Abstract
International health experts agree that China is on the verge of an AIDS crisis. In response, the Chinese government initiated the “Four Frees and One Care” policy in 2003 to decrease economic barriers and increase access to antiretroviral therapies for people with HIV. However, long-term treatment success requires not only access, but high rates of medication adherence. This qualitative interview study with 29 persons receiving HIV care at Beijing’s Ditan Hospital identified barriers to and facilitators of medication adherence. The interviews were guided by an a priori conceptual model of adherence with four components: access, knowledge about medications, motivation, and proximal cues to action. Barriers to adherence were related to stigma and fear of discrimination; the medications themselves (including side effects and complicated dosing regimens); and other economic issues (i.e., costs of transportation, lab tests, and hospitalizations). Facilitators included participants’ strong will to live, use of electronic reminders, and family support. These results support the conceptual model and suggest that successful interventions must minimize stigma as it negatively affects all components of the model for adherence.
Keywords: antiretroviral adherence, qualitative research, China, conceptual models
Introduction
Current reports of the AIDS epidemic in China estimate that approximately 650,000 people are infected with HIV; of these, about 75,000 people are living with AIDS. Approximately 80 percent of people who are HIV-positive do not know they are infected and the epidemic is spreading from high risk groups to the general population (Kaufman & Jing, 2002; China Ministry of Health [MOH] et al, 2006). The Chinese government’s response to the epidemic has been to implement the “Four Frees and One Care” policy (see Table 1 for services offered to eligible citizens), which is financed by the Central Government’s Treasury department and implemented by local governments through rural clinics and regional pharmacies. Eligibility is based on the hukou or township where an individual holds a residency card (China MOH, 2003; China MOH et al, 2006; Wu et al, 2006).
Table 1.
Components of the Four Frees and One Care policy
|
The ART is a combination therapy involving three classes of antiretroviral agents: nucleoside reverse transcriptase inhibitors (NRTIs); non-NRTIs; and protease inhibitors.
The effort to increase economic access to antiretroviral therapy (ART) is vital to the success of treatment programs. Yet it is equally important to establish parallel programs to assure high rates of long-term medication adherence (95–100% of prescribed doses) to maximize health benefits and minimize drug resistance (Friedland & Williams, 1999). Theoretical work on antiretroviral adherence has focused on separate but related factors that are correlated with ART adherence including social support (Bontempi, Burleson, & Lopez, 2004; Gonzalez et al., 2004; Simoni, Frick, Lockhart, & Liebovitz, 2002); self-efficacy (Johnson et al., 2006; Reynolds et al., 2004); coping styles (Catz, Kelly, Bogart, Benotsch, & McAuliffe, 2000; Gore-Felton et al., 2005; Weaver et al., 2005); andART-related information, motivation, and behavior (Amico, Toro-Alfonso, & Fisher, 2005; Fisher, Fisher, Amico, & Harman, 2006; Kalichman, Malow, Devieux, Stein, & Piedman, 2005). It is not yet clear how these various factors function and interact in the social context within which HIV infection and treatment occur in China, which has a strong familial orientation that is characterized by a reliance on interpersonal relations (Yang, 1995).
