Abstract
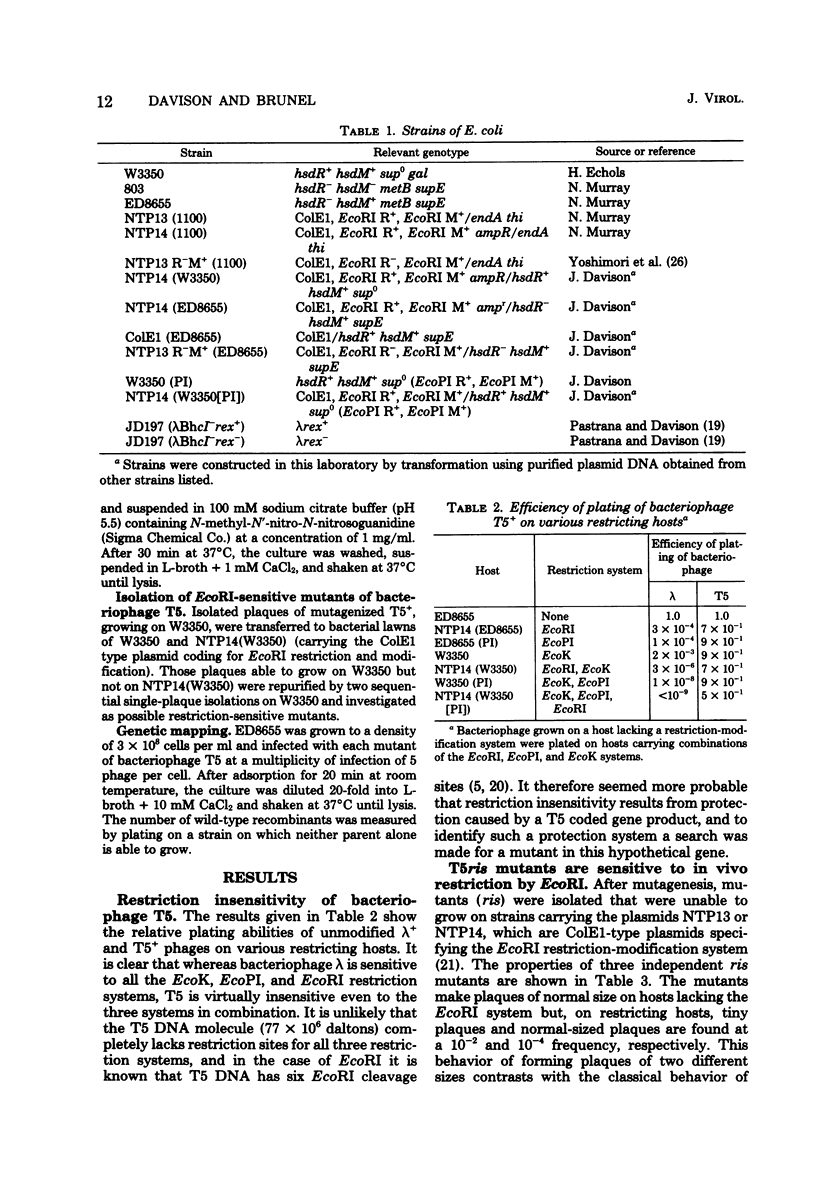
Unmodified bacteriophage T5 is able to grow normally on bacterial hosts carrying three different Escherichia coli restriction systems, EcoK, EcoPI, and EcoRI. Under the same conditions, the plating efficiency of bacteriophage gamma is less than 10(-9). At least in the case of EcoRI, this lack of in vivo restriction is not due to lack of restriction sites on the T5 DNA molecule. These observations suggest that bacteriophage T5 specifies one or more restriction protection systems. Mutants (ris) of T5 have been isolated which confer sensitivity to EcoRI restriction but not to EcoK or EcoPI. The mutations are located in the pre-early region of the genetic map but are too far apart to be alleles of a single gene. Complementation studies show that the ris mutants can be helped to grow on the EcoRI-restricting host by coinfection with T5+. This result provides evidence for a restriction protection function but does not necessarily show that the ris mutants are defective in such a system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. HOST SPECIFICITY OF DNA PRODUCED BY ESCHERICHIA COLI V . THE ROLE OF METHIONINE IN THE PRODUCTION OF HOST SPECIFICITY. J Mol Biol. 1965 Feb;11:247–256. doi: 10.1016/s0022-2836(65)80055-9. [DOI] [PubMed] [Google Scholar]
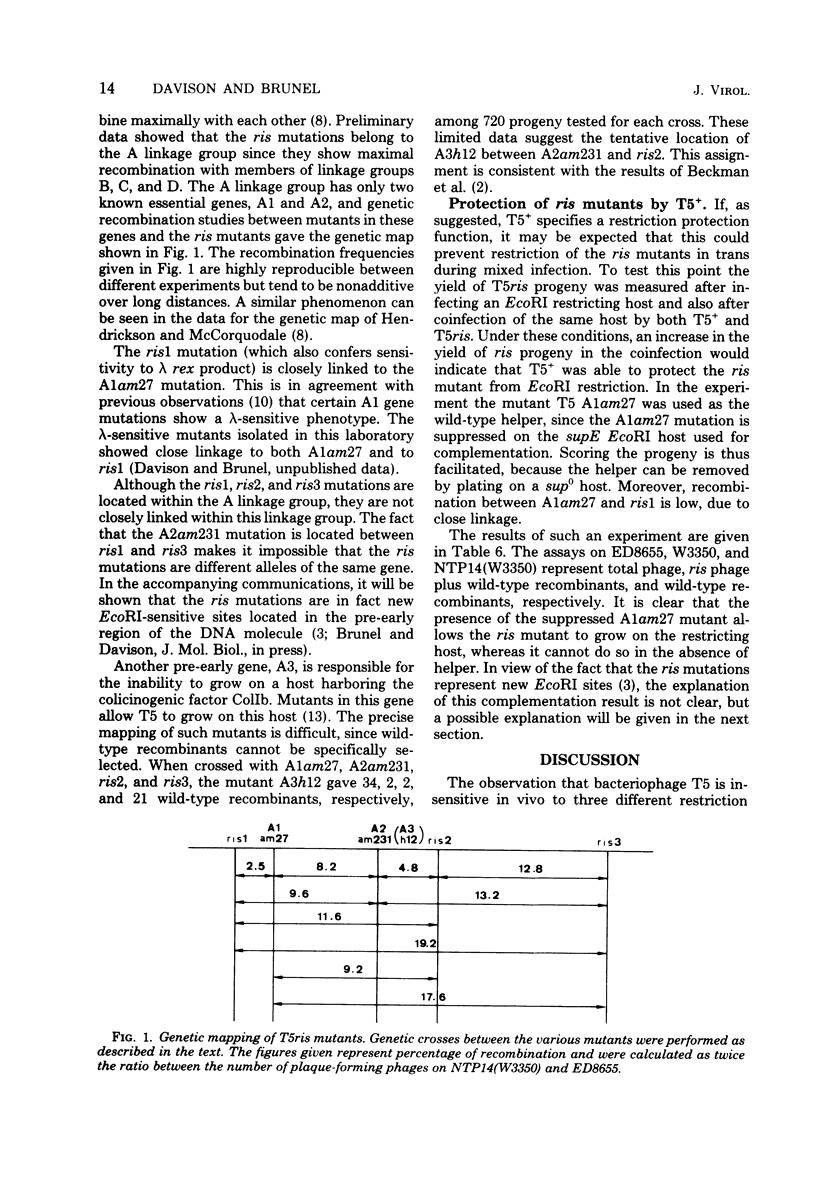
- Beckman L. D., Anderson G. C., McCorquodale D. J. Arrangement on the chromosome of the known pre-early genes of bacteriophages T5 and BF23. J Virol. 1973 Nov;12(5):1191–1194. doi: 10.1128/jvi.12.5.1191-1194.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison J., Brunel F. Restriction insensitivity in bacteriophage T5. II. Lack of EcoRI modification in T5+ and T5ris mutants. J Virol. 1979 Jan;29(1):17–20. doi: 10.1128/jvi.29.1.17-20.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R. Synthesis of an S-adenosylmethionine-cleaving enzyme in T3-infected Escherichia coli and its disturbance by co-infection with enzymatically incompetent bacteriophage. J Virol. 1967 Feb;1(1):57–63. doi: 10.1128/jvi.1.1.57-63.