Abstract
We evaluated the antifungal activities of amphotericin B, fluconazole, and flucytosine, alone and in combination, in a murine model of cryptococcal meningitis. The objectives were to determine the greatest antifungal effects achievable with these drugs alone or in combination. Meningitis was established in male BALB/c mice weighing 23 to 25 g by intracerebral injection of Cryptococcus neoformans. Treatment was started on day 2. Amphotericin B was tested at 0.3 to 1.3 mg/kg of body weight/day by slow intravenous injection. Fluconazole at 10 to 40 mg/kg/day and flucytosine at 20 to 105 mg/kg/day were administered in the sole source of drinking water. The mice were killed at 16 days, and the numbers of fungal colonies in the brain were quantified. The association between the response and the dose combination was evaluated by local nonparametric response surface methods; 99% confidence intervals were used to evaluate the antifungal effects. Ninety-five percent of the mice treated with amphotericin B at 0.5 mg/kg survived to the end of the experiment, regardless of the fluconazole or flucytosine dose used. The greatest activity was seen with amphotericin B plus fluconazole with or without flucytosine. However, the addition of flucytosine did not increase the antifungal activity. Given the widespread availability of amphotericin B and fluconazole and the relative safety profile of fluconazole compared to that of flucytosine, the full potential of this two-drug combination deserves further evaluation.
Cryptococcus neoformans is one of the most common central nervous system pathogens worldwide, courtesy of the AIDS epidemic. Worldwide, it is estimated that 25 to 30% of persons with AIDS will die as a consequence of cryptococcal meningitis (12, 14, 23, 27, 28). Fortunately, the widespread availability of testing for human immunodeficiency virus (HIV) infection combined with antiviral therapy has markedly diminished the frequency of cryptococcal meningitis in persons with AIDS in the United States and Western Europe (6). However, even in the United States and Western Europe, some 20% of persons infected with HIV are unaware of their HIV infection status. In addition, among individuals who progress to severe immunosuppression, i.e., AIDS, about 2 to 5% will develop cryptococcal meningitis annually (16). As recently shown in a prospective clinical trial, the overall outcome of cryptococcal meningitis in the United States is similar to that in medically less affluent societies, e.g., only 202 of 381 (53%) had been successfully treated by one of the four treatment strategies by 10 weeks (31). This is strikingly similar to the 55% success rate reported by White and colleagues in Thailand (27).
Progress in antifungal drug treatment for cryptococcal meningitis has been terribly slow. Amphotericin B was introduced in the late 1950s and remains the cornerstone of therapy (29). While alternative formulations of the drug are now available (lipid encapsulation), the parent compound remains the active agent in these lipid-based drugs (20). Flucytosine was introduced in the late 1970s, and the azoles itraconazole and fluconazole were introduced in the late 1980s and early 1990s. In total, these are the principal agents upon which clinicians must rely. New additions to the antifungal drug armament either have no demonstrated activity against C. neoformans (echinocandins) or have not been tested for their activities against human cryptococcal disease (voriconazole, an expanded-spectrum azole). Improvements in the treatment of cryptococcal meningitis have come primarily from the use of combination antifungal chemotherapy and attention to raised intracranial pressure (21, 25, 26). However, the frequent need to use toxic agents, particularly the combination of amphotericin B and flucytosine, and the recent emergence of fluconazole-resistant isolates of C. neoformans highlight the need for improved treatment regimens and the use of combination therapy to forestall the emergence of resistant organisms (1, 2, 5, 13). This study evaluated the combinations of fluconazole, flucytosine, and amphotericin B in a murine model of cryptococcal meningitis.
(This study was presented in part at the 5th International Conference on Cryptococcus and Cryptococcosis, Adelaide, Australia, March 2002.)
MATERIALS AND METHODS
Animal protocol
Male BALB/c mice weighing 23 to 25 g were used in both experiments. The animals were tagged and weighed individually, housed in isolation cages at four or five mice per cage, and given free access to food and water. The mice were briefly anesthetized with isoflurane and challenged intracerebrally with approximately 700 CFU of C. neoformans (University of Southern California clinical isolate 1597). As described previously (9, 10, 19), the inoculum was delivered in a volume of 0.06 ml through a 27-gauge needle by direct puncture through the cranial vault approximately 6 mm posterior to the orbit. The animal protocol was approved by the University of Southern California Institutional Animal Care and Use Committee.
Chemotherapy
Four or five mice were randomly to each of the treatment combinations 2 days after intracerebral challenge, when treatment with the assigned concentrations of fluconazole with or without flucytosine dissolved in the sole source of drinking water was initiated as described previously (9, 10, 19). Amphotericin B was reconstituted in sterile water and diluted with sterile 5% dextrose on the day of administration, and the assigned dose level was delivered in a volume of 0.2 ml. Amphotericin B was administered daily by slow intravenous push via the lateral tail vein. The original experimental plan was to test amphotericin B alone at 0.3 to 1.3 mg/kg of body weight/day and to test amphotericin B at 0.7 mg/kg/day combined with fluconazole at 10 to 40 mg/kg/day with or without flucytosine at 20 to 105 mg/kg/day. However, due to the number of deaths observed during or immediately after the first dose of amphotericin B at 0.7 mg/kg or higher (see Results), all subsequent doses of amphotericin B were administered at 0.5 mg/kg daily.