Conceptual model of adherence in China
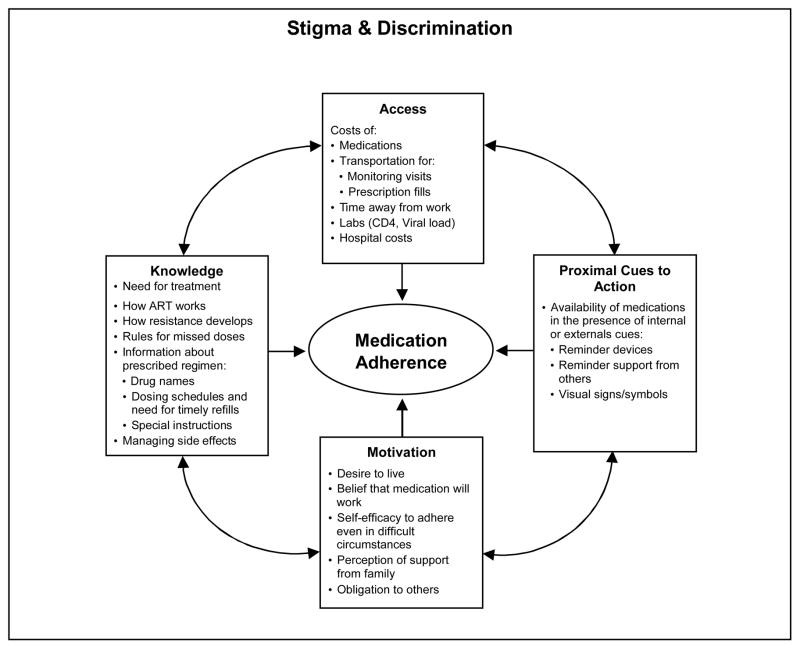
We developed a preliminary conceptual model to better understand what is needed to promote optimal ART adherence in China (Figure 1), based largely on the information, motivation, and behavioral (IMB) model described by Fisher and colleagues (2006). Our conceptual model suggests that there are four components that are necessary for consistent medication adherence. First, persons with HIV/AIDS must have access to medications and other supports to allow for ongoing monitoring of health and treatment regimens (a structural component). Second, they require correct knowledge (i.e., about dosing schedules, resistance, potential side effects) to take their medications (a cognitive component). Third, they must be motivated to take the medications (a psychological component). Fourth, they need to have the medication available and remember to take the medication at the appropriate time (an internal cue) or be prompted by a family member or an alarm (an external cue). These external cues represent a social/mechanical component. The components all affect each other and collectively work to enhance medication adherence.
Figure 1.
Conceptual model to guide adherence interventions in China
The model in Figure 1 is situated inside a larger box that signifies the social environment in which the threat of stigma and discrimination are constant factors that affect all components of the model. This is particularly true in China, where general public knowledge about HIV transmission remains low, and suspicion of and discrimination against persons with HIV/AIDS is still common (Cao, Pang, & Wu, 2005; Yang, Zhang, Chan, & Reidpath, 2005). In the present study, as the first part of a larger project to promote HIV-medication adherence in Beijing, we conducted a qualitative study to explore barriers to and facilitators of ART adherence to inform our conceptual model.
Methods
Setting
This cross-sectional qualitative interview study was conducted at Ditan Hospital, which is regarded as one of Beijing’s premier specialist hospitals for HIV/AIDS care. Ditan Hospital serves about 1200 patients with HIV/AIDS, of whom about one third (31%) are on ART. The ratio of male to female patients is 5 to 1 and about 80% of patients come from outside the city limits. The average age of patients is 35 years (range 5–68). Seventeen percent of patients are men who have sex with men.
Recruitment and Consent Procedures
We recruited two groups of persons with HIV/AIDS: those who had been on ART for at least four months (“ART-experienced,” n=21) and those who had started ART during the current hospitalization or a recent outpatient visit (“ART-naïve,” n=8). Six physicians and one senior nurse in the Ditan HIV/AIDS section (who all had existing relationships with the patients) recruited potential study participants from both the inpatient and outpatient wards during a hospitalization or usual care visit. These clinicians told potential participants about the study and referred them to study personnel for a one-time, semi-structured in-person interview. Interested participants reviewed and signed a written consent form after being assured that no data linking names with study identifiers would be maintained. Participants received a small monetary incentive of 150 RMB (~USD$20) for their time. All study procedures were reviewed and approved by the institutional review boards of Ditan Hospital, China CDC, and the University of Washington.
Data Collection, Management, and Analysis
The research interviews were conducted one-on-one between the participant and one interviewer. Interviewers included physicians and a nurse educator from the Ditan Hospital and two of the study investigators. The semi-structured interviews were conducted in Mandarin and covered the following topics: (1) the patient’s treatment and side effect history withART; (2) current medications and adherence behaviors; (3) knowledge of and instructions about medication taking, how the medications work, and the consequences of missed doses; (4) barriers and facilitators to adherence; and (5) any hopes or worries about the medications.
