Abstract
Research in industrialized countries has demonstrated that a key factor limiting the control of hypertension is poor patient adherence and that the most successful interventions for long-term adherence employ multiple strategies. Very little data exist on this question in low-income countries, where medication-taking behavior may be less well developed. We conducted a treatment adherence trial of 544 subjects (mean age ~63 years, mean BP ~168/92 mmHg) with previously untreated hypertension in urban and rural Nigeria. Eligible participants were randomized to one of two arms: clinic management only, or clinic management + home visits. Both interventions included three elements: a community based, nurse-led treatment program with physician backup; facilitation of clinic visits and health education; and the use of diuretics plus a beta blocker as needed. After initial diagnosis, the management protocol was implemented by a nurse with physician backup. Participants were evaluated monthly for 6 months. Medication adherence was assessed with pill count and urine testing. Dropout rates, by treatment group, ranged from 12% to 28%. Among participants who completed the 6 month trial, overall adherence was high (~77% of participants took > 98% of prescribed pills). Adherence did not differ by treatment arm, but was better at the rural than the urban site and among those with higher baseline BP. Hypertension control (BP < 140/90 mmHg) was achieved in ~66% of participants at 6 months. This community-based intervention confirms relatively modest default rates compared to industrialized societies, and suggests that medication adherence can be high in developing world settings in clinic attenders.
Keywords: anti-hypertensive, adherence, compliance trial
Introduction
Hypertension is the most common cardiovascular condition in the world and accounts for a substantial proportion of adult mortality[1, 2]. Although its prevalence varies widely among countries, the challenge to establish a culturally appropriate treatment and control strategy is shared by all societies. Prevention is, of course, the most effective response to common disease where the exposure is near universal, however prevention at the population level has been difficult to implement for hypertension and drugs are still the mainstay of control efforts. The public health impact of anti-hypertensive therapy is now widely appreciated.[3] In the US, for example, drug therapy for hypertension is one of the few medical therapies introduced in the last half of the 20th century with a sufficient impact to be registered in the vital statistics trends.[3]
The single most pressing challenge in the field of hypertension is therefore to improve the treatment and control rate of patients with hypertension. Rates of hypertension control range from 5–40% in industrialized country.[4] Nonetheless, most cases of severe hypertension have now been eliminated in countries with population-wide detection and treatment strategies.[3] In sub-Saharan Africa and in most developing countries progress has been much slower. In virtually every resource-poor country for which data have been reported treatment and control rates are no more than 1 – 3%.[5–10] The reasons for this failure are manifold, including limited access to health services as well as an inadequate scientific framework to guide locally sustainable treatment programs. Although the economic barriers are paramount in these societies, additional health services research is also needed before strategies that are appropriate for poor countries can be developed. Pill taking is a learned behavior and treatment success for chronic disease is determined by a variety of complex factors related to the patient, the healthcare provider and the healthcare system. The impact of these factors varies substantially from culture to culture. Just as oral rehydration therapy dramatically improved the treatment of infantile diarrhea in poor countries, similar tailored methods may be required to achieve higher rates of adherence to medications prescribed for chronic diseases under conditions of limited resources.
The primary goal of this study was to expand the evidence base necessary to guide hypertension treatment and control programs in Africa. Over a six month period we quantified adherence to a standard anti-hypertensive regimen and compared the effect of a nurse-led clinic-based care management strategy versus a similar program plus home visits, on medication adherence among patients with hypertension.
Methods
Study participants and setting
This study was conducted in an urban and a rural setting in the Yoruba-speaking region of southwestern Nigeria. The rural sample was drawn from the farming towns of Igbo-ora and Idere, with a combined population of approximately 50,000 people. The local residents are engaged primarily in subsistence farming and petty trading. The urban sample was drawn from the Idikan neighborhood of Ibadan, a city of approximately 3 million people, where most individuals are engaged in the informal economy. Both communities have participated in numerous prior studies of hypertension and other chronic diseases.
Study participants were recruited through community screening. Eligibility included adults age 40 years and older with BP ≥ 140/80 mmHg on three consecutive occasions one week apart or BP ≥ 160/90 mmHg on two consecutive occasions one week apart; not on any antihypertensive medication at the time of enrollment, no known comorbidity or complications of hypertension; no plans to move from the community for at least one year from the date of enrollment; and willingness to comply with the study protocol. Pregnant women were excluded, and participants who became pregnant during the trial were also dropped. The study protocol was reviewed and approved by the institutional review boards at Loyola University Medical Center in the US and the University of Ibadan in Nigeria. All participants provided written or verbal informed consent for the study.
