Abstract
We compared various amphotericin B formulations (no treatment or 0.8 mg of Fungizone [conventional deoxycholate amphotericin B] per kg of body weight, or 0.8, 4, or 8 mg of Amphocil, AmBisome, or Abelcet per kg of body weight) for treatment of systemic murine aspergillosis. In two studies, all formulations prolonged survival, with the results for AmBisome nearly equivalent to those for Fungizone; Amphocil and Abelcet were less effective or equivalent depending on the severity of infection. No survivors were cured in both kidneys and brain, but each formulation showed efficacy, especially in the kidneys. Although higher doses could be given, no lipid-based formulation showed consistent superiority over Fungizone or over each other.
In spite of present therapeutic options, the mortality rate from invasive aspergillosis is high (13-15). Despite the introduction of newer agents into the therapy of aspergillosis, conventional deoxycholate-formulated amphotericin B (AMB; Fungizone) remains a principal therapeutic and comparator in clinical trials. However, its well-described toxicities can limit its usefulness (19).
The following lipid-carried formulations of AMB have reduced toxicities (20, 24, 30): Amphotec (Amphocil [ABCD]; Intermune, Inc., Burlingame, Calif.), a discoidal complex of cholesteryl sulfate and AMB (21, 22); AmBisome (AmBi; Gilead Sciences, Foster City, Calif.), a true unilamellar liposome (1, 33); and Abelcet (ABLC; Enzon, Fairfield, N.J.), a ribbon form of dimyristoyl phosphatidyl choline and dimyristoyl phosphatidyl glycerol with AMB (27, 35). Each formulation has been reported to have efficacy against aspergillosis equal to or better than that of conventional AMB (3, 4, 18, 25, 29, 32). However, no studies comparing the four formulations against aspergillosis have been done.
Systemic model
Murine models of systemic aspergillosis were established in 6-week-old female CD-1 mice (average weight, 24.2 g) by intravenous injection of 8 × 106 (high inoculum) or 3.6 × 106 (low inoculum) conidia of Aspergillus fumigatus isolate 10AF (16, 23). Therapy began 1 day after infection, with groups of 10 or 11 mice receiving either no treatment, 0.8 mg of conventional AMB per kg of body weight (a dose just below toxicity in this model), or 0.8, 4, or 8 mg of ABCD, AmBi, or ABLC per kg. All dosages were based on an equivalent number of milligrams of AMB per kilogram of body weight. Four doses were administered intravenously every other day, as AMB has a long half-life in all four preparations (4, 21, 30, 33).
Deaths were tallied through 9 days of infection. All surviving mice were then euthanatized by CO2 asphyxia, and the number of CFU of A. fumigatus in the brain and kidneys was determined by quantitative plating of organ homogenates (16, 23); the dilution procedures eliminated drug carryover.
Statistics
Evaluation of survival was done by using a log-rank test and a comparison of the number of CFU by a Mann-Whitney U test. For the quantification of CFU burdens, to ensure that death was considered as a worse outcome than survival with any amount of burden, a log value of 5 was assigned to data points missing due to death from infection (28). This value has been determined to be the approximate number of CFU in the organ just prior to death (16, 23).
