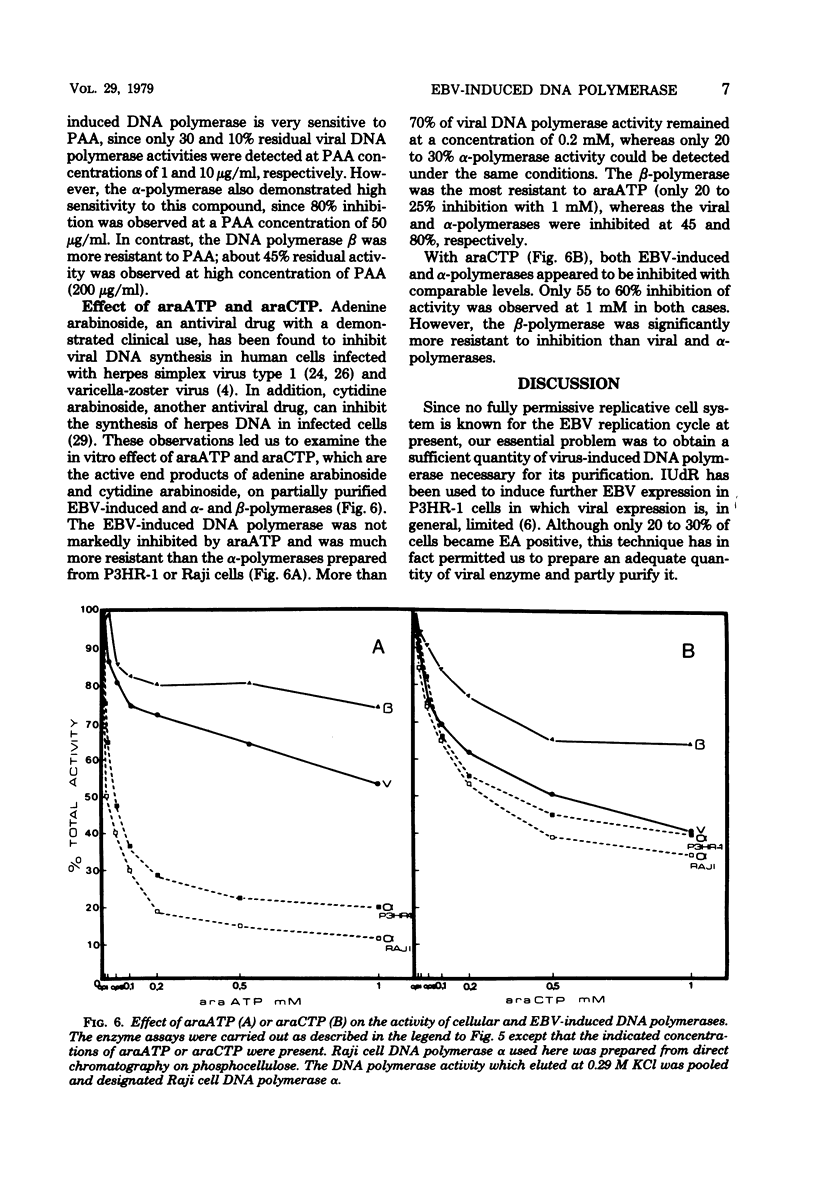
Abstract
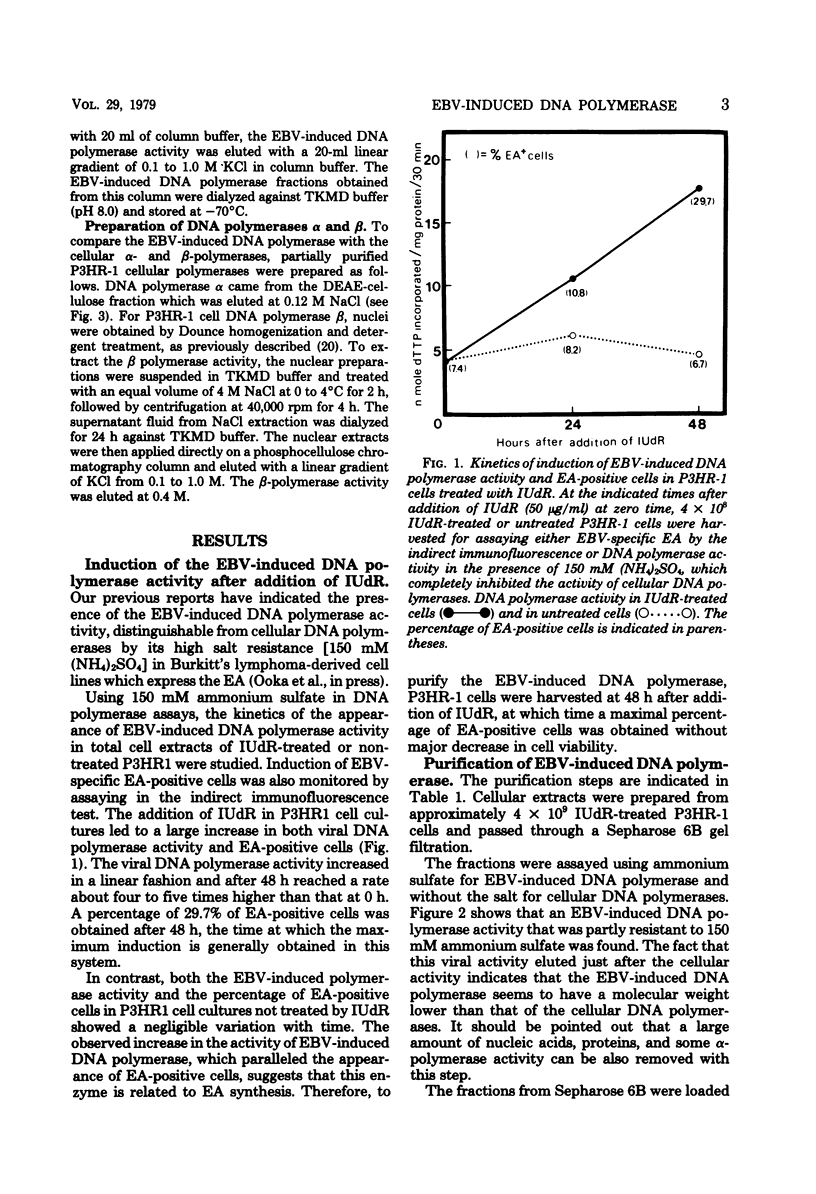
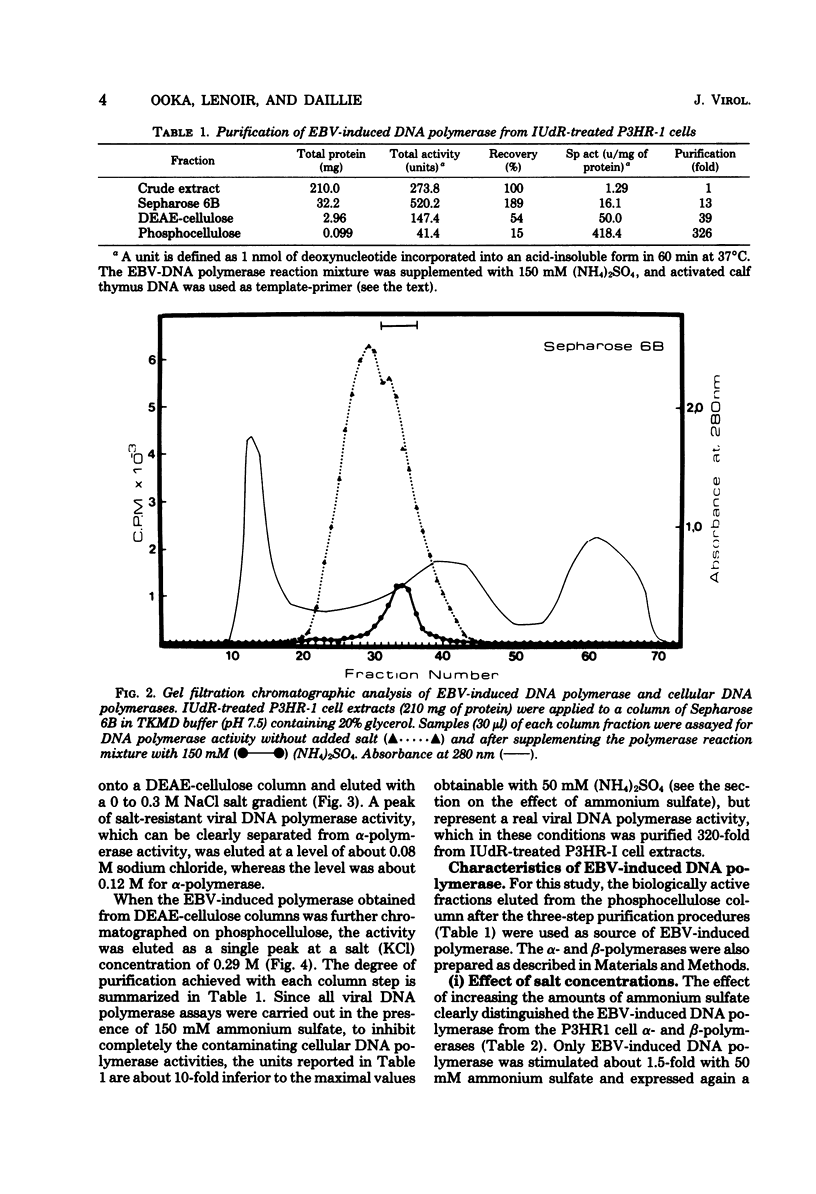
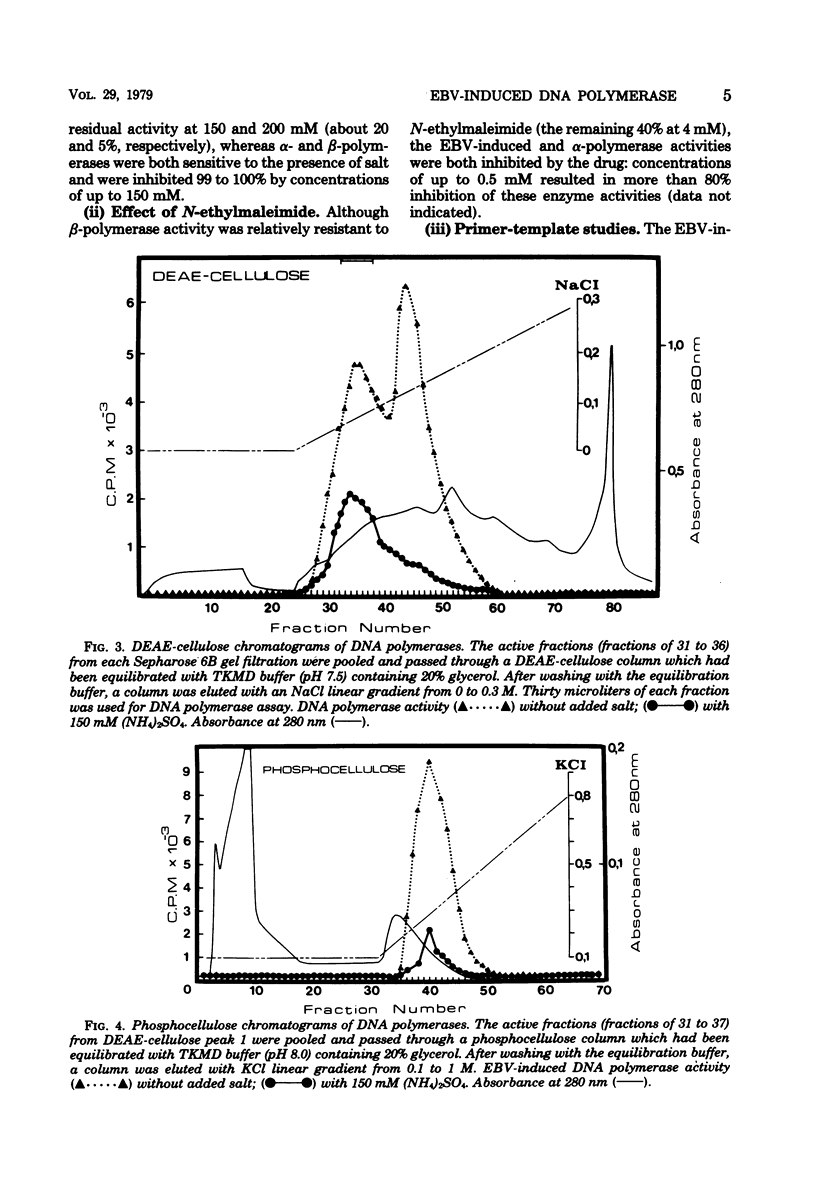
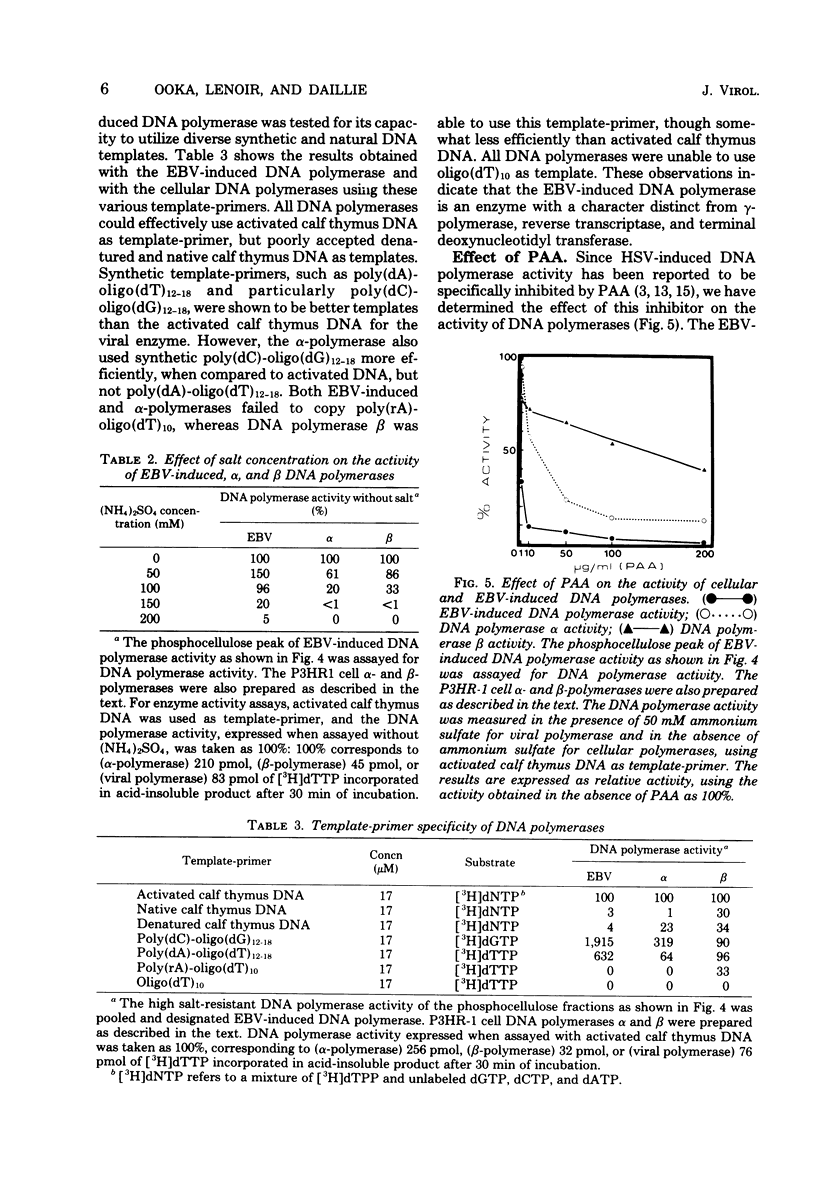
The addition of iododeoxyuridine to P3HR-I cell cultures led to a large increase in both Epstein-Barr virus (EBV)-induced DNA polymerase activity and early antigen-positive cells. This EBV-induced DNA polymerase was separated from the cellular alpha- and beta-polymerases by sequential column chromatography on Sepharose 6B, DEAE-cellulose, and phosphocellulose, resulting in partial purification of about 320-fold. The partially purified-EBV DNA polymerase could be distinguished from the cellular DNA polymerases by its activation by salts, its catalytic properties, and its degree of sensitivity to N-ethylmaleimide, phosphonoacetic acid, araATP, and araCTP. The viral polymerase showed properteis similar to those reported for other herpesvirus DNA polymerases. The enzyme exhibited optimal activity for copying activated calf DNA in the presence of 50 mH (NH4)2SO4 and was resistant to 150 mM (NH4)2SO4. It utilized with high efficiency template-primer poly(dC)-oligo(dG)12-18 or poly(dA)-oligo(dT)12-18, but failed to copy poly(rA)-oligo(dT)10 and oligo(dT)10, indicating that this enzyme has characters distinct from DNA polymerase gamma, reverse transcriptase, and terminal deoxynucleotidyl