Two experiments were conducted: each experiment included untreated controls and tested amphotericin B alone, fluconazole alone at 10 to 40 mg/kg/day, and amphotericin B plus fluconazole. In the first experiment, fluconazole was tested in combination with flucytosine at 20 to 105 mg/kg/day with or without amphotericin B. The second experiment included amphotericin B at 0.5 mg/kg/day plus flucytosine at 20 to 100 mg/kg/day. Flucytosine was not tested alone in either experiment since no animals treated with flucytosine alone survived to the end of the experiment in our previous experiments with this isolate (9, 10). Table 1 shows the actual dose combinations of the three drugs tested. In general, four to five mice were used to test each combination, based on design principles developed by Bauer et al. (M. Bauer, A. M. Thomas, J. R. Graybill, and R. A. Larsen, Proc. 2001 S-Plus User Conf., 2001). In both studies, all animals received 6 mg of acetaminophen per kg in the drinking water for pain relief. All treatments were initiated on day 2 and were continued for 14 days.
TABLE 1.
Survival at day 16 by dose of fluconazole with or without flucytosine and without or with amphotericin B
| Fluconazole dose (mg/kg/day) | % of mice alive at day 16 after treatment with flucytosine at (mg/kg/day):
|
||||||
|---|---|---|---|---|---|---|---|
| 0 | 20 | 35 | 50 | 65 | 90 | 105 | |
| Without amphotericin B | |||||||
| 0 | 0 | ||||||
| 10 | 50 | 25 | 0 | 50 | 0 | ||
| 16 | 75 | 75 | 100 | 100 | |||
| 24 | 100 | 75 | 100 | ||||
| 32 | 100 | 100 | 100 | 100 | |||
| 40 | 100 | 100 | 75 | 100 | 100 | ||
| With amphotericin B | |||||||
| 0 | 70 | 75 | 75 | 100 | 100 | 100 | |
| 10 | 100 | 100 | 100 | 67 | 100 | ||
| 16 | 100 | 100 | 100 | 100 | |||
| 24 | 100 | 100 | 100 | ||||
| 32 | 100 | 100 | 100 | 100 | |||
| 40 | 100 | 100 | 75 | 100 | 100 | ||
Mycologic procedures
The C. neoformans isolate used in the study (isolate USC 1597) was obtained from a patient with AIDS-associated cryptococcal meningitis who responded promptly to treatment with fluconazole and flucytosine. For measurement of the brain fungal burden, the animals were killed and the brains were removed, weighed, and homogenized in 1.0 ml of normal saline. Serial dilutions of the whole-brain homogenate were prepared for quantitative counts on Sabouraud dextrose agar, the plates were incubated at 35°C for 72 to 96 h, and the numbers of organisms were recorded. In addition, the remaining original whole-brain homogenate was plated on a large pour plate and diluted with 30 ml of molten agar (48°C) to assess the samples for low colony counts or sterility.
Measurement of efficacy
As described previously (9, 10), treatment activity was measured by mycologic evaluation of brain tissue and the weight change relative to the initial weight for animals alive at the end of treatment. Analysis of antifungal activity for treated animals was based on the numbers of CFU of C. neoformans per gram of brain tissue.
Statistical analysis
The primary objective of these experiments was to determine the greatest antifungal effect that could be achieved with the drugs used alone or in combination. Data from the two experiments were combined, and local nonparametric regression (“loess”) was used to estimate the dose-response surface, as described previously (7, 8, 9, 10, 19). Pointwise 99% confidence intervals (CIs) for the estimated response were used to identify the range of doses with the greatest response (3). The 99% CIs were also used to evaluate the differences between the two experiments. The duration of survival for untreated controls was estimated by the Kaplan-Meier method (18). CIs for the proportion of mice surviving up to a particular time point were estimated by Greenwood's formula by the method described elsewhere (15, 22). Descriptive statistics were based on medians and robust CIs (17). All statistical analyses were performed by using S-Plus software (32; S-Plus guide to statistical and mathematical analysis, S-Plus 2000 for Windows; Insightful, Inc., Seattle, Wash.).
RESULTS
C. neoformans susceptibility
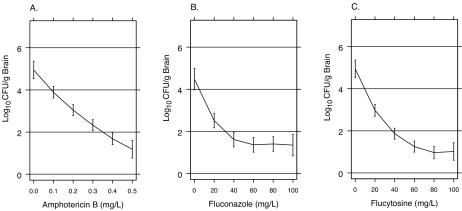
The susceptibility of C. neoformans isolate 1597 to amphotericin B, fluconazole, and flucytosine was measured by the National Committee for Clinical Laboratory Standards broth macrodilution method (24). The numbers of viable CFU per milliliter after 48 h for each drug concentration were assessed quantitatively by plating serial dilutions on Sabouraud dextrose agar (Fig. 1).