High-inoculum model
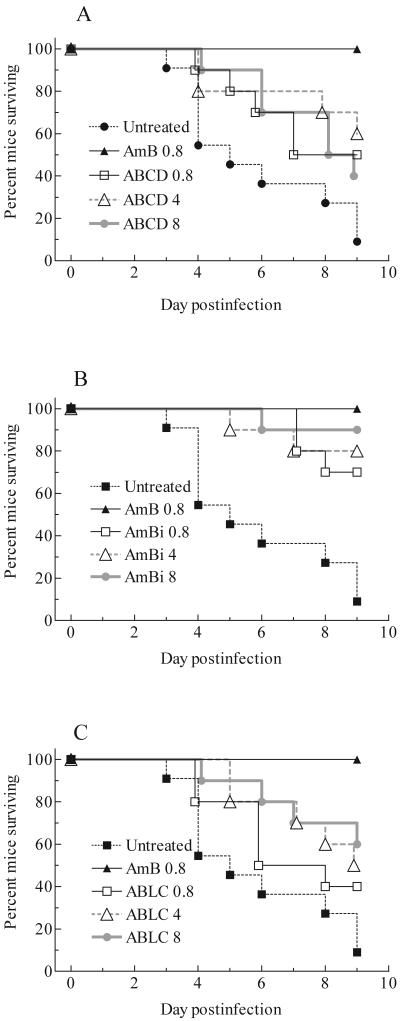
The first deaths occurred on day 3 and continued through day 9. Ten of 11 untreated animals died, whereas all animals treated with conventional AMB survived (Fig. 1). The survival for those given a lipid-based formulation varied from 40 to 90%, with no formulation providing complete protection, even at 8 mg of AMB per kg. In comparison with results for the controls, 8 mg of ABCD per kg and 0.8 mg of ABLC per kg showed no significant prolongation of survival (P > 0.05). All other regimens provided significant prolongation (P values of 0.05 to 0.0002, depending on the comparison). At equal doses of AMB, the lipid formulations were not different. A dose of 0.8 mg of conventional AMB per kg was superior to all doses of ABCD or ABLC (P values of 0.029 to 0.002, depending on the comparison). Each dose of AmBi was equivalent to 0.8 mg of conventional AMB per kg. A dose of 8 mg of AmBi per kg was superior to 8 mg of ABCD per kg (P = 0.025) and 0.8 mg of ABLC per kg (P = 0.02).
FIG. 1.
Cumulative mortality of mice infected with a high inoculum of A. fumigatus and given no treatment or treated with conventional amphotericin B (AmB) and ABCD (A), AmBi (B), or ABLC (C). For the untreated control group, n = 11; for all other groups, n = 10.
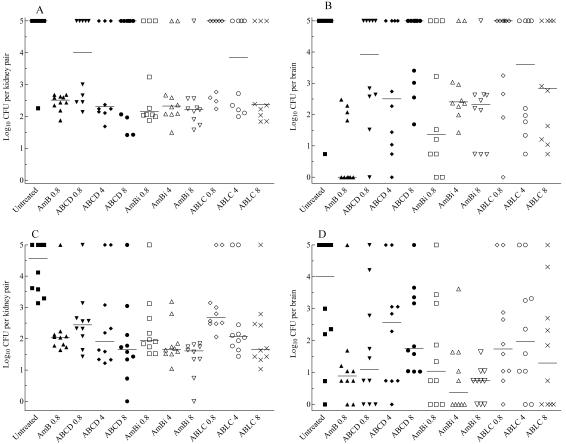
No survivor was free of detectable infection in the kidneys (primary target organ), whereas six mice given conventional AMB had no detectable infection in the brain, compared with two or fewer animals given any lipid-based AMB regimen (Fig. 2 A and B). Conventional AMB was superior to all other treatments in clearing the brain (P values of 0.01 to 0.0004, depending on the comparison), except for a dose of 0.8 mg of AmBi per kg (P > 0.05); no regimen of ABCD or ABLC was superior to results for the controls. Among lipid formulations, 8 mg of AmBi per kg was superior to ABCD at all doses (P values of 0.04 to 0.008). Except for the preceding comparison, at equal AMB doses, the three lipid-based formulations were equivalent (e.g., 0.8 mg of ABCD, AmBi, or ABLC per kg had equivalent results).
FIG. 2.
CFU recovered from the organs of each surviving mouse in the high-inoculum model of infection (A and B) and CFU recovered from the organs of each surviving mouse in the low-inoculum model of infection (C and D). The values of 5 represent mice that succumbed to infection. A value of 0 indicates that there was no detectable infection, which is approximately 10 or fewer CFU per organ. The bars represent the medians. In the untreated control group in panels A and B, n = 11; for all other groups, n = 10.
In the kidneys, conventional AMB proved superior to equivalent dosages of ABCD and ABLC (P values of 0.023 and 0.011, respectively), whereas all other treatment regimens of ABCD, AmBi, or ABLC were equivalent to treatment with conventional AMB. Comparisons among the lipid formulations based on equal doses showed them equivalent (P > 0.05). ABCD and ABLC were effective in clearing the kidneys at doses of 4 and 8 mg/kg, respectively (P ≤ 0.03 versus untreated), but only AmBi was effective at all three doses (P ≤ 0.01). No dose responsiveness was observed in the reduction of CFU for any formulation (Fig. 2).