Study design
The study was conducted in two phases. During the start-up phase we completed extensive open-ended interviews and observations of study participants to determine current knowledge and attitudes toward hypertension and the use of medications for chronic diseases. The study procedures were piloted in order to define optimal intervals between study visits, how many pills to provide at one time, monitoring procedures, etc. Population screening in the targeted communities was conducted simultaneously to identify potential participants. The 2-arm randomized controlled trial was designed to evaluate the effect of a nurse-led intervention plus home visits compared to nurse-led intervention alone on medication adherence and BP control among participants from both rural and urban settings. After enrollment and baseline assessment, patients were followed monthly for a period of 6 months.
If patients default from treatment programs after a short period of time the effort invested in screening and detection are undermined and they cannot be justified as cost effective. We therefore focused exclusively on the six month outcomes to assure that we were assessing a reasonably long interval of follow-up.
Participant recruitment and baseline assessments
The protocol was supervised by one of the authors - a physician from University College Hospital in Ibadan (AA). Nurses were employed to provide care during routine clinic visits. Survey staff from the local community, conducted the recruitment and patient assessments. The survey staff was trained to administer a brief questionnaire, measure participants’ BP and height and weight in a standardized fashion, and explain the study protocol to participants. The methods used for standardization of BP measurement have been described in detail elsewhere.[11] In brief, after a 5 minute rest in the sitting position, three consecutive BP readings were taken in the right arm with an automated device, and the mean of the last two readings was used in the analyses.[11]. Height and weight were measured in light clothing. At baseline, trained study staff confirmed each patient’s eligibility, assessed their demographic status, reviewed the study protocol with the participants and obtained their BP, height and weight.
Randomization
Once eligibility was confirmed and study participants had been consented, they were randomized on 1:1 basis into one of two treatment groups: (a) clinic-based care management intervention or (b) clinic-based care management intervention plus home visits. Given the nature of the intervention, and as is typical for most behavioral interventions, blinding of study participants was not possible.
Description of the treatment groups
The clinic-based care management intervention involved three components: clinic-based treatment administered by trained nurses; provision of free antihypertensive medications; and provision of funds to reimburse participants for transportation costs for monthly clinic visits. The clinic-based treatment was standardized, with a thiazide diuretic (hydrochlorothiazide) as first line anti-hypertensive and the addition of a beta-blocker (atenolol) as a second drug if BP remained uncontrolled. Titration was left to the discretion of the physicians. Participants randomized to the clinic-based care management plus home visits received all three elements including scheduled home visits by the nurses.
Follow-up assessments
The study staff conducted monthly clinic visits with participants for a period of 6 months during which the adherence and BP measurements were performed. Data collection at the final study visit was similar to that at the baseline visit.
Outcomes and measurements
Adherence to prescribed anti-hypertensive medications was assessed in two ways. The primary measure of adherence was pill counts, while the secondary adherence measure was the use of biological assay with a urinary riboflavin tracer.[12–14] Pill counts were done at each clinic visit; participants returned all medications and counts were recorded. Pill count adherence was defined as the proportion of pills taken divided by the total number of pills prescribed during the assessment period. For the biological assay assessment, each participant was instructed to take 50 mg riboflavin daily with the morning dose of antihypertensive medication. A single dose of 50 mg riboflavin can be reliably detected in urine under UV light for 18–24 hours.[14] Therefore, we could use riboflavin fluorescence under ultraviolet (UV) light as a biomarker of adherence to prescribed antihypertensive medication in this low resource environment.[13] Urine samples were collected at each monthly clinic visit and/or home visits.[15] The spot urine samples were tested within 2 hours of collection for the presence of riboflavin using a UV lamp and scored as “positive”, “indeterminate” or “negative”, using blank samples as controls.[16] Mean adherence rates were calculated for each measure across the 6-month study duration. Adherence was defined with a composite measure based on both assessments such that participants were categorized as nonadherent if they had a pill count of zero or tested negative on the urinary riboflavin tracer. Participants were categorized as adherent if they miss no more than 2 doses per month or if they tested positive on the urinary riboflavin tracer test.