FIG. 1.
In vitro susceptibility of C. neoformans isolate USC 1597 to amphotericin B (A), fluconazole (B), and flucytosine (C). The curves show the loess fits of the association between drug concentration and the numbers of CFU per milliliter (log10) after 48 h of incubation. Vertical lines above and below the curves show the 99% CIs for log10 CFU per milliliter.
Amphotericin B toxicity
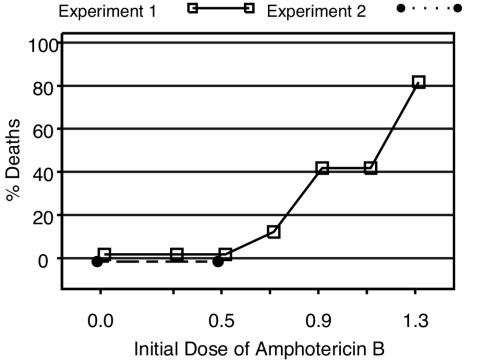
The plan for the first experiment was to test amphotericin B alone at 0.3 to 1.3 mg/kg/day and to test amphotericin B at 0.7 mg/kg/day in combination with fluconazole with or without flucytosine. However, the administration of amphotericin B at doses of 0.7 mg/kg/day or higher resulted in an unacceptable number of deaths within the first 24 h (Fig. 2). All untreated mice and mice treated with amphotericin B at 0.3 to 0.5 mg/kg survived more than 24 h. Six percent of mice treated with amphotericin B at 0.7 mg/kg, 40% of mice treated with amphotericin B at 0.9 mg/kg, and 80% of mice treated with amphotericin B at ≥1.1 mg/kg died during or immediately following the first dose. Consequently, animals originally assigned to receive amphotericin B at doses ≥0.7 mg/kg were treated at 0.5 mg/kg/day after the first dose. A total of 98% of the assigned amphotericin B doses were administered successfully.
FIG. 2.
Proportion of deaths within the first 24 h of administration of the first dose of amphotericin B by dose level.
C. neoformans growth curve for untreated control mice
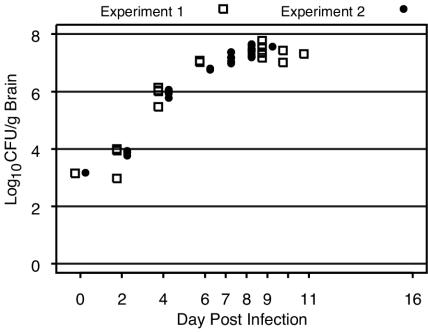
Figure 3 shows the in vivo growth curve for C. neoformans by day postinfection for untreated control animals. The median inoculum level was 670 CFU of C. neoformans (99% CI, 642 to 697 CFU) for the first experiment and 660 CFU (99% CI, 610 to 709 CFU) for the second experiment. Untreated mice tolerated untreated infection for up to 7 days, with the counts being ∼107 CFU/g of brain tissue before the mice exhibited signs of distress or death. The median length of survival was 8 days (99% CI, 7 to 9 days) in each experiment (data not shown). No untreated mice survived beyond day 11 in either experiment.
FIG. 3.
Numbers of CFU of C. neoformans per gram of brain tissue by day postinfection for untreated control animals. The symbols at day 0 represent the median inoculum level (log10 CFU per gram of brain tissue) in experiments 1 and 2.
Survival of treated animals
Table 1 shows the proportion of mice alive at the end of treatment with fluconazole alone, amphotericin B alone, fluconazole and flucytosine, fluconazole and amphotericin B, and all three drugs (Table 1). As stated above, no mice were treated with flucytosine alone. Twenty-nine percent of mice treated with fluconazole at 10 mg/kg/day and 96% of mice treated with fluconazole at ≥16 mg/kg/day without amphotericin B (Table 1) were alive at the end of treatment, regardless of the dose of flucytosine used. Seventy percent of mice treated with amphotericin B at 0.5 mg/kg/day alone and 100% of mice treated with amphotericin B at 0.5 mg/kg/day combined with flucytosine at ≥65 mg/kg/day survived to day 16 (Table 1). Ninety-eight percent of mice treated with amphotericin B at 0.5 g/kg/day plus fluconazole at ≥10 mg/kg/day (Table 1) were alive at the end of the experiment, regardless of the flucytosine dose.
Weight change
Untreated mice lost weight rapidly, losing more than 25% of their initial weight within 7 days (data not shown). Animals treated with amphotericin B at 0.5 mg/kg/day alone or combined with fluconazole with or without flucytosine maintained their initial weight. Animals treated with fluconazole at doses ≥24 mg/kg/day alone or combined with flucytosine showed some weight gain.