Low-inoculum model
In a second study, the infection was less severe and lethal. Fewer control animals died (i.e., only 50%), with deaths occurring between days 5 and 7, and 80 to 100% of treated animals survived. Doses of 0.8 and 8 mg of ABCD per kg, 4 or 8 mg of AmBi per kg, and 8 mg of ABLC per kg provided significant prolongation of survival compared to results for the controls (P values of 0.05 to 0.012, depending on the comparison). Although 80 or 90% of the animals in the other regimens survived, there was no advantage, either by day of death or number of survivors, compared to results for the controls (P > 0.05). The lipid formulations were equivalent in prolonging survival.
All survivors in all regimens carried residual infection in one or both organs (Fig. 2C and D). In the brain, conventional AMB at a dose of 0.8 mg/kg and AmBi at a dose of 4 or 8 mg/kg were efficacious compared to no treatment (P values of <0.05 to 0.01, depending on the comparison); no regimen of ABCD or ABLC proved better than no treatment (P > 0.05). Additionally, the four preparations were equivalent at equal doses of AMB except for AmBi; 4 or 8 mg of AmBi per kg was superior to ABCD at the same dose (P < 0.05). No dose of a lipid preparation was superior to 0.8 mg of conventional AMB per kg.
In the kidneys, no treatment cured more than a single animal, and all regimens proved efficacious in the clearance of infection (P values of <0.01 to 0.001, depending on the comparison) (Fig. 2). However, only a dose of 8 mg of AmBi per kg was better than 0.8 mg of conventional AMB per kg (P < 0.001). Neither ABCD nor AmBi showed dose responsiveness in the clearance of infection. ABLC showed modest dose responsiveness, with results for doses of both 4 and 8 mg/kg superior to results for the dose of 0.8 mg/kg (P value of <0.05 or 0.01, respectively). In comparisons of equal doses of AMB, none of the preparations had significantly different results except that conventional AMB was superior (P < 0.01) to ABLC.
Each of these preparations has been reported to be useful for the treatment of aspergillosis (18, 24, 31, 32, 34, 36, 37, 39). In the murine model, the primary target of infection is the kidney, with central nervous system disease also occurring; this is a model of acutely fatal disease, with most deaths occurring between days 3 to 11 (16, 23). Central nervous system infection spontaneously clears in mice that survive 15 to 20 days of infection; kidney infection clears to a lesser extent (16, 23). Because we wished to assess therapeutic efficacy with respect to the clearance of the infectious burden, the studies were ended on day 9 after infection to minimize the difficulties in interpretation presented by spontaneous clearance of fungal burden.
Overall, these studies indicate that the lipid preparations are equivalent in efficacy when given at equal doses of AMB. No formulation was consistently superior in either the prolongation of survival or clearance of infection, and no formulation at any dosage effected a cure of any animals in either study. No formulation showed a consistent clear dose responsiveness over the 10-fold range tested, further indicating that the question of optimal dosing of lipid-based formulations of AMB for treatment of aspergillosis remains to be answered (for a review, see reference 17). In comparison with conventional AMB, ABCD and ABLC might be considered about 10-fold less efficacious, whereas AmBi might be considered equivalent for the prolongation of survival and (if equal doses are compared) as <10-fold as efficacious (if 8 mg of AmBi per kg is compared with 0.8 mg of AMB per kg). However, clearance of the infectious burden showed that no regimen of a lipid preparation was superior to conventional AMB, even at 10 times the dose, in both studies. Taken together, the results suggest that ABCD and ABLC are less potent than conventional AMB on the basis of milligrams of AMB per kilogram of body weight, whereas AmBi was nearly equivalent to conventional AMB in potency.
These results are similar to those we described in a study of cryptococcosis, although the lipid formulations showed greater efficacy against brain infection, particularly at the higher dosages (12). The difference may be the progressive meningitis caused by Cryptococcus neoformans compared to more acute parenchymal abscess formation in aspergillosis or the result of the different treatment duration and dosing schedule (i.e., four doses rather than six or more and given every other day rather than daily), or it may reflect how refractory A. fumigatus is to treatment.