Measurements of BP was conducted every month as described earlier; and the mean BP level was calculated across the 6-month study duration for each participant and the proportion of those with BP<140/80 mm Hg was noted.
Statistical methods
Data forms were reviewed manually for completeness and accuracy and combined in a single computer database. Outliers were identified by examining values exceeding three standard deviations and corrected or removed. Means, standard deviations and frequencies were calculated for each variable at each visit and the normality of the distributions of continuous variables was examined. Analysis was conducted on an “intention to treat” basis. Comparison of sample characteristics between treatment groups was performed by use of Student’s t-test for independent samples and comparison of compliance between treatment groups was done by use of chi-squared test. Logistic regression models were fitted to estimate the effect of intervention on adherence and also to identify predictors of adherence among baseline characteristics. All statistical analyses were performed using the software Stata SE (Release 10, College Station, TX).
Results
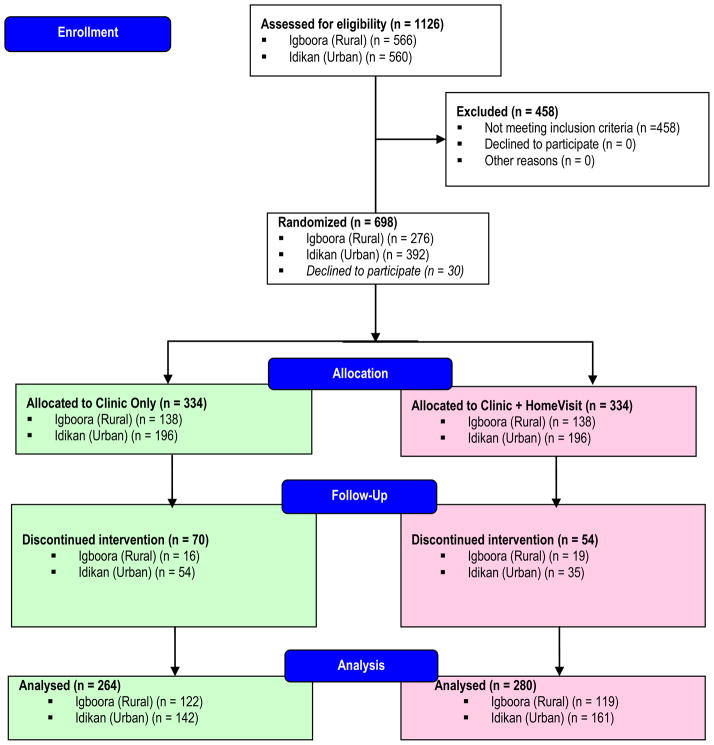
A total of 1126 potentially eligible individuals were screened, of which 698 were randomized; 668 subsequently entered the treatment phase. Of the 668 participants who started the trial, 544 (81%) completed the 6 months of follow-up and 124 (19%) did not complete the trial (Figure 1). The known reasons for non-completion were as follows: death (n=7), severe cardiovascular events (comprising 2 stroke and 1 congestive cardiac failure), referral due to uncontrollable high blood pressure (n=11), side-effects of treatment (excessive micturition, dizziness, etc.) (n=9), lost interest in participating (n=5) and preference for alternative medicine (n=3). The others simply defaulted and the reasons for this were not known. Randomization for both the urban and rural sites yielded comparable groups of participants (Table 1). In the urban site, participants in the ‘clinic-based’ arm were slightly older and had systolic BP’s that were 5 mmHg higher (p<.05). In the rural site ‘clinic-based’ participants also had modestly higher diastolic BP’s. All participants had low relative weight.
Figure 1.
Participants enrollment flow diagram
Table 1.