Antifungal activity
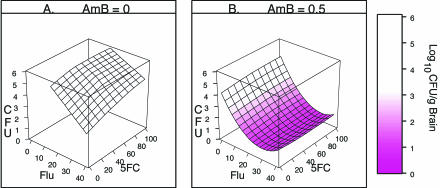
The dose-response surfaces in Fig. 4 show the loess fit of the association between the numbers of CFU per gram of brain tissue and the dose combination for mice alive at day 16. The response surfaces were fit separately because of the effect of amphotericin B on the fluconazole dose range that provided 100% survival (Table 1). Figure 4A shows the loess response surface for fluconazole at 16 to 40 mg/kg/day with or without flucytosine and without amphotericin B obtained by use of a regression model that was locally linear for each drug. Figure 4B shows the loess response surface for fluconazole with or without flucytosine combined with amphotericin B obtained by use of a model that was locally quadratic for fluconazole and locally linear for flucytosine.
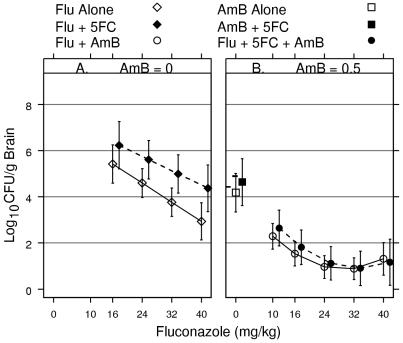
FIG. 4.
Response surfaces showing the association between numbers of CFU per gram of brain tissue and dose combinations of fluconazole (Flu) with or without flucytosine (5FC) and without amphotericin B (AmB) (A) and fluconazole with or without flucytosine and with amphotericin B (B). The color key shows the levels of response (numbers of log10 CFU per gram of brain tissue) associated with shades of pink.
The shades of pink in Fig. 4 show the level of response (numbers of log10 CFU per gram of brain tissue), with the deepest pink showing responses below 1.5 log10 CFU/g of brain tissue and white showing responses >3.5 log10 CFU/g of brain tissue. The left front edge of the response surface in Fig. 4A shows the dose-response curve for fluconazole alone; the right back edge shows the dose-response curve for fluconazole combined with flucytosine at 105 mg/kg/day. Similarly, in Fig. 4B the left front edge of the surface shows the dose-response curve for fluconazole with amphotericin B but without flucytosine, and the right back edge shows the dose-response curve for fluconazole with amphotericin B combined with flucytosine at 105 mg/kg/day.
Figure 4A shows that the numbers of CFU per gram increase as the dose of flucytosine increases; Fig. 4B shows that flucytosine has no apparent effect on the level of response. In order to assess the significance of the effects of flucytosine and amphotericin B on the fluconazole dose-response, the graphs need to include information regarding the uncertainty of the response. Figure 5A and B shows the dose-response curves for fluconazole alone and fluconazole combined with flucytosine at the highest dose tested (105 g/kg/day) based on the response surfaces shown in Fig. 4A and B, respectively. The vertical lines extending above and below the symbols represent the limits of the 99% CIs for the fitted response. If the vertical lines do not overlap, the CIs do not overlap. If the CIs for one combination do not overlap the 99% CIs for the other combinations, the difference is significant at the 0.01 level. If the 99% CIs do overlap, the magnitude of the difference is not significant at the 0.01 level. Thus, CIs provide the level of significance as well as information about the location, magnitude, and direction of the treatment effect.
FIG. 5.
Dose-response curves for fluconazole (Flu) without flucytosine (5FC) (open symbols) and for flucytosine at 105 mg/kg/day (filled symbols) without amphotericin B (AmB) (A) and with amphotericin B (B). The lines above and below the symbols represent the 99% CIs for the numbers of CFU per gram of brain. Horizontal references lines are shown at 2, 4, 6, and 8 log10 CFU/g to facilitate comparison of CIs among the dose regimens.
Antifungal effect of amphotericin B
The effect of amphotericin B depended on the range of fluconazole doses used. Amphotericin B combined with fluconazole at >10 mg/kg/day was better than fluconazole alone or fluconazole plus flucytosine (P < 0.01) since the 99% CIs do not overlap. In Fig. 5A, the 99% CIs for fluconazole with or without flucytosine are above the horizontal line at 2 log10 CFU/g of brain tissue for all fluconazole doses. In contrast, in Fig. 5B, the 99% CIs for fluconazole plus amphotericin B are below the horizontal line at 2 log10 CFU/g for fluconazole doses of >10 mg/kg/day.
Amphotericin B plus fluconazole at all dose levels was better than amphotericin B alone or amphotericin B combined with flucytosine (P < 0.01) since the 99% CIs for fluconazole plus amphotericin B are below the 99% CIs for amphotericin B alone and for amphotericin B plus flucytosine (Fig. 5B).
The effect of amphotericin B alone or combined with flucytosine (Fig. 5B) was not significantly different at the 0.01 level from that of fluconazole alone or fluconazole combined with flucytosine (Fig. 5A) since the CIs overlap.