The lack of cure indicates that additional studies using different treatment durations and schedules are needed to determine if cure can be attained. The lack of dose responsiveness may reflect the attainment of the maximal activity against A. fumigatus at a relatively low dosage or altered pharmacokinetics in the presence of infection.
No preparation showed overt toxicity at the doses administered, and all provided some measure of protection, similar to the results of previous work (2-4, 27, 32, 36, 38). Whether the early deaths in the high-inoculum study in the ABCD group dosed at 8 mg/kg may have been due in part to drug toxicity is unknown. More frequent dosing might magnify potential drug toxicities that would appear during severe infection. Regardless, all formulations were nontoxic at dosages 10-fold greater than a near-toxic dosage of conventional AMB, similar to data previously reported against other models of fungal infection (5-12, 26). Lastly, each formulation showed efficacy at dosages similar to those in present clinical usage recommended for treatment of aspergillosis (34). Although we found no differences in the effectiveness of the lipid formulations, head-to-head clinical studies will be required to determine whether there is any advantage of a particular formulation. If there are differences for the preparations relative to each other in their pharmacokinetics in humans, compared to their pharmacokinetics in mice, the application of the present observations to clinical practice would need to be guarded.
Acknowledgments
These studies were funded in part by a grant from Sequus Pharmaceuticals, Inc., Menlo Park, Calif.
REFERENCES
- 1.Adler-Moore, J., and R. T. Proffitt. 2002. AmBisome: liposomal formulation, structure, mechanism of action and pre-clinical experience. J. Antimicrob. Chemother. 49 (Suppl. A):21-30. [DOI] [PubMed] [Google Scholar]
- 2.Allen, S. D., K. N. Sorensen, M. J. Nejdl, C. Durrant, and R. T. Proffit. 1994. Prophylactic efficacy of aerosolized liposomal (AmBisome) and non-liposomal (Fungizone) amphotericin B in murine pulmonary aspergillosis. J. Antimicrob. Chemother. 34:1001-1013. [DOI] [PubMed] [Google Scholar]
- 3.Allende, M. C., J. W. Lee, P. Francis, K. Garrett, H. Dollenberg, J. Berenguer, C. A. Lyman, P. A. Pizzo, and T. J. Walsh. 1994. Dose-dependent antifungal activity and nephrotoxicity of amphotericin B colloidal dispersion in experimental pulmonary aspergillosis. Antimicrob. Agents Chemother. 38:518-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark, J. M., R. R. Whitney, S. J. Olsen, R. J. George, M. R. Swerdel, L. Kunselman, and D. P. Bonner. 1991. Amphotericin B lipid complex therapy of experimental fungal infections in mice. Antimicrob. Agents Chemother. 35:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clemons, K. V., E. Brummer, and D. A. Stevens. 1994. Cytokine treatment of central nervous system infection: efficacy of interleukin-12 alone and synergy with conventional antifungal therapy in experimental cryptococcosis. Antimicrob. Agents Chemother. 38:460-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemons, K. V., R. A. Sobel, P. L. Williams, and D. A. Stevens. 2001. Comparative toxicities and pharmacokinetics of intrathecal lipid (amphotericin B colloidal dispersion) and conventional deoxycholate formulations of amphotericin B in rabbits. Antimicrob. Agents Chemother. 45:612-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemons, K. V., and D. A. Stevens. 1991. Comparative efficacies of amphotericin B lipid complex and amphotericin B deoxycholate suspension against murine blastomycosis. Antimicrob. Agents Chemother. 35:2144-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemons, K. V., and D. A. Stevens. 1991. Comparative efficacy of amphotericin B colloidal dispersion and amphotericin B deoxycholate suspension in treatment of murine coccidioidomycosis. Antimicrob. Agents Chemother. 35:1829-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemons, K. V., and D. A. Stevens. 1992. Efficacies of amphotericin B lipid complex (ABLC) and conventional amphotericin B against murine coccidioidomycosis. J. Antimicrob. Chemother. 30:353-363. [DOI] [PubMed] [Google Scholar]