Baseline Characteristics by Intervention Group over the 6 month Trial Period
| Igboora (rural)
|
Idikan (urban)
|
|||||
|---|---|---|---|---|---|---|
| Clinic only (n=122) | Clinic + HmV (n=119) | p¶ | Clinic only (n=142) | Clinic + HmV (n=161) | p¶ | |
| Sex (Male/Female) | 42/80 | 46/73 | 0.495 | 47/95 | 51/110 | 0.792 |
| Age (years) | 61.3 (10.1) | 61.7 (11.2) | 0.780 | 64.9 (9.3) | 62.7 (9.4) | 0.042 |
| Systolic BP (mmHg) | 169.5 (18.9) | 166.8 (16.6) | 0.242 | 169.4 (21.6) | 164.6 (19.1) | 0.043 |
| Diastolic BP (mmHg) | 94.8 (12.7) | 91.0 (12.7) | 0.021 | 91.5 (12.7) | 90.4 (11.4) | 0.444 |
| Pulse (per minute) | 79.6 (15.5) | 77.4 (15.1) | 0.248 | 78.5 (12.3) | 74.5 (12.1) | 0.005 |
| Body mass index (kg/m2) | 22.7 (4.6) | 23.1 (4.1) | 0.539 | 24.3 (5.0) | 24.7 (5.4) | 0.455 |
| Waist circumference (cm) | 75.8 (8.6) | 76.4 (8.5) | 0.541 | 81.1 (9.5) | 82.0 (10.6) | 0.466 |
P value for t-test for independent samples or chi-square test (sex only)
At the six-month study end point less than half of randomized participants, specifically 219 of 544 were continuing to attend their appointments. In the rural site, 88% and 86% of participants continued in the study in the ‘clinic-based’ and the ‘home visit’ arms, respectively (Figure 1). In the urban site, in the same study arms, 72% and 82% were still attending.
Among participants who remained in the study until the six month end point adherence to prescribed medications was high throughout the trial (Table 2). In the rural sample almost half of the participants had no excess pills remaining when they returned for interval clinic visits, and nearly 90% had either < 2 excess pills at each monthly visit or a “satisfactory” urine assay for the riboflavin tracer. A second drug (i.e., a beta blocker) was required in well over half of participants, and almost three-quarters had BP’s < 140/90 after 6 months of treatment.
Table 2.
Treatment, Compliance and Control by Intervention Group over the 6 month Trial Period
| Igboora (rural)
|
Idikan (urban)
|
|||||
|---|---|---|---|---|---|---|
| Clinic only (n=122) | Clinic + HmV | P¶ | Clinic only (n=142) | Clinic + HmV | P | |
| Strict compliance (zero pill | 50.0 | (n=119) 45.4 | 0.473 | 35.9 | (n=161) 32.9 | 0.584 |
| Pill count <= 2 per month count) | 89.3 | 76.5 | 0.008 | 75.4 | 69.6 | 0.261 |
| “Satisfactory” urine tests* | 89.3 | 91.6 | 0.552 | 78.2 | 78.9 | 0.880 |
| Treated with 2 drugs | 59.8 | 61.3 | 0.811 | 83.1 | 82.6 | 0.910 |
| BP controlled at 6 months** | 71.3 | 68.1 | 0.584 | 62.0 | 62.7 | 0.891 |
All values are percentages. HmV = home visits
P value for chi-squared test
“Satisfactory” urine scores defined as at least 3 positive urine samples
BP < 140/90 mmHg after 6 months of treatment
Treatment arm assignment (i.e., ‘clinic-based’ vs. ‘clinic + home visit’) had no impact on adherence for 2 of the outcome measures, although the ‘clinic + home visit’ group had a significantly greater proportion of participants with excess pills < 2 (p<.01) (Table 3). Among the predictors of adherence, as was apparent from the descriptive findings, those who were enrolled in the rural site had significantly higher rates of pill taking (Table 4). Group assignment likewise retained significance in this model, and a significant but small effect was observed for initial systolic BP and BMI.
Table 3.
Effect of Intervention Group on Adherence
| Outcome | Crude OR (SE) | p | Site-adjusted OR (SE) | p | * Fully adjusted OR (SE) | p |
|---|---|---|---|---|---|---|
| Strict compliance (pill count | 0.839 (0.147) | 0.317 | 0.854 (0.151) | 0.373 | 0.823 (0.148) | 0.278 |
| Pill count <= 2 per month zero) | 0.586 (0.122) | 0.010 | 0.594 (0.125) | 0.013 | 0.524 (0.114) | 0.003 |
| Satisfactory urine tests | 1.073 (0.250) | 0.763 | 1.111 (0.263) | 0.654 | 1.050 (0.255) | 0.841 |
Adjusted for site, age, sex, number of drugs, initial systolic BP, initial diastolic BP, initial BMI
Table 4.