Effect of flucytosine
The effect of the addition of flucytosine to fluconazole was not significant at the 0.01 level since the 99% CIs for fluconazole alone (Fig. 5A) overlap the 99% CIs for fluconazole combined with flucytosine at 105 mg/kg/day (Fig. 5A). The magnitude of the differences between these two regimens at all fluconazole dose levels are within the limits of these 99% CIs, indicating that there were no statistically significant differences between the two regimens at the 0.01 level.
The effect of the addition of flucytosine to amphotericin B was not significant at the 0.01 level since the 99% CI for amphotericin B plus flucytosine at 105 mg/kg/day (Fig. 5B) overlaps the 99% CI for amphotericin B alone (Fig. 5B).
The effect of the addition of flucytosine to amphotericin B plus fluconazole was not significant at the 0.01 level since the 99% CIs for all dose levels of fluconazole plus amphotericin B combined with flucytosine (Fig. 5B) overlap the CIs for fluconazole plus amphotericin B (Fig. 5B).
Due to the strong association between the growth curve for C. neoformans and the times of death (Fig. 3), analysis of antifungal activity was based on the numbers of mice alive at the end of the experiment. Thus, since no untreated animals survived beyond day 11, it was not possible to compare any of the treated groups to the untreated controls. Furthermore, evaluation of the response to fluconazole without amphotericin B (Fig. 4A and 5A) was based on data for animals treated with fluconazole at ≥16 mg/kg/day, due to the low proportion of animals alive at day 16 (29%; Table 1) for animals treated with fluconazole at <16 mg/kg/day without amphotericin B.
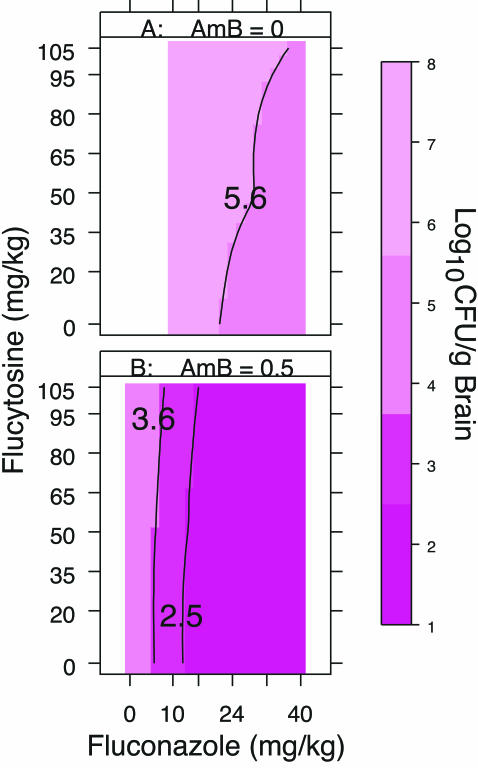
The plots in Fig. 6 show the contours of the upper limits of the 99% CIs for the numbers of log10 CFU per gram of brain tissue. The contours and shades of pink were used to identify the dose combinations that resulted in similar levels of response. For example, in Fig. 6A, the upper limits of the 99% CIs for dose combinations within the contour labeled 5.6 are <5.6 log10 CFU/g. As seen in Fig. 5A, the CIs for these dose combinations overlap the CIs for the best response obtained with fluconazole without amphotericin B. Thus, the responses to fluconazole in the range 24 to 40 mg/kg/day were similar, regardless of the dose of flucytosine used.
FIG. 6.
Contour plots of the upper limits of the 99% CIs for the numbers of CFU per gram of brain tissue for fluconazole with or without flucytosine and without amphotericin B (A) and with amphotericin B (B). The contours and shades of pink identify dose combinations with similar levels of response. The color key shows the CI level (numbers of log10 CFU per gram of brain tissue) associated with each shade of pink.
The darkest pink region in Fig. 6B, outlined by the black line labeled 2.5, shows the dose combinations for which the upper limit of the 99% CIs is <2.5 log10 CFU/g. As seen in Fig. 5B, the CIs for these dose combinations overlap the CIs for the best response. Thus, all combinations of amphotericin B at 0.5 mg/kg/day plus fluconazole at >10 mg/kg/day resulted in similar levels of response, regardless of the dose of flucytosine used.