All but six of the interviews were audio recorded and transcribed; for the other six, the interviewer took detailed notes and immediately typed them in transcript form. All transcripts were translated into English by four bi-lingual research staff and were then coded using Atlas.ti (Scientific Software Development, 2005). After initial coding into broad topical categories, we generated reports for a given topic across all the participants and reviewed them to identify ways in which the narratives corresponded to the components of the conceptual model and new themes that emerged from the analysis.
Results
Sample characteristics
Characteristics of the study sample are reported in Table 2. Thirty-eight percent of the participants did not know (n = 3) or did not report (n = 8) the source of their HIV infection. The reported transmission routes included blood products (n=8) and sexual contact (n=10).
Table 2.
Participant characteristics
| ART-experienced | ART-naïve | All | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Total | 21 | 72 | 8 | 28 | 29 | 100 |
| Male | 15 | 71 | 7 | 88 | 22 | 76 |
| Average age in years (sd) | 39 | (9) | 36 | (16) | 38 | (11) |
| Han ethnicity | 18 | 86 | 8 | 100 | 26 | 90 |
| Beijing resident | 18 | 86 | 4 | 50 | 22 | 76 |
| Employment/Occupation | ||||||
| Unskilled Labor (factory worker, farmer, driver) | 3 | 14 | 2 | 25 | 5 | 17 |
| Skilled Labor (trades, cashier, sales) | 7 | 33 | 2 | 25 | 9 | 31 |
| Technical (accounting, journalism, advertising, web design) | 6 | 29 | 1 | 13 | 7 | 24 |
| Management (professional, small business) | 5 | 24 | 2 | 25 | 7 | 24 |
| Missing data | 0 | 0 | 1 | 13 | 1 | 3 |
| Education | ||||||
| Less than high school | 4 | 19 | 4 | 50 | 8 | 28 |
| High school diploma | 9 | 43 | 2 | 25 | 11 | 38 |
| Night school, professional or vocational training | 2 | 10 | 0 | 0 | 2 | 7 |
| College (includes undergraduate and graduate education) | 6 | 29 | 2 | 25 | 8 | 28 |
| HIV transmission route | ||||||
| Blood products | 6 | 29 | 2 | 25 | 8 | 28 |
| Sexual contact | 6 | 29 | 4 | 50 | 10 | 34 |
| Unknown | 2 | 10 | 1 | 13 | 3 | 10 |
| Not reported/missing | 7 | 33 | 1 | 13 | 8 | 28 |
| Medication regimen | ||||||
| NNRTI* | 17 | 81 | 6 | 75 | 23 | 80 |
| Other** | 4 | 19 | 2 | 25 | 20 | 6 |
| Mean weeks on ART, (sd) | 120.5 | 91.6 | 2.9 | 1.1 | 88.0 | 94.1 |
NNRTI = non-nucleoside reverse transcriptase inhibitor;
Other includes reverse transcriptase inhibitor only (n=3), dual nucleosides (n=1), protease inhibitors (n=1), and unknown (n=1).
Adherence barriers and facilitators
Table 3 presents the main themes from the analysis of barriers and facilitators, organized according to the components of the conceptual model. All of the participants reported that their adherence was generally very good and that they rarely missed doses. When they did miss doses, they usually remembered to make up the dose within a few hours. Participants reported that the main barriers to adherence were related to stigma and fear of discrimination, the medications themselves (including side effects and complicated dosing regimens), and financial issues. Facilitators included participants’ motivations and use of proximal cues to action. In the next section, we present participants’ quotes to illustrate how they described barriers and facilitators to adherence. Quotes are followed by identifier codes and information about the participant’s ART group, subject number, sex, and age.
Table 3.