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgpeth J., Goodman H. M., Boyer H. W. DNA nucleotide sequence restricted by the RI endonuclease. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3448–3452. doi: 10.1073/pnas.69.11.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K., Zinder N. D. Cleavage of bacteriophage fl DNA by the restriction enzyme of Escherichia coli B. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3220–3224. doi: 10.1073/pnas.69.11.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin-Sablon A., Lanni Y. T. Lambda-repressed mutants of bacteriophage T5. I. Isolation and genetical characterization. Virology. 1973 Nov;56(1):230–237. doi: 10.1016/0042-6822(73)90302-4. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Schroeder C., Hansen S., Rosenthal H. A. Active protection by bacteriophages T3 and T7 against E. coli B- and K-specific restriction of their DNA. Mol Gen Genet. 1977 May 20;153(1):99–106. doi: 10.1007/BF01036001. [DOI] [PubMed] [Google Scholar]
- Labedan B., Crochet M., Legault-Demare J., Stevens B. J. Location of the first step transfer fragment and single-strand interruptions in T5stO bacteriophage DNA. J Mol Biol. 1973 Apr 5;75(2):213–234. doi: 10.1016/0022-2836(73)90017-x. [DOI] [PubMed] [Google Scholar]
- MCCORQUODALE D. J., LANNI Y. T. MOLECULAR ASPECTS OF DNA TRANSFER FROM PHAGE T5 TO HOST CELLS. I. CHARACTERIZATION OF FIRST-STEP-TRANSFER MATERIAL. J Mol Biol. 1964 Oct;10:10–18. doi: 10.1016/s0022-2836(64)80023-1. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- McCorquodale D. J. The T-odd bacteriophages. CRC Crit Rev Microbiol. 1975 Dec;4(2):101–159. doi: 10.3109/10408417509111574. [DOI] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Meselson M., Yuan R., Heywood J. Restriction and modification of DNA. Annu Rev Biochem. 1972;41:447–466. doi: 10.1146/annurev.bi.41.070172.002311. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Murray K. Manipulation of restriction targets in phage lambda to form receptor chromosomes for DNA fragments. Nature. 1974 Oct 11;251(5475):476–481. doi: 10.1038/251476a0. [DOI] [PubMed] [Google Scholar]