DISCUSSION
Individuals who develop cryptococcal meningitis face a perilous course. The disease has an appreciable short-term mortality rate (about 25% in 10 weeks) and can leave a devastating aftermath following eradication of the organism from the central nervous system, including blindness, deafness, and/or an impaired sensorium. Antifungal drug treatments are toxic and frequently ineffective in eradicating central nervous system infection even after weeks of use. The toxicities of amphotericin B and flucytosine are particularly noteworthy since side effects are of sufficient magnitude and severity to limit the use of these drugs in nearly 50% of those who require 4 weeks of treatment (4, 30). This has led to the common practice of using amphotericin B with or without flucytosine for a relatively brief period (2 weeks), followed by azole therapy (31). Unfortunately, only a minority of subjects become culture negative by 2 weeks, and those who remain culture positive have a much higher probability of serious health consequences (28). The combination of amphotericin B plus fluconazole may ameliorate some of these difficulties. While both fluconazole and flucytosine are excreted renally and can be expected to accumulate as renal function deteriorates during amphotericin B use, the safety profile of fluconazole is far superior to that of flucytosine, even when high doses of fluconazole are used (11). Thus, if renal function deteriorates as a result of amphotericin B use, the increasing fluconazole levels are not likely to lead to adverse clinical effects. Additionally, the increased potencies of amphotericin B and fluconazole may lead to more rapid sterilization of the central nervous system, thus reducing the time during which amphotericin B must be administered.
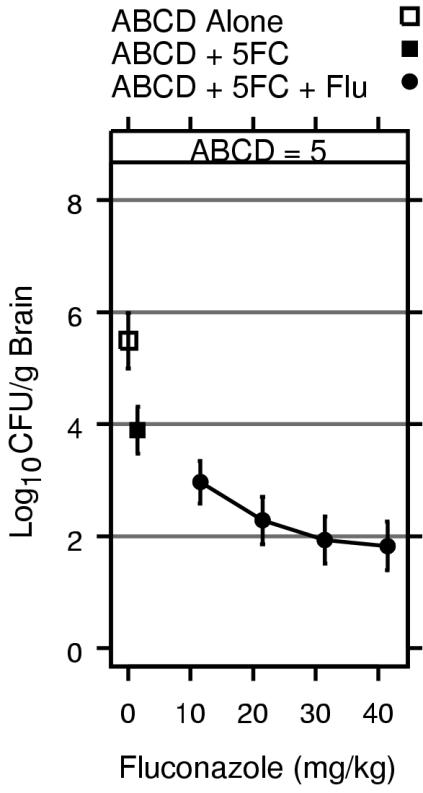
This study demonstrates that amphotericin B plus fluconazole is better than amphotericin B plus flucytosine and that the addition of flucytosine does not improve the antifungal effect of amphotericin B plus fluconazole. We have reported similar findings from studies with amphotericin B colloidal dispersion (ABCD) combined with fluconazole and flucytosine against the same isolate (9). However, we did not formally test the two-drug combination of amphotericin B plus fluconazole. Figure 7 shows that the greatest effect was seen with ABCD at 5.0 mg/kg/day plus fluconazole at 40 mg/kg/day combined with flucytosine at 100 mg/kg/day. The 99% CIs for the best response achieved with ABCD combined with fluconazole plus flucytosine in the study of Diamond et al. (9) (Fig. 7) overlap the 99% CIs for the best response achieved with amphotericin B plus fluconazole in the present study (Fig. 5B). However, in the present study, the best response was seen over a broader range of fluconazole doses.
FIG. 7.
Response for ABCD at 5 mg/kg/day alone, ABCD with flucytosine (5FC) at 100 mg/kg, and ABCD with flucytosine and fluconazole (Flu). The lines above and below the symbols represent the 99% CIs for the numbers of CFU per gram of brain tissue. Horizontal references lines are shown at 2, 4, 6, and 8 log10 CFU/g to facilitate comparison of CIs. Data are from Diamond et al. (9).
In the present study, we found that adding flucytosine to fluconazole alone did not improve the antifungal effect of fluconazole. This is in contrast to the findings of an earlier study (19), in which the best response (percentage of animals whose brains were sterile) required flucytosine in the range of 40 to 100 mg/kg/day combined with fluconazole at 24 to 40 mg/kg/day. We believe that these differences are due to the procedures used to assess the effects of antifungals on brain tissues. The previous study (19) focused on the drugs' abilities to render the surface of the brain sterile, i.e., assessment of the response to meningeal infection. In those experiments, response was measured by culturing the intact brain to determine the presence or absence of growth. However, having sterilized the surface provides no guarantee that the central nervous system has been sterilized. In our subsequent experiments (including those described in the present report) we have used brain homogenates, which more accurately reflect the antifungal effects on cryptococcal meningoencephalitis.
However, the role of flucytosine may be more complex than that reflected in these studies with an inoculum level that produces relatively mild meningitis. We used the delay in the onset of treatment to study the effect of the severity of meningitis on the antifungal activities of fluconazole with or without flucytosine (10). In that experiment, fluconazole alone at high doses was effective when treatment was started when the cryptococcal disease was mild. However, flucytosine at moderate doses combined with high doses of fluconazole was required to achieve the greatest effect in the setting of more severe meningitis. Furthermore, the maximum level of response that could be achieved was decreased as the severity of meningitis increased. Given the severity of meningitis seen in humans (27, 28), further investigations are warranted to evaluate the roles of the severity of meningitis and amphotericin B combined with flucytosine and fluconazole.