Barriers and facilitators to ART adherence
| Barriers: | Facilitators: |
|---|---|
Stigma
|
Motivation
|
Stigma and discrimination
Worries about stigma and discrimination underscored many of the barriers. Eight participants mentioned negative experiences with discrimination related to their HIV status, the consequences of which included being isolated by and not receiving treatment from health care providers, being shunned by friends and family, and losing jobs (and thus the source of income and health insurance). All of the participants were concerned about the negative social costs of disclosure beyond (and sometimes including) their immediate social circle. Ten participants reported being very careful about hiding their medication-taking when they were in the presence of others who did not know their HIV status. This was especially a barrier for working persons. For example, one man reported,
I don’t like other people [at work] to see [me take my medications] because I think that if other people see that, they would ask what I am taking and if I feel well or something, and why I have to take medicine everyday. There will be a series of questions. So I try to hide from others when I am taking the medicine. …So sometimes I take my small medicine box and go to the bathroom. I lock the door and take the medicine there. (ART-Exp-20, male, age 42)
Several participants mentioned considering changing jobs due to travel or scheduling difficulties. Two people discussed deliberating about returning to work because of the dual problem of interference with their medication schedule and the risk of discovery of their HIV status by co-workers or clients. Those who took their medications in front of others told them they were vitamins or supports for the immune system. Some transferred pills to unmarked bottles to avoid questions; however, one person described how this strategy worked against him:
Once I used a vitamin box to hold [my pills] and my coworkers saw it and asked for it. I didn’t give it to them, and they were not happy with that. …If other people take it, it would be bad for both me and others. (ART-Naïve-6, male, age 28)
Access
Before the Four Frees and One Care policy was implemented, cost posed a significant barrier for eight of the ART-experienced patients, with monthly medication expenses frequently costing 2–3 times their monthly incomes. One participant reported that she and her HIV-positive husband had to sell their house to cover their medication costs, and four other participants considered selling their houses if necessary. Currently, most costs for first line therapies and for measuring CD4 cell counts are covered by the Four Frees policy. However, costs for labs for viral loads, transportation, and the time away from work for physician visits and prescription refills remain economic and social barriers. This is particularly onerous for those who travel from distant provinces for care in Beijing.
Health insurance is not universally available in China. Of the nine participants who reported having insurance, only five said they used it. The others chose not to out of concern that either their insurance company or employer would disclose their HIV status. In addition, most participants said they skip getting expensive lab tests, including CD4 counts and viral loads, unless they access them through research studies or the hospital on special days when tests are free. One man’s story reflects all these issues:
Why did I say that I would come to the hospital tomorrow? Because I want to get tested for CD4 again. Tomorrow, it is arranged that the carriers in Beijing will get free testing. …The medication I am taking now is free. But I have to pay for the [tests and] treatment out of pocket. Medical insurance is connected with the jobs. I don’t have a job right now. Other patients I know who have jobs and insurance won’t use the insurance for this disease [because they don’t want other people to find out about their HIV]. …The insurance company examines the drug very carefully. They can tell what disease you have based on what meds have been prescribed. (ART-Exp-7, male, age 46)
Another man described how he lost access to medications during the SARS epidemic when the public health infrastructure closed down to minimize the spread of infection:
In April [2003] we got the SARS epidemic, which interrupted all my connections with others and my treatment plan [that] was interrupted totally from April to July. …Because of the SARS epidemic, there was no one on the street basically, and no one I knew that I could turn to either. …My health was declining again. I made plenty of phone calls. Finally, in July, Ditan re-opened the outpatient department and the hotline for consultation was re-opened too. But the outpatient department had been moved from the general wards [and] I couldn’t find them. …Eventually I got the right information, and they told me that I could go to the hospital for my next prescription. (ART-exp-17, male, age 36)
Knowledge
All but two of the patients reported receiving medication-taking instructions, and information about how the drugs work, managing side effects and the consequences of missed doses. However, the two ART-experienced people who had not received instruction at the initiation of their therapy attributed their poor adherence and subsequent problems to this lack of knowledge. One of these men had the additional disadvantage of language barriers that made it difficult to obtain the information he needed.