- 10.Clemons, K. V., and D. A. Stevens. 1993. Comparison of a liposomal amphotericin formulation (AmBisome) and deoxycholate amphotericin (Fungizone) for the treatment of paracoccidioidomycosis. J. Med. Vet. Mycol. 31:387-394. [Google Scholar]
- 11.Clemons, K. V., and D. A. Stevens. 1993. Therapeutic efficacy of a liposomal formulation of amphotericin B (AmBisome) against murine blastomycosis. J. Antimicrob. Chemother. 32:465-472. [DOI] [PubMed] [Google Scholar]
- 12.Clemons, K. V., and D. A. Stevens. 1998. Comparison of Fungizone, Amphotec, AmBisome, and Abelcet for treatment of systemic murine cryptococcosis. Antimicrob. Agents Chemother. 42:899-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denning, D. W. 1994. Treatment of invasive aspergillosis. J. Infect. 28 (Suppl. 1):25-33. [DOI] [PubMed] [Google Scholar]
- 14.Denning, D. W. 1996. Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis. 23:608-615. [DOI] [PubMed] [Google Scholar]
- 15.Denning, D. W., and D. A. Stevens. 1990. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev. Infect. Dis. 12:1147-1201. [DOI] [PubMed] [Google Scholar]
- 16.Denning, D. W., and D. A. Stevens. 1991. Efficacy of cilofungin alone and in combination with amphotericin B in a murine model of disseminated aspergillosis. Antimicrob. Agents Chemother. 35:1329-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis, M. 2000. Amphotericin B preparations: a maximum tolerated dose in severe invasive fungal infections? Transpl. Infect. Dis. 2:51-61. [DOI] [PubMed] [Google Scholar]
- 18.Francis, P., J. W. Lee, A. Hoffman, J. Peter, A. Francesconi, J. Bacher, J. Shelhamer, P. A. Pizzo, and T. J. Walsh. 1994. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar d-mannitol and serum galactomannan as markers of infection. J. Infect. Dis. 169:356-368. [DOI] [PubMed] [Google Scholar]
- 19.Gallis, H. A., R. H. Drew, and W. W. Pickard. 1990. Amphotericin B: 30 years of clinical experience. Rev. Infect. Dis. 12:308-329. [DOI] [PubMed] [Google Scholar]
- 20.Groll, A. H., S. C. Piscitelli, and T. J. Walsh. 1998. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv. Pharmacol. 44:343-495. [DOI] [PubMed] [Google Scholar]
- 21.Guo, L. S. 2001. Amphotericin B colloidal dispersion: an improved antifungal therapy. Adv. Drug Deliv. Rev. 47:149-163. [DOI] [PubMed] [Google Scholar]
- 22.Guo, L. S. S., R. M. Fielding, D. D. Lasic, R. L. Hamilton, and D. Mufson. 1991. Novel antifungal drug delivery: stable amphotericin B-cholesteryl sulfate discs. Int. J. Pharmaceut. 75:45-54. [Google Scholar]
- 23.Hanson, L. H., K. V. Clemons, D. W. Denning, and D. A. Stevens. 1995. Efficacy of oral saperconazole in systemic murine aspergillosis. J. Med. Vet. Mycol. 33:311-317. [DOI] [PubMed] [Google Scholar]
- 24.Hiemenz, J. W., and T. J. Walsh. 1996. Lipid formulations of amphotericin B: recent progress and future directions. Clin. Infect. Dis. 22 (Suppl. 2):S133-S144. [DOI] [PubMed] [Google Scholar]
- 25.Hospenthal, D. R., J. C. Byrd, and R. B. Weiss. 1995. Successful treatment of invasive aspergillosis complicating prolonged treatment-related neutropenia in acute myelogenous leukemia with amphotericin B lipid complex. Med. Pediatr. Oncol. 25:119-122. [DOI] [PubMed] [Google Scholar]