Predictors of Adherence with Anti-Hypertensive Medications, Rural and Urban Sites Combined
| Outcome
|
||||||
|---|---|---|---|---|---|---|
| Zero pill count
|
Pill count <= 2 per month
|
Satisfactory urine tests
|
||||
| OR (SE) | P | OR (SE) | P | OR (SE) | p | |
| Intervention group | 0.823 (0.148) | 0.278 | 0.524 (0.114) | 0.003 | 1.050 (0.255) | 0.841 |
| Site | 0.533 (0.102) | 0.001 | 0.457 (0.107) | 0.001 | 0.433 (0.119) | 0.002 |
| Age | 1.015 (0.010) | 0.141 | 1.007 (0.012) | 0.526 | 1.007 (0.014) | 0.609 |
| Sex | 1.070 (0.207) | 0.726 | 1.058 (0.240) | 0.804 | 1.306 (0.333) | 0.295 |
| Treated with 2 drugs | 0.944 (0.217) | 0.797 | 1.184 (0.325) | 0.539 | 0.559 (0.194) | 0.093 |
| Initial SBP | 0.995 (0.007) | 0.457 | 0.987 (0.007) | 0.017 | 0.988 (0.008) | 0.124 |
| Initial DBP | 1.005 (0.010) | 0.623 | 1.004 (0.012) | 0.749 | 1.021 (0.014) | 0.119 |
| Initial BMI | 1.037 (0.020) | 0.056 | 1.063 (0.025) | 0.011 | 1.010 (0.026) | 0.700 |
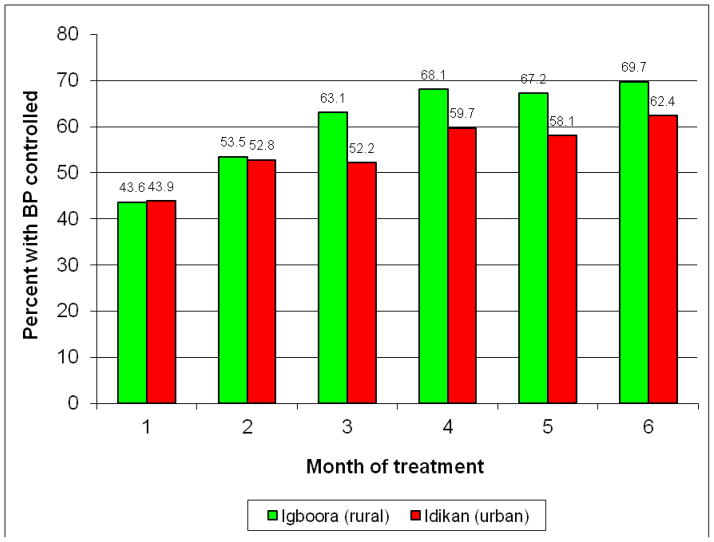
In this treatment-naïve sample with excellent adherence, the decline in BP during the trial was very substantial (Figures 2 and 3). BP’s trended downward over the entire 6 month period of observation and 62% and 70% of participants reached goal in the urban and rural sites, respectively. The mean decline in systolic BP was approximately 30 mmHg and for diastolic BP 15 mmHg, all participants combined (Table 5). As anticipated from use of the beta-blocker, average pulse rates fell significantly as well. Very modest changes in weight and circumference were observed, that were not of clinical significance.
Figure 2.
Changes in Blood Pressure over 6 month trial period, stratified by Urban and Rural Sites
Figure 3.
Trends in Blood Pressure Control over 6 month trial period
Table 5.
Changes in Blood Pressure, Pulse and Anthropometric Values During the Intervention Phase, All Participants Combined.