- Pastrana R., Davison J. Control of transcription of the rex-cl region of bacteriophage lambda. Mol Gen Genet. 1974;131(3):223–232. doi: 10.1007/BF00267962. [DOI] [PubMed] [Google Scholar]
- Rhoades M. Cleavage of T5 DNA by the Escherichia coli R-I restriction endonuclease. Virology. 1975 Mar;64(1):170–179. doi: 10.1016/0042-6822(75)90089-6. [DOI] [PubMed] [Google Scholar]
- Smith H. R., Humphreys G. O., Willshaw G. A., Anderson E. S. Characterisation of plasmids coding for the restriction endonuclease EcoRI. Mol Gen Genet. 1976 Feb 2;143(3):319–325. doi: 10.1007/BF00269410. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Movva N. R. SAMase gene of bacteriophage T3 is responsible for overcoming host restriction. J Virol. 1976 Jul;19(1):136–145. doi: 10.1128/jvi.19.1.136-145.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Toussaint A. The DNA modification function of temperate phage Mu-1. Virology. 1976 Mar;70(1):17–27. doi: 10.1016/0042-6822(76)90232-4. [DOI] [PubMed] [Google Scholar]
- WYATT G. R., COHEN S. S. The bases of the nucleic acids of some bacterial and animal viruses: the occurrence of 5-hydroxymethylcytosine. Biochem J. 1953 Dec;55(5):774–782. doi: 10.1042/bj0550774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori R., Roulland-Dussoix D., Boyer H. W. R factor-controlled restriction and modification of deoxyribonucleic acid: restriction mutants. J Bacteriol. 1972 Dec;112(3):1275–1279. doi: 10.1128/jb.112.3.1275-1279.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R., Bickle T. A., Ebbers W., Brack C. Multiple steps in DNA recognition by restriction endonuclease from E. coli K. Nature. 1975 Aug 14;256(5518):556–560. doi: 10.1038/256556a0. [DOI] [PubMed] [Google Scholar]
- von Gabain A., Hayward G. S., Bujard H. Physical mapping of the HindIII, EcoRI, Sal and Sma restriction endonuclease cleavage fragments from bacteriophage T5 DNA. Mol Gen Genet. 1976 Feb 2;143(3):279–290. doi: 10.1007/BF00269404. [DOI] [PubMed] [Google Scholar]