transferase. Phosphonacetic acid inhibited not only EBV DNA polymerase, but also, to a lesser degree, the cellular polymerase alpha. AraATP did not severely inhibit viral activity, whereas the polymerase alpha was inhibited most effectively. Both EBV polymerase and polymerase alpha were inhibited at a comparable level by araCTP.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. P., O'Callaghan D. J., Randall C. C. Purification and characterization of equine herpesvirus-induced DNA. Virology. 1977 Jan;76(1):395–408. doi: 10.1016/0042-6822(77)90311-7. [DOI] [PubMed] [Google Scholar]
- Bolden A., Aucker J., Weissbach A. Synthesis of herpes simplex virus, vaccinia virus, and adenovirus DNA in isolated HeLa cell nuclei. I. Effect of viral-specific antisera and phosphonoacetic acid. J Virol. 1975 Dec;16(6):1584–1592. doi: 10.1128/jvi.16.6.1584-1592.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson Y. J., Connor J. D. In vitro susceptibility of varicella zoster virus to adenine arabinoside and hypoxanthine arabinoside. Antimicrob Agents Chemother. 1976 Mar;9(3):540–543. doi: 10.1128/aac.9.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Perdue M. L., Randall C. C., O'Callaghan D. J. Replication of equine herpesvirus type I: resistance to hydroxyurea. Virology. 1975 Sep;67(1):56–67. doi: 10.1016/0042-6822(75)90402-x. [DOI] [PubMed] [Google Scholar]
