifluorophenyl 8-chloro quinolone (B) in 0.1 M phosphate buffer in the presence or absence of excess chloride ions.
DISCUSSION
In this study, the phototoxic potentials of newly synthesized FQs with various substituents at position 1 were determined systematically. The most important new conclusion from this study is that the phototoxic potentials of FQs are affected not only by the substituent at position 8 but also by that at position 1.
Several clinical and experimental studies have reported that FQs with a halogen atom at position 8 cause severe phototoxicity (2, 5, 9, 13, 15, 18, 19), while 8-hydrogen quinolones cause much milder phototoxicity (5, 8, 9, 19, 20). On the contrary, the 8-methoxy quinolones did not cause any significant phototoxicity (12, 13, 15). In the present study, we examined the structure-phototoxicity relationships of a series of 7-(3-aminopyrrolidinyl) quinolones with various substituents at position 1 (in which R8 contained a hydrogen or halogen) using a mouse model. The degree of phototoxicity of FQs with a difluorophenyl group at position 1 changed from mild to severe when a hydrogen atom was replaced by a halogen at position 8. On the other hand, the 1-cyclopropyl or the 1-ethyl quinolones were severely phototoxic, regardless of the substituents at position 8, and caused severe phototoxicity comparable to those of the reference 8-halogeno quinolones. Furthermore, the 1-aminodifluorophenyl and the 1-isoxazolyl quinolones caused mild phototoxicity immediately after irradiation, which disappeared by 48 h, despite the substitution of a halogen atom at position 8. These results indicate that the phototoxic potentials of FQs are influenced not only by the substituent at position 8 but also by that at position 1.
It is well known that the substitution of a halogen atom at position 8 expands the spectrum of antibacterial activity and improves the oral bioavailability (5). However, a halogen atom has rarely been used as a substituent at position 8 in the FQs that have been developed due to the severe phototoxicity caused by this substitution. Recently, some FQs with a methoxy group at position 8 have been developed to avoid the appearance of phototoxicity. In fact, these FQs did not show any phototoxicity in experimental or clinical studies (12, 13, 15). However, there are some problems with 8-methoxy quinolones. In general, the introduction of this group resulted in a decrease in antibacterial activity, especially against gram-negative bacteria such as Pseudomonas aeruginosa (6, 7). In this study, we found that the 8-halogen quinolone derivatives with an aminodifluorophenyl group or an isoxazolyl group at position 1 showed mild phototoxicity. An example of such a derivative is levofloxacin, which clinical studies have shown is a safe FQ in terms of its phototoxicity (4). Furthermore, FQs with these two novel groups had improved antibacterial activities (see the introduction). Therefore, the discovery of these substituents paves the way for the design of new types of 8-halogeno quinolones with high levels of antibacterial activity but without severe phototoxicity.
Araki et al. (1) reported that a carbon-centered radical ( · C) was generated at position 8 by scission of the C—Cl bond of an 8-chloro quinolone under UV irradiation, and excess chloride ions immediately reacted with it. As a result, the C—Cl bond was recovered and the apparent photostability was increased. In this study, excess chloride ions did not inhibit the photodegradation of the 1-aminodifluorophenyl 8-chloro quinolone, suggesting that · C might react with a 1-aminodifluorophenyl group faster than it does when excess chloride ions are present. The carbon radicals should trigger unknown subsequent chemical reactions that lead to toxic biological reactions. Therefore, the protective effect of this N-1 substituent against phototoxicity might be due to this putative intramolecular scavenger effect.
In conclusion, the phototoxic potentials of FQs were influenced not only by the substituent at position 8 but also by that at position 1. This finding provides new information on the structure-phototoxicity relationships of FQs. In particular, we believe that the discovery of the novel substituents aminodifluorophenyl and probably isoxazolyl is important for the design of new types of 8-halogeno quinolones.
Acknowledgments
We thank Ken Hashimoto for helpful discussions and critical reading of the manuscript and Hirotaka Amano and Takayuki Amago for technical assistance. We also thank Robert Crawford for critical reading of the manuscript.