| Idikan (urban) (n=123)
|
Igbo-Ora (rural) (n= 106)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Start Mean(SD) | End Mean(SD) | Change Mean (95% CI) | P | Start Mean(SD) | End Mean(SD) | Change Mean (95% CI) | P | |
| SBP | 166.6 (20.9) | 138.1 (21.5) | −28.5 (−32.0,−25.1) | <0.0001 | 169.3 (18.1) | 134.6 (22.1) | −34.7 (−38.8, −30.6) | <0.0001 |
| DBP | 90.3 (12.8) | 79.1 (10.8) | −11.8 (−13.3, −9.1) | <0.0001 | 94.2 (13.0) | 76.2 (12.4) | −18.1 (−20.3, −15.9) | <0.0001 |
| Pulse | 78.0 (13.4) | 73.5 (12.5) | −4.5 (−6.8, −2.2) | 0.0002 | 78.8 (15.9) | 70.1 (13.1) | −8.7 (−11.3, −6.1) | <0.0001 |
| BMI | 24.1 (4.9) | 24.5 (5.0) | +0.4 (+0.2, +0.7) | 0.003 | 22.4 (4.1) | 22.6 (4.0) | +0.2 (0.0, 0.5) | 0.062 |
| Waist | 82.7 (11.7) | 83.0 (9.4) | +0.2 (−0.8, +1.3) | 0.657 | 76.3 (7.8) | 75.5 (8.5) | −0.8 (−1.3, −0.2) | 0.007 |
Discussion
This systematic adherence study demonstrated that – across the urban and rural settings - 12 to 27% of patients with newly diagnosed hypertension would default from treatment in the first six months. While these are difficult to compare directly to studies in developed countries, large scale electronic data bases which track prescription refills generally show larger rates of default for treatment of high BP and cholesterol[17, 18]. Likewise, in most industrialized countries, less than one-third of hypertensive patients in the community are actively taking treatment and are at goal [18]. Overall, therefore, the degree of non-attendance at six months is substantially lower, although this comparison can be only approximate. Likewise, among participants who continued in the study adherence was extremely high.
The findings from our “optimal scenario” intervention support the general experience that, perhaps contrary to expectations, patients who previously had little or no exposure to education campaigns for chronic disease, or prior contact with organized modern medical care, will readily adhere to recommended therapy. We acknowledge that our study was designed to identify the upper threshold of pill-taking behavior and some aspects, such as home visits, cannot be supported in routine care. However, in the absence of prior evidence we chose to establish the “gold standard” against which more cost-effective programs could be compared. This evidence strongly suggests that widespread use of basic antihypertensive therapy in rural and urban Africa would meet with high levels of patient acceptance, assuming the economic barriers were removed.
In sub-Saharan Africa (SSA) prevalence of hypertension ranges from 10–15%, yielding an estimated 10–20 million patients.[6, 9] Effective treatment could forestall 250,000 deaths/yr.[6] Stroke and heart failure are common occurrences in the virtual absence of treatment. In the cohort we followed in the small farming area where this trial was conducted population attributable risk from hypertension for deaths from all causes was 5%[9]. The Adult Morbidity and Mortality Project in Tanzania reported stroke deaths rates 8–10 times higher than in the UK, an outcome attributed to the absence of hypertension treatment.[10] CV diseases will undoubtedly increase in frequency in SSA over the coming decades. Like most tropical countries, Nigeria is undergoing a transition and 35% of the population is now urbanized.
While considerable progress has been made toward hypertension control in industrialized countries, very little has been accomplished in developing countries[4–10]. In the US and Europe treatment and control rates in the general population range from 15–35% [3–10, 19, 20]. A national survey of 14,000 persons in Mexico documented a treatment and control rate of 2% [8]. In Tanzania and Senegal 10% of hypertensives were being treated and <1% controlled[4]. The control rates among clinic attenders are also very low in Africa, although successful interventions have been demonstrated. A previous cluster-randomized trial to improve hypertension treatment through use of structured guidelines was recently conducted in Nigeria[21]. Attendance in the intervention group at 12 months was high (i.e., 90%), however the mean decline in BP was smaller than observed in our study (i.e., 11 mmHg systolic); control rates were not reported. In South Africa only 18% of hypertensive patients were still attending one year after their initial visit, and less than half of those were controlled[22]. By contrast, in a large Canadian cohort 78% of patients persisted in treatment after one year[19]; ironically the Canadian investigators characterized this outcome as “remarkably poor”. The single over-riding theme throughout this extensive literature is the need to develop strategies that take account of the social and cultural factors to boost adherence.
The question of whether hypertension control is a priority for developing countries has been viewed from different perspectives. In the past, some authorities have disputed its value in Africa, when so many competing health emergencies exist. A World Bank document in the 1990’s, for example, suggested that hypertension treatment is not cost effective in Africa, citing an expense of up to $1.5 million to prevent a fatal event [23]. Unfortunately, this recommendation was based on the cost to prevent a death in a middle-aged American, which is likely to be much higher than in Africa given physician fees and ancillary costs, and the report used no empirical data on the effectiveness of programs in SSA [6]. We accept the proposition that under many circumstances in Africa hypertension treatment may not qualify as a priority. On the other hand, this recommendation can hardly be universal, and it must be grounded in data. Furthermore, the purpose of health research is to provide options for policy; understanding how to treat and control chronic disease in the long term is an essential health need in all countries, and if not now a priority will become one in the future.