- Hampar B., Derge J. G., Tanaka A., Nonoyama M. Sequence of Epstein-Barr virus productive cycle in human lymphoblastoid cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):811–815. doi: 10.1101/sqb.1974.039.01.094. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y., Grace J. T., Jr Cloning of immunoglobulin-producing human leukemic and lymphoma cells in long-term cultures. Proc Soc Exp Biol Med. 1967 Jan;124(1):107–111. doi: 10.3181/00379727-124-31677. [DOI] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. III. Virus-induced DNA polymerase. J Virol. 1975 Aug;16(2):298–310. doi: 10.1128/jvi.16.2.298-310.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. IV. Specific inhibition of virus-induced DNA polymerase activity and viral DNA replication by phosphonoacetic acid. J Virol. 1975 Dec;16(6):1560–1565. doi: 10.1128/jvi.16.6.1560-1565.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir H. M., Subak-Sharpe H., Shedden W. I., Watson D. H., Wildy P. Immunological evidence for a specific DNA polymerase produced after infection by herpes simplex virus. Virology. 1966 Sep;30(1):154–157. doi: 10.1016/s0042-6822(66)81022-x. [DOI] [PubMed] [Google Scholar]
- Kemp M. C., Perdue M. L., Rogers H. W., O'Callaghan D. J., Randall C. C. Structural polypeptides of the hamster strain of equine herpes virus type 1: products associated with purification. Virology. 1974 Oct;61(2):361–375. doi: 10.1016/0042-6822(74)90274-8. [DOI] [PubMed] [Google Scholar]