[When I first got the medications,] I didn’t know that there is a strict time requirement, [that] I should take it at 6:00 in the morning and 6:00 in the afternoon. I didn’t understand it. …I couldn’t understand Mandarin very well. So every time [I see the doctor], I have to write down what I want to say. …I am still not very clear on a lot of things about this disease. One is about the medicine. The second is about my situation. I don’t know. The doctor doesn’t tell me. …I just found out that I have drug resistance. (ART-Exp-5, male, age 66)
Motivation
The motivation for adherence mentioned most often was feeling the health benefits after taking medications. The positive effects gave participants courage and stamina to cope with short- or long-term side effects:
The power of living is much more powerful than the side effects. Aside from this, the instruction from the doctors has indicated that there are side effects, and those side effects will disappear after a period of time, so I am mentally prepared. (ART-Exp-7, male, age 46)
The health benefits also helped to offset worries about possible long-term toxic effects to the liver, kidney, and immune system. As one man said,
[The most helpful thing for me is my] confidence in the medicine. Just believing that the medicine will work. It is necessary for my health. I have to take it no matter what even though it is poison. It is very toxic. But I still have to take it. Otherwise my health will get bad. (ART-Exp-14, male, age 40)
Another set of motivators included having a strong will to live (or fear of death) and a desire to live long enough to take advantage of a perceived eventual cure. Many participants had been seriously ill for months to years before receiving a diagnosis of HIV/AIDS. Once they understood that the medications could give them a second chance on life, they wanted to do all they could to sustain their health.
The fear of drug resistance motivated high adherence for several reasons, including avoiding expensive monitoring lab tests, lacking access to second- and third-line therapies (which often require paying out of pocket and having international connections to acquire the imported medicines), and fear of running out of viable options to sustain long-term health. The following reflects many of these concerns:
I heard that this medicine can only be effective for two or three years. Then the drug resistance will appear. I am using the first line medicine. The second line medicine has not shown up yet. What if we developed drug resistance? …It is very expensive to test the drug resistance. If the CD4 count goes down, it must be the drug resistance. No medicine to change to. I am still taking first line. Some people are already taking the second, or third line. They don’t have any [more] medicine. No matter how much they would like to spend. (ART-Exp-6, male, age 30)
Finally, participants reported that their obligations to family members were important reasons to maintain good adherence. For most, this was an additional motivator among many, but for one man who was not coping well with the shame he felt he had brought to himself and his family, it was the only motivation he had: “If I didn’t have a family and was only by myself, I wouldn’t care anymore. I would not be afraid of death. I wouldn’t treat it.” (ART-Exp-10, male, age 40)
Proximal cues to action
Almost everyone reported using a mix of one or more internal and external cues for adherence. Fifteen participants reported that their own good habits and discipline were their primary reminders. Their good habits, coupled with a routine schedule, once- or twice-daily regimens, and a reminder—an alarm, a pillbox, or another person—made it fairly easy to maintain good adherence.
Family members won’t always be with me. I can only ask my parents to remind me. But, if I go to work or on a business trip, they are not likely to follow me everywhere. My cell phone is always with me, which is most convenient. [It is also] necessary to keep in mind the importance of good adherence. You attach importance to it, and you are more likely to practice. (ART-Naïve-8, male, age 32)
Conversely, having irregular schedules or a 3–4 times daily dosing schedule made it more likely that people would miss doses or be late taking their medications. Six participants admitted to simply forgetting a dose while watching TV or visiting with friends.
Discussion
In this empirical investigation of antiretroviral medication adherence in Beijing, China, we conducted semi-structured interviews with 29 persons with HIV/AIDS from Ditan Hospital. We found substantial support for the four components of our proposed model of adherence—access, knowledge, motivation, and cues to action. In addition, our data on barriers and facilitators revealed some challenging issues for long-term adherence interventions based on larger structural factors. First, while the Four Frees and One Care policy is meant to improve access to counseling and treatment, the residency requirement may need to be reconsidered given the legitimate concerns participants had about confidentiality and fear of stigma and discrimination in the tight-knit hukou communities. These concerns created incentives for participants to forego using free local services and insurance (when they had it) and incur significant costs for transportation to urban centers, lost time from work, hospitalizations, and viral load lab tests. Therefore, intervention efficacy may depend on addressing these important structural factors.