REFERENCES
- 1.Araki, T., Y. Kawai, I. Ohta, and H. Kitaoka. 2002. Photochemical behavior of sitafloxacin, fluoroquinolone antibiotic, in an aqueous solution. Chem. Pharm. Bull. 50:229-234. [DOI] [PubMed] [Google Scholar]
- 2.Arata, J., T. Horio, R. Soejima, and K. Ohara. 1998. Photosensitivity reactions caused by lomefloxacin hydrochloride: a multicenter survey. Antimicrob. Agents Chemother. 42:3141-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blondeau, J. M. 1999. Expanded activity and utility of the new fluoroquinolones: a review. Clin. Ther. 21:3-40. [DOI] [PubMed] [Google Scholar]
- 4.Boccumini, L. E., C. L. Fowler, T. A. Campbell, L. F. Puertolas, and K. H. Kaidbey. 2000. Photoreaction potential of orally administered levofloxacin in healthy subjects. Ann. Pharmacother. 34:453-458. [DOI] [PubMed] [Google Scholar]
- 5.Domagala, J. M. 1994. Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J. Antimicrob. Chemother. 33:685-706. [DOI] [PubMed] [Google Scholar]
- 6.Fass, R. J. 1993. In vitro activity of Bay y 3118, a new quinolone. Antimicrob. Agents Chemother. 37:2348-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fass, R. J. 1997. In vitro activity of Bay 12-8039, a new 8-methoxyquinolone. Antimicrob. Agents Chemother. 41:1818-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson, J., and B. E. Johnson. 1990. Ciprofloxacin-induced photosensitivity: in vitro and in vivo studies. Br. J. Dermatol. 123:9-20. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson, J. 1995. Fluoroquinolone photosensitization: a review of clinical and laboratory studies. Photochem. Photobiol. 62:954-958. [Google Scholar]
- 10.Horio, T., H. Miyauchi, Y. Asada, Y. Aoki, and M. Harada. 1994. Phototoxicity and photoallergenicity of quinolones in guinea pigs. J. Dermatol. Sci. 7:130-135. [DOI] [PubMed] [Google Scholar]
- 11.Iwamoto, Y., A. Kurita, T. Shimizu, T. Masuzawa, K. Uno, M. Yagi, T. Kitagawa, T. Oku, and Y. Yanagihara. 1994. DNA strand-breaking activities of quinolone antimicrobial agents under visible light irradiation. Biol. Pharm. Bull. 17:654-657. [DOI] [PubMed] [Google Scholar]
- 12.Kuninishi, Y., M. Nagata, H. Ogata, A. Omotera, Y. Nomoto, and K. Tsuru. 1998. Phototoxicity studies of gatifloxacin in guinea pigs and mice. Jpn. Pharmacol. Ther. 26:1651-1654. [Google Scholar]
- 13.Man, I., J. Murphy, and J. Ferguson. 1999. Fluoroquinolone phototoxicity: a comparison of moxifloxacin and lomefloxacin in normal volunteers. J. Antimicrob. Chemother. 43(Suppl. B):77-82. [DOI] [PubMed] [Google Scholar]
- 14.Martinez, L. J., G. Li, and C. F. Chignell. 1997. Photogeneration of fluoride by the fluoroquinolone antimicrobial agents lomefloxacin and fleroxacin. Photochem. Photobiol. 65:599-602. [DOI] [PubMed] [Google Scholar]
- 15.Marutani, K., M. Matsumoto, Y. Otabe, M. Nagamuta, K. Tanaka, A. Miyoshi, T. Hasegawa, H. Nagano, S. Matsubara, R. Kamide, T. Yokota, F. Matsumoto, and Y. Ueda. 1993. Reduced phototoxicity of a fluoroquinolone antibacterial agent with a methoxy group at the 8 position in mice irradiated with long-wavelength UV light. Antimicrob. Agents Chemother. 37:2217-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto, M., K. Kojima, H. Nagano, S. Matsubara, and T. Yokota. 1992. Photostability and biological activity of fluoroquinolones substituted at the 8 position after UV irradiation. Antimicrob. Agents Chemother. 36:1715-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morimura, T., T. Ohno, H. Matsukura, and Y. Nobuhara. 1995. Photodegradation kinetics of the new antibacterial fluoroquinolone derivative, orbifloxacin, in aqueous solution. Chem. Pharm. Bull. 43:1000-1004. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira, H. S., M. Goncalo, and A. C. Figueiredo. 2000. Photosensitivity to lomefloxacin. A clinical and photobiological study. Photodermatol. Photoimmunol. Photomed. 16:116-120. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez, J. P., R. D. Gogliotii, J. M. Domagala, S. J. Gracheck, M. D. Huband, J. A. Sesnie, M. A. Cohen, and M. A. Shapiro. 1995. The synthesis, structure-activity, and structure-side effect relationships of a series of 8-alkoxy- and 5-amino-8-alkoxyquinolone antibacterial agents. J. Med. Chem. 38:4478-4487. [DOI] [PubMed] [Google Scholar]
- 20.Vousden, M., J. Ferguson, J. Richards, N. Bird, and A. Allen. 1999. Evaluation of phototoxic potential of gemifloxacin in healthy volunteers compared with ciprofloxacin. Chemotherapy (Basel) 45:512-520. [DOI] [PubMed] [Google Scholar]
- 21.Wagai, N., F. Yamaguchi, M. Sekiguchi, and K. Tawara. 1990. Phototoxic potential of quinolone antibacterial agents in Balb/c mice. Toxicol. Lett. 54:299-308. [DOI] [PubMed] [Google Scholar]
- 22.Wagai, N., and K. Tawara. 1991. Quinolone antibacterial-agent-induced cutaneous phototoxicity: ear swelling reactions in Balb/c mice. Toxicol. Lett. 58:215-223. [DOI] [PubMed] [Google Scholar]