The addition of amphotericin B has a striking effect on the antifungal effect of fluconazole. Given this dramatic increase in antifungal activity and the widespread availability of these two drugs, the full potential of this two-drug combination deserves further evaluation. Although the dose-limiting toxicity associated with amphotericin B prevents testing of higher doses of the standard formulation, it would be worthwhile to evaluate the potential of liposomal formulations in combination with fluconazole.
Acknowledgments
This work was supported in part by the National Institute of Allergy and Infectious Diseases (grants N01-AI-85351 and N01-AI-15082).
REFERENCES
- 1.Alves, S. H., J. O. Lopes, J. M. Costa, and C. Klock. 1997. Development of secondary resistance to fluconazole in Cryptococcus neoformans isolated from a patient with AIDS. Rev. Inst. Med. Trop. Sao Paulo 39:359-361. [DOI] [PubMed] [Google Scholar]
- 2.Armengou, A. C., Porcar, J. Mascaro, and F. Garcia-Bragado. 1996. Possible development of resistance to fluconazole during suppressive therapy for AIDS-associated cryptococcal meningitis. Clin. Infect. Dis. 23:1337-1338. [DOI] [PubMed] [Google Scholar]
- 3.Bauer, M., A. M. Thomas, J. R. Graybill, and R. A. Larsen. 1998. Display of confidence intervals for response surfaces in combination drug experiments, p. 100-105. In Proceedings of statistical graphics, 1997 JSM. American Statistical Association, Alexandria, Va.
- 4.Bennett, J. E., W. E. Dismukes, R. J. Duma, G. Medoff, M. A. Sande, H. Gallis, J. L. Leonard, B. T. Fields, M. Bradshaw, H. Haywood, Z. A. McGee, T. R. Cate, C. G. Cobbs, J. F. Warner, and D. W. Alling. 1979. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptococcal meningitis. N. Engl. J. Med. 301:126-131. [DOI] [PubMed] [Google Scholar]
- 5.Birley, H. D., E. M. Johnson, P. McDonald, C. Parry, P. B. Carey, and D. W. Warnock. 1995. Azole drug resistance as a cause of clinical relapse in AIDS patients with cryptococcal meningitis. Int. J. STD AIDS 6:353-355. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1997. Update: trends in AIDS incidence. Morbid. Mortal. Wkly. Rep. 46:165-173. [PubMed] [Google Scholar]
- 7.Cleveland, W. S., E. Grosse, and W. M. Shyu. 1991. Local regression models, p. 309-376. In J. M. Chambers, and T. Hastie (ed.), Statistical models in S. Chapman & Hall, New York, N.Y.
- 8.Cleveland, W. S. 1993. Visualizing data. Hobart, Summit, N.J.
- 9.Diamond, D. M., M. Bauer, B. E. Daniel, M. A. Leal, D. Johnson, B. K. Williams, A. M. Thomas, J. C. Ding, L. Najvar, J. R. Graybill, and R. A. Larsen. 1998. Amphotericin B colloidal dispersion combined with flucytosine with or without fluconazole for treatment of murine cryptococcal meningitis. Antimicrob. Agents Chemother. 42:528-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding, J. C., M. Bauer, D. M. Diamond, M. E. Leal, D. Johnson, A. M. Thomas, L. Navjar, J. R. Graybill, and R. A. Larsen. 1997. Effects of delayed treatment on the fungicidal activity of flucytosine combined with fluconazole in murine cryptococcal meningitis. Antimicrob. Agents Chemother. 41:1589-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duswald, K. H. A. Pend, and L. Pittrow. 1997. High-dose therapy with fluconazole > or ≥ 800 mg/day. Mycoses 40:267-277. [DOI] [PubMed] [Google Scholar]
- 12.Ford, B. J. 2000. AIDS and Africa. Biologist 47:224. [PubMed] [Google Scholar]
- 13.Friese, G., T. Discher, R. Fussle, A. Schmalreck, and J. Lohmeyer. 2001. Development of azole resistance during fluconazole maintenance therapy for AIDS-associated cryptococcal disease. AIDS 15:2344-2345. [DOI] [PubMed] [Google Scholar]
- 14.Gangaidzo, I. T., J. Mielke, and J. A. Matenga. 1999. Ethical considerations in the care of the patient with HIV/AIDS. Central Afr. J. Med. 45:51-53. [PubMed] [Google Scholar]
- 15.Greenwood, M. 1926. The natural duration of cancer. In Reports of public health and medical subjects, vol. 33. Her Majesty's Stationery Office, London, United Kingdom.
- 16.Hajjeh, R. A., L. A. Conn, D. S. Stephens, W. Baughman, R. Hamill, E. Graviss, P. G. Pappas, C. Thomas, A. Reingold, G. Rothrock, L. C. Hutwagner, A. Schuchat, M. E. Brandt, R. W. Pinner, et al. 1999. Cryptococcosis: population-based multistate active surveillance and risk factors in human immunodeficiency virus-infected persons. J. Infect. Dis. 179:449-454. [DOI] [PubMed] [Google Scholar]
- 17.Hoaglin, D. C., F. Mosteller, and J. W. Tukey. 1983. Understanding robust and exploratory data analysis. John Wiley & Sons, Inc., New York, N.Y.