We recognize that the observed declines in BP are in excess of the findings from most previous treatment trials. While we conducted a series of ‘run-’in visits it is possible that we had not abolished the orienting response and since no placebo group was included we cannot determine what trend would have occurred in the absence of treatment. In addition, as noted, a treatment-naïve sample with good adherence to this degree has not been previously studied, and diuretics are likely to be particularly effective in this set of primarily elderly patients. We also note that the provision of free medication is expected to have a positive impact on adherence. This is especially relevant in a resource-poor community where the cost of such medications can be prohibitive if they are not subsidized. Therefore, “real world” studies of adherence without this component may not achieve similar adherence and/or BP control levels.
Hypertension is not only the most common CV disease by far, but the most treatable. Although primary prevention is an important long-term goal, programs must be developed to make drug treatment available in the interim. The challenge is to develop treatment methods that are effective in the African social setting, as with oral rehydration and directly observed therapy for TB. This recommendation has been made repeatedly in recent years, eg : “Hypertension is ideally suited to be the initial component of a CVD control program…in developing countries[24]; “Low-cost hypertension control programs are needed in developing countries” [7]. What is missing is an evidence base upon which to build these programs. The data presented here provide one starting point from which large-scale, community-based programs can proceed.
Acknowledgments
We acknowledge the valuable contributions of Professor O. O. Omotade, Dr. A. Adebiyi, Dr. O. Odeku, Dr. E. Owoaje and Dr. M. Ladipo. Many thanks to the research and field staff at the Institute of Child Health, University of Ibadan, and the staff and participants at the field sites in Igbo-Ora, Idere and Idikan. O.O. was supported in part, by Fogarty Training Grant D43TW009140.
Sources of funding: This work was supported by National Institutes of Health (NIH) grant from the NHLBI (R01 HL67883).
Footnotes
Conflicts of Interest/Disclosure Statement: NONE
References
- 1.Edwards R, Unwin N, Mugusi F, Whiting D, Rashid S, Kissima J, et al. Hypertension prevalence and care in an urban and rural area of Tanzania. J Hypertens. 2000;18(2):145–52. doi: 10.1097/00004872-200018020-00003. [DOI] [PubMed] [Google Scholar]
- 2.Fuentes R, Ilmaniemi N, Laurikainen E, Tuomilehto J, Nissinen A. Hypertension in developing economies: a review of population-based studies carried out from 1980 to 1998. J Hypertens. 2000;18(5):521–9. doi: 10.1097/00004872-200018050-00003. [DOI] [PubMed] [Google Scholar]
- 3.Pobee JO. Community-based high blood pressure programs in sub-Saharan Africa. Ethn Dis. 1993;3(Suppl):S38–45. [PubMed] [Google Scholar]
- 4.Walker RW, McLarty DG, Kitange HM, Whiting D, Masuki G, Mtasiwa DM, et al. Stroke mortality in urban and rural Tanzania. Adult Morbidity and Mortality Project. Lancet. 2000;355(9216):1684–7. doi: 10.1016/s0140-6736(00)02240-6. [DOI] [PubMed] [Google Scholar]
- 5.Arroyo P, Fernandez V, Loria A, Kuri-Morales P, Orozco-Rivadeneyra S, Olaiz G, et al. Hypertension in urban Mexico: the 1992–93 national survey of chronic diseases. J Hum Hypertens. 1999;13(10):671–5. doi: 10.1038/sj.jhh.1000909. [DOI] [PubMed] [Google Scholar]
- 6.Erdine S. How well is hypertension controlled in Europe? J Hypertens. 2000;18(9):1348–9. doi: 10.1097/00004872-200018090-00025. [DOI] [PubMed] [Google Scholar]
- 7.Karppanen H, Mervaala E. Adherence to and population impact of non-pharmacological and pharmacological antihypertensive therapy. J Hum Hypertens. 1996;10(Suppl 1):S57–61. [PubMed] [Google Scholar]