- Lee L. F., Boezi J. A., Blakesley R. W., Koenig M., Towle H. C. Marek's disease herpesvirus-induced DNA polymerase. J Virol. 1974 Nov;14(5):1209–1219. doi: 10.1128/jvi.14.5.1209-1219.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinbach S. S., Reno J. M., Lee L. F., Isbell A. F., Boezi J. A. Mechanism of phosphonoacetate inhibition of herpesvirus-induced DNA polymerase. Biochemistry. 1976 Jan 27;15(2):426–430. doi: 10.1021/bi00647a029. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E. Mode of inhibition of herpes simplex virus DNA polymerase by phosphonoacetate. Biochemistry. 1975 Dec 16;14(25):5475–5479. doi: 10.1021/bi00696a015. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Glaser R., Rapp F. Studies of an Epstein-Barr virus-induced DNA polymerase. Virology. 1977 Feb;76(2):494–502. doi: 10.1016/0042-6822(77)90232-x. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Rapp F. Varicella-zoster virus-induced DNA polymerase. J Gen Virol. 1977 Sep;36(3):515–524. doi: 10.1099/0022-1317-36-3-515. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Zahn R. K., Falke D. Variation of DNA polymerase and RNA polymerase activities in cells infected with herpes simplex virus type 1. Virology. 1978 Feb;84(2):320–330. doi: 10.1016/0042-6822(78)90251-9. [DOI] [PubMed] [Google Scholar]