Findings from this study suggest that many participants have family members who are already actively assisting with their medications. More research is needed to determine the extent of knowledge and skills family members have in relation to promoting effective medication adherence. Family-based interventions aimed at providing education and instruction about HIV treatment as well as strengthening problem-solving and communication skills may be promising. However, the development of such interventions must be tailored to account for variations that exist in terms of availability and type of familial support and the extent to which the person living with HIV desires such involvement (Li et al., 2006).
The findings from this study must be interpreted in the context of some methodological limitations. First, all participants were recruited from one hospital in Beijing that is recognized for its high quality HIV care. Patients recruited from care facilities with less specialized knowledge or located in rural environments may report different barriers and facilitators to adherence. For example, our sample included no active injection drug users, who characterize the epidemic in the southern provinces of China. Additionally, interviews were conducted and transcribed in Mandarin but then translated and analyzed in English creating the possibility of shifts in the intended meaning of participants’ responses. We addressed this by sharing our results with clinicians and researchers in Beijing who generally concurred with our interpretation of these findings. Finally, physicians and nurses conducted most of the interviews, which may have led to either socially desirable responses on the part of the participants or more candid responses. The participants in this study did express trust and respect for their providers, based on longstanding relationships and the provider’s knowledge and comfort discussing HIV.
Future research is needed to explore further how generic models of adherence are influenced by specific cultural contexts, especially in resource-constrained settings experiencing expanded access to antiretroviral treatment. Focusing on structural factors that affect both access and adherence, as well as the cognitive and psychological components, will be crucial in these contexts.
In our ongoing work in Beijing with ART-naïve and -experienced patients, we are testing the feasibility of a nurse-delivered counseling intervention that encourages family involvement and offers the choice of electronic reminders. Given the difficulties of identifying specific strategies that are most efficacious (Simoni et al., 2006), this line of work, bolstered by qualitative findings such as those reported here, promises to yield solutions that may enable individuals in developing countries to achieve and maintain the long-term adherence ART requires.
Acknowledgments
This study was supported by R34-MH074364 from the U.S. National Institute of Mental Health (NIMH) to Dr. Simoni. The views expressed are those of the authors and do not necessarily reflect the institutional opinions or positions of NIMH, the University of Washington, the China CDC, or Ditan Hospital. We would like to acknowledge and thank Li Se, Wenhui Lun, Kerong Wang, Xiaojing Wang, Yan Wu, and Chunhui Wang for conducting the interviews, and Amiel Dan Ying, Chunhui Wang, and Chengshi Shiu for their translations of the transcripts.
References
- Amico KR, Toro-Alfonso J, Fisher JD. An empirical test of the information, motivation and behavioral skills model of antiretroviral therapy adherence. AIDS Care. 2005;17(6):661–673. doi: 10.1080/09540120500038058. [DOI] [PubMed] [Google Scholar]
- Bontempi JM, Burleson L, Lopez MH. HIV medication adherence programs: the importance of social support. Journal of Community Health Nursing. 2004;21(2):111–122. doi: 10.1207/s15327655jchn2102_05. [DOI] [PubMed] [Google Scholar]
- Cao XB, Pang L, Wu ZY. Reasons and patterns of AIDS stigma and intervention strategies. Chinese Journal of AIDS/STD. 2005;11(3):243–245. [Google Scholar]
- Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychology. 2000;19(2):124–133. [PubMed] [Google Scholar]