- 26.Hostetler, J. S., K. V. Clemons, L. H. Hanson, and D. A. Stevens. 1992. Efficacy and safety of amphotericin B colloidal dispersion compared with those of amphotericin B deoxycholate suspension for treatment of disseminated murine cryptococcosis. Antimicrob. Agents Chemother. 36:2656-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janoff, A. S., L. T. Boni, M. C. Popescu, S. R. Minchey, P. R. Cullis, T. D. Madden, T. Taraschi, S. M. Gruner, E. Shyamsunder, M. W. Tate, R. Mendelsohn, and D. Bonner. 1988. Unusal lipid structures selectively reduce the toxicity of amphotericin B. Proc. Natl. Acad. Sci. USA 85:6122-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lachin, J. M. 1999. Worst-rank score analysis with informatively missing observations in clinical trials. Control. Clin. Trials 20:408-422. [DOI] [PubMed] [Google Scholar]
- 29.Lister, J. 1996. Amphotericin B lipid complex (Abelcet) in the treatment of invasive mycoses: the North American experience. Eur. J. Haematol. 56:18-23. [DOI] [PubMed] [Google Scholar]
- 30.Lyman, C. A., and T. J. Walsh. 1992. Systemically administered antifungal agents a review of their clinical pharmacology and therapeutic applications. Drugs 44:9-35. [DOI] [PubMed] [Google Scholar]
- 31.Oppenheim, B. A., R. Herbrecht, and S. Kusne. 1995. The safety and efficacy of amphotericin B colloidal dispersion in the treatment of invasive mycoses. Clin. Infect. Dis. 21:1145-1153. [DOI] [PubMed] [Google Scholar]
- 32.Patterson, T. F., P. Miniter, J. Dijkstra, F. C. Szoka, Jr., J. L. Ryan, and V. T. Andriole. 1989. Treatment of experimental invasive aspergillosis with novel amphotericin B/cholesterol-sulfate complexes. J. Infect. Dis. 159:717-724. [DOI] [PubMed] [Google Scholar]
- 33.Proffitt, R. T., A. Satorius, S. Chiang, L. Sullivan, and J. P. Adler-Moore. 1991. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J. Antimicrob. Chemother. 28 (Suppl. B):49-61. [DOI] [PubMed] [Google Scholar]
- 34.Stevens, D. A., V. L. Kan, M. A. Judson, V. A. Morrison, S. Dummer, D. W. Denning, J. E. Bennett, T. J. Walsh, T. F. Patterson, and G. A. Pankey. 2000. Practice guidelines for diseases caused by Aspergillus. Infectious Diseases Society of America. Clin. Infect. Dis. 30:696-709. [DOI] [PubMed] [Google Scholar]
- 35.Swenson, C. E., W. R. Perkins, P. Roberts, I. Ahmad, R. Stevens, D. A. Stevens, and A. S. Janoff. 1998. In vitro and in vivo antifungal activity of amphotericin B lipid complex: are phospholipases important? Antimicrob. Agents Chemother. 42:767-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walsh, T. J., K. Garrett, E. Feurerstein, M. Girton, M. Allende, J. Bacher, A. Francesconi, R. Schaufele, and P. A. Pizzo. 1995. Therapeutic monitoring of experimental invasive pulmonary aspergillosis by ultrafast computerized tomography, a novel, noninvasive method for measuring responses to antifungal therapy. Antimicrob. Agents Chemother. 39:1065-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh, T. J., J. W. Hiemenz, N. L. Seibel, J. R. Perfect, G. Horwith, L. Lee, J. L. Silber, M. J. DiNubile, A. Reboli, E. Bow, J. Lister, and E. J. Anaissie. 1998. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin. Infect. Dis. 26:1383-1396. [DOI] [PubMed] [Google Scholar]
- 38.Walsh, T. J., A. J. Jackson, J. W. Lee, M. Amantea, T. Sein, J. Bacher, and L. Zech. 2000. Dose-dependent pharmacokinetics of amphotericin B lipid complex in rabbits. Antimicrob. Agents Chemother. 44:2068-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh, T. J., N. L. Seibel, C. Arndt, R. E. Harris, M. J. Dinubile, A. Reboli, J. Hiemenz, and S. J. Chanock. 1999. Amphotericin B lipid complex in pediatric patients with invasive fungal infections. Pediatr. Infect. Dis. J. 18:702-708. [DOI] [PubMed] [Google Scholar]