- Ooka T., Daillie J. Studies on changes in DNA polymerase activity during the cell cycle in synchronized KB cells. Biochimie. 1975;57(2):235–246. doi: 10.1016/s0300-9084(75)80170-2. [DOI] [PubMed] [Google Scholar]
- Overby L. R., Robishaw E. E., Schleicher J. B., Rueter A., Shipkowitz N. L., Mao J. C. Inhibition of herpes simplex virus replication by phosphonoacetic acid. Antimicrob Agents Chemother. 1974 Sep;6(3):360–365. doi: 10.1128/aac.6.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Purifoy D. J. Nonstructural proteins of herpes simplex virus. I. Purification of the induced DNA polymerase. J Virol. 1977 Nov;24(2):618–626. doi: 10.1128/jvi.24.2.618-626.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz P. M., Shipman C., Jr, Drach J. C. Antiviral activity of arabinosyladenine and arabinosylhypoxanthine in herpes simplex virus-infected KB cells: selective inhibition of viral deoxyribonucleic acid synthesis in the presence of an adenosine deaminase inhibitor. Antimicrob Agents Chemother. 1976 Jul;10(1):64–74. doi: 10.1128/aac.10.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebeck T., Shaw J. E., Pagano J. S. Synthesis of Epstein-Barr virus DNA in vitro: effects of phosphonoacetic acid, N-ethylmaleimide, and ATP. J Virol. 1977 Jan;21(1):435–438. doi: 10.1128/jvi.21.1.435-438.