- China MOH. A joint assessment of HIV/AIDS prevention, treatment and care in China. Beijing, China: MOH; 2003. China Ministry of Health & UN Theme Group on HIV/AIDS in China. [Google Scholar]
- China MOH, Joint United Nations Programme on HIV/AIDS, & World Health Organization. 2005 update on the HIV/AIDS epidemic and response in China. Beijing, China: 2006. [Google Scholar]
- Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychology. 2006;25(4):462–473. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- Friedland GH, Williams A. Attaining higher goals in HIV treatment: the central importance of adherence. AIDS. 1999;13(Suppl 1):S61–72. [PubMed] [Google Scholar]
- Gonzalez JS, Penedo FJ, Antoni MH, Duran RE, McPherson-Baker S, Ironson G, et al. Social support, positive states of mind, and HIV treatment adherence in men and women living with HIV/AIDS. Health Psychology. 2004;23(4):413–418. doi: 10.1037/0278-6133.23.4.413. [DOI] [PubMed] [Google Scholar]
- Gore-Felton C, Rotheram-Borus MJ, Weinhardt LS, Kelly JA, Lightfoot M, Kirshenbaum SB, et al. The Healthy Living Project: an individually tailored, multidimensional intervention for HIV-infected persons. AIDS Education and Prevention. 2005;17(1 Suppl A):21–39. doi: 10.1521/aeap.17.2.21.58691. [DOI] [PubMed] [Google Scholar]
- Johnson MO, Chesney MA, Goldstein RB, Remien RH, Catz S, Gore-Felton C, et al. Positive provider interactions, adherence self-efficacy, and adherence to antiretroviral medications among HIV-infected adults: A mediation model. AIDS Patient Care and STDS. 2006;20(4):258–268. doi: 10.1089/apc.2006.20.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman S, Malow R, Devieux J, Stein JA, Piedman F. HIV risk reduction for substance using seriously mentally ill adults: test of the information-motivation-behavior skills (IMB) model. Community Mental Health Journal. 2005;41(3):277–290. doi: 10.1007/s10597-005-5002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Jing J. China and AIDS-The Time to Act Is Now. Science. 2002;296:2339–2340. doi: 10.1126/science.1074479. [DOI] [PubMed] [Google Scholar]
- Li L, Wu S, Wu Z, Sun S, Cui H, Jia M. Understanding family support for people living with HIV/AIDS in Yunnan, China. AIDS and Behavior. 2006;10(5):509–517. doi: 10.1007/s10461-006-9071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds NR, Testa MA, Marc LG, Chesney MA, Neidig JL, Smith SR, et al. Factors influencing medication adherence beliefs and self-efficacy in persons naive to antiretroviral therapy: a multicenter, cross-sectional study. AIDS and Behavior. 2004;8(2):141–150. doi: 10.1023/B:AIBE.0000030245.52406.bb. [DOI] [PubMed] [Google Scholar]
- Scientific Software Development. Atlas.ti (Version 5.0, Build 66) Berlin: 2005. [Google Scholar]
- Simoni JM, Frick PA, Lockhart D, Liebovitz D. Mediators of social support and antiretroviral adherence among an indigent population in New York City. AIDS Patient Care and STDS. 2002;16(9):431–439. doi: 10.1089/108729102760330272. [DOI] [PubMed] [Google Scholar]
- Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load: A meta-analytic review of randomized controlled trials. Journal of Acquired Immune Deficiency Syndromes. 2006;43(Suppl 1):S23–S35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver KE, Llabre MM, Duran RE, Antoni MH, Ironson G, Penedo FJ, et al. A stress and coping model of medication adherence and viral load in HIV-positive men and women on highly active antiretroviral therapy (HAART) Health Psychology. 2005;24(4):385–392. doi: 10.1037/0278-6133.24.4.385. [DOI] [PubMed] [Google Scholar]
- Wu Z, Rou K, Cui H. The HIV/AIDS Epidemic in China: History, Current Strategies and Future Challenges. AIDS Education and Prevention. 2004;16(3 Suppl A):7–17. doi: 10.1521/aeap.16.3.5.7.35521. [DOI] [PubMed] [Google Scholar]
- Yang KS. Chinese social orientation: An integrative analysis. In: Lin TY, Tseng WS, Yeh EK, editors. Chinese societies and mental health. New York: Oxford University Press; 1995. [Google Scholar]
- Yang Y, Zhang KL, Chan KY, Reidpath DD. Institutional and structural forms of HIV-related discrimination in health care: a study set in Beijing. AIDS Care. 2005;17(Suppl 2):S129–140. doi: 10.1080/09540120500119874. [DOI] [PubMed] [Google Scholar]