- 8.Kaufman JS, Rotimi CN, Brieger WR, Oladokum MA, Kadiri S, Osotimehin BO, et al. The mortality risk associated with hypertension: preliminary results of a prospective study in rural Nigeria. J Hum Hypertens. 1996;10(7):461–4. [PubMed] [Google Scholar]
- 9.Klungel OH, de Boer A, Paes AH, Seidell JC, Nagelkerke NJ, Bakker A. Undertreatment of hypertension in a population-based study in The Netherlands. J Hypertens. 1998;16(9):1371–8. doi: 10.1097/00004872-199816090-00018. [DOI] [PubMed] [Google Scholar]
- 10.Reddy KS. Hypertension control in developing countries: generic issues. J Hum Hypertens. 1996;10(Suppl 1):S33–8. [PubMed] [Google Scholar]
- 11.Cooper R, Puras A, Tracy J, Kaufman J, Asuzu M, Ordunez P, et al. Evaluation of an electronic blood pressure device for epidemiological studies. Blood Press Monit. 1997;2(1):35–40. [PubMed] [Google Scholar]
- 12.Dubbert PM, King A, Rapp SR, Brief D, Martin JE, Lake M. Riboflavin as a tracer of medication compliance. J Behav Med. 1985;8(3):287–99. doi: 10.1007/BF00870315. [DOI] [PubMed] [Google Scholar]
- 13.Hungerbuhler P, Bovet P, Shamlaye C, Burnand B, Waeber B. Compliance with medication among outpatients with uncontrolled hypertension in the Seychelles. Bull World Health Organ. 1995;73(4):437–42. [PMC free article] [PubMed] [Google Scholar]
- 14.Kapur S, Ganguli R, Ulrich R, Raghu U. Use of random-sequence riboflavin as a marker of medication compliance in chronic schizophrenics. Schizophr Res. 1991;6(1):49–53. doi: 10.1016/0920-9964(91)90020-r. [DOI] [PubMed] [Google Scholar]
- 15.Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, Hunter DJ. Rationale and design of the Tanzania Vitamin and HIV Infection Trial. Control Clin Trials. 1999;20(1):75–90. doi: 10.1016/s0197-2456(98)00045-2. [DOI] [PubMed] [Google Scholar]
- 16.Schaid DJ, Ingle JN, Wieand S, Ahmann DL. A design for phase II testing of anticancer agents within a phase III clinical trial. Control Clin Trials. 1988;9(2):107–18. doi: 10.1016/0197-2456(88)90032-3. [DOI] [PubMed] [Google Scholar]
- 17.Degli Esposti L, Saragoni S, Batacchi P, Benemei S, Geppetti P, Sturani A, et al. Adherence to statin treatment and health outcomes in an Italian cohort of newly treated patients: results from an administrative database analysis. Clin Ther. 2012;34(1):190–9. doi: 10.1016/j.clinthera.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Corrao G, Zambon A, Parodi A, Poluzzi E, Baldi I, Merlino L, et al. Discontinuation of and changes in drug therapy for hypertension among newly-treated patients: a population-based study in Italy. Journal of hypertension. 2008;26(4):819–24. doi: 10.1097/HJH.0b013e3282f4edd7. [DOI] [PubMed] [Google Scholar]
- 19.Caro JJ, Salas M, Speckman JL, Raggio G, Jackson JD. Persistence with treatment for hypertension in actual practice. CMAJ. 1999;160(1):31–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Saunders LD, Ntoane C, Wilson TD. Why don’t patients return for antihypertensive treatment in Soweto? S Afr Med J. 1983;64(6):208–10. [PubMed] [Google Scholar]
- 21.Mendis S, Johnston SC, Fan W, Oladapo O, Cameron A, Faramawi MF. Cardiovascular risk management and its impact on hypertension control in primary care in low-resource settings: a cluster-randomized trial. Bulletin of the World Health Organization. 2010;88(6):412–9. doi: 10.2471/BLT.08.062364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper RS, Osotimehin B, Kaufman JS, Forrester T. Disease burden in sub-Saharan Africa: what should we conclude in the absence of data? Lancet. 1998;351(9097):208–10. doi: 10.1016/S0140-6736(97)06512-4. [DOI] [PubMed] [Google Scholar]
- 23.Miller NH. Compliance with treatment regimens in chronic asymptomatic diseases. Am J Med. 1997;102(2A):43–9. doi: 10.1016/s0002-9343(97)00467-1. [DOI] [PubMed] [Google Scholar]
- 24.Cooper RS, Muna W, Kingue S, Osotimehin B, Kadiri S, Rotimi C, et al. The burden of hypertension in rural Africa: results from the international collaborative study on hypertension in Blacks (ICSHIB) Trop Cardiol. 1996;22:69–75. [Google Scholar]