- 18.Kaplan, G., and P. Meier. 1958. Non-parametric estimation from incomplete observations. J. Am. Stat. Assoc. 53:457-481. [Google Scholar]
- 19.Larsen, R. A., M. Bauer, J. M. Weiner, D. M. Diamond, M. E. Leal, J. C. Ding, M. G. Rinaldi, and J. R. Graybill. 1996. Effect of fluconazole on fungicidal activity of flucytosine in murine cryptococcal meningitis. Antimicrob. Agents Chemother. 40:2178-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leenders, A. C., P. Reiss, P. Portegies, K. Clezy, W. C. Hop, J. Hoy, J. C. Borleffs, T. Allworth, R. H. Kauffmann, P. Jones, F. P. Kroon, H. A. Verbrugh, and S. de Marie. 1997. Liposomal amphotericin B (AmBisome) compared with amphotericin B both followed by oral fluconazole in the treatment of AIDS-associated cryptococcal meningitis. AIDS 11:1463-1471. [DOI] [PubMed] [Google Scholar]
- 21.Liliang, P. C., C. L. Liang, W. N. Chang, K. Lu, and C. H. Lu. 2002. Use of ventriculoperitoneal shunts to treat uncontrollable intracranial hypertension in patients who have cryptococcal meningitis without hydrocephalus. Clin. Infect. Dis. 34:E64-E68. [DOI] [PubMed] [Google Scholar]
- 22.Link, C. L. 1984. Confidence intervals for the survival function using Cox's proportional hazards model with covariates. Biometrics 40:601-610. [PubMed] [Google Scholar]
- 23.Mwaba, P., J. Mwansa, C. Chintu, J. Pobee, M. Scarborough, S. Portsmouth, and A. Zumla. 2001. Clinical presentation, natural history, and cumulative death rates of 230 adults with primary cryptococcal meningitis in Zambian AIDS patients treated under local conditions. Postgrad. Med. J. 77:769-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 2nd ed. M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 25.Newton, P. N., H. Thai le, N. Q. Tip, J. M. Short, W. Chierakul, A. Rajanuwong, P. Pitisuttithum, S. Chasombat, B. Phonrat, W. Maek-A-Nantawat, R. Teaunadi, D. G. Lalloo, and N. J. White. 2002. A randomized, double blind, placebo-controlled trial of acetazolamide for the treatment of elevated intracranial pressure in cryptococcal meningitis. Clin. Infect. Dis. 35:769-772. [DOI] [PubMed] [Google Scholar]
- 26.Park, M. K., D. R. Hospenthal, and J. E. Bennett. 1999. Treatment of hydrocephalus secondary to cryptococcal meningitis by use of shunting. Clin. Infect. Dis. 28:629-633. [DOI] [PubMed] [Google Scholar]
- 27.Pitisuttithum, P., S. Tansuphasawadikul, A. J. Simpson, P. A. Howe, and N. J. White. 2001. A prospective study of AIDS-associated cryptococcal meningitis in Thailand treated with high-dose amphotericin B. J. Infect. 43:226-233. [DOI] [PubMed] [Google Scholar]
- 28.Robinson, P. A., M. Bauer, M. A. Leal, S. G. Evans, P. D. Holtom, D. A. Diamond, J. M. Leedom, and R. A. Larsen. 1999. Early mycological treatment failure in AIDS-associated cryptococcal meningitis. Clin. Infect. Dis. 28:82-92. [DOI] [PubMed] [Google Scholar]
- 29.Saag, M. S., J. R. Graybill, R. A. Larsen, P. G. Pappas, J. R. Perfect, W. G. Powderly, J. D. Sobel, and W. E. Dismukes. 2000. Practice guidelines for the management of cryptococcal disease. Clin. Infect. Dis. 30:710-718. [DOI] [PubMed] [Google Scholar]
- 30.Stamm, A. M., R. B. Diasio, W. E. Dismukes, S. Shadomy, G. A. Cloud, C. A. Bowles, G. H. Karam, and A. Espinel-Ingroff. 1987. Toxicity of amphotericin B plus flucytosine in 194 patients with cryptococcal meningitis. Am. J. Med. 83:236-242. [DOI] [PubMed] [Google Scholar]
- 31.van der Horst, C. M., M. S. Saag, G. A. Cloud, R. J. Hamill, J. R. Graybill, J. D. Sobel, P. C. Johnson, C. U. Tuazon, T. Kerkering, B. L. Moskovitz, W. G. Powderly, W. E. Dismukes, et al. 1997. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N. Engl. J. Med. 337:15-21. [DOI] [PubMed] [Google Scholar]
- 32.Venables, W. N., and B. D. Ripley. 1999. Modern applied statistics with S-PLUS, 3rd ed. Springer-Verlag, Inc., New York, N.Y.