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman C., Jr, Smith S. H., Carlson R. H., Drach J. C. Antiviral activity of arabinosyladenine and arabinosylhypoxanthine in herpes simplex virus-infected KB cells: selective inhibition of viral deoxyribonucleic acid synthesis in synchronized suspension cultures. Antimicrob Agents Chemother. 1976 Jan;9(1):120–127. doi: 10.1128/aac.9.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. C., Klein G. Inhibition of Epstein-Barr virus DNA synthesis and late gene expression by phosphonoacetic acid. J Virol. 1976 Apr;18(1):151–155. doi: 10.1128/jvi.18.1.151-155.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D., Strominger J. L. Transformation of human lymphocytes by Epstein-Barr virus is inhibited by phosphonoacetic acid. Nature. 1976 Sep 23;263(5575):332–334. doi: 10.1038/263332a0. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Stevens J. G. Effect of cytosine arabinoside on viral-specific protein synthesis in cells infected with herpes simplex virus. J Virol. 1975 Jan;15(1):71–80. doi: 10.1128/jvi.15.1.71-80.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach A., Hong S. C., Aucker J., Muller R. Characterization of herpes simplex virus-induced deoxyribonucleic acid polymerase. J Biol Chem. 1973 Sep 25;248(18):6270–6277. [PubMed] [Google Scholar]
- Yajima Y., Tanaka A., Nonoyama M. Inhibition of productive replication of Epstein-Barr virus DNA by phosphonoacetic acid. Virology. 1976 May;71(1):352–354. doi: 10.1016/0042-6822(76)90119-7. [DOI] [PubMed] [Google Scholar]



