Abstract
Context
A history of depression may increase risk for developing Alzheimer disease (AD) later in life. Clarifying this relation might improve understanding of risk factors for and disease mechanisms in AD.
Objective
To systematically review and complete a meta-analysis on the relation of depression and AD.
Data Sources
We conducted electronic bibliographic searches of MEDLINE, PsychLit, EMBASE, and BIOSIS using search terms sensitive to studies of etiology combined with searches on terms related to depression and AD and reviewed reference lists of articles.
Study Selection
Studies with data contrasting depressed vs nondepressed patients who did and did not later develop AD were included. Studies that related continuous measures of depression and cognitive status were excluded.
Data Extraction
Numerical data were independently extracted by 3 reviewers. They also rated studies on a scale that assessed quality indicators for observational studies. Data on the interval between observation of depression and the diagnosis of AD were collected when available.
Data Synthesis
Meta-analytic evaluation with random-effects models resulted in pooled odds ratios of 2.03 (95% confidence interval, 1.73–2.38) for case-control and of 1.90 (95% confidence interval, 1.55–2.33) for cohort studies. Findings of increased risk were robust to sensitivity analyses. Interval between diagnoses of depression and AD was positively related to increased risk of developing AD, suggesting that rather than a prodrome, depression may be a risk factor for AD.
Conclusions
A history of depression may confer an increased risk for later developing AD. This relation may reflect an independent risk factor for the disease.
Depression is a critically important issue for those working with the elderly and especially those working with older persons with Alzheimer disease (AD). Depression affects a large number of elderly people1 and has been associated with poor cognitive function.2 Its treatment has been associated with improved functional status.3 It is a significant behavioral aspect of the symptomatology of AD4 that affects the cognitive and functional status of patients with AD.5 A better understanding of the relation of AD and depression thus might have important clinical and research implications.
A personal history of depression has been related to increased risk for developing AD later in life, although this finding has not been universal. For example, some case-control studies have found a relation between a history of depression and risk for AD.6–9 A number of these studies, however, have potential biases that limit their interpretability. Most notably, case-control studies that are inherently retrospective may be biased by a greater recall of history of depression in patients with AD (the cases), who often undergo more careful evaluation than do control subjects. Even some case-control studies have yielded negative results.10–14 Cohort studies, in which a group of persons undergoes evaluation and then prospective follow-up, have also yielded inconsistent results, with some indicating a statistically significant increased risk for AD with history of depression15–19 and others not finding it.20–26 Because depression affects a large number of patients with AD and has been associated with increased morbidity and mortality in the elderly, an improved understanding of the relation of depression to AD may have important public health implications.
The relation between depression and risk for later development of dementia is thus unclear. Other study-related factors may have affected these studies’ outcomes. Some studies of depression and AD evaluated the relation between the number of depressive symptoms (rather than categorical diagnosis) and diagnosis of AD,27 whereas others studied the relation of symptoms and measures of cognitive function, such as the Mini-Mental State Examination,28 rather than AD diagnosis. Two studies19,29 found that the relation between depression and risk for AD existed only for men, although other studies found a relation between number of depressive symptoms and subsequent poorer cognitive function only in women.2,30
Understanding the relation of depression and AD is further complicated by the possibility that depression may be a prodromal symptom of AD, so that its appearance before the recognition of other illness-defining symptoms of AD constitutes a harbinger of rather than a risk factor for the disease. One reviewer,31 for example, concluded that there was insufficient evidence to determine whether depression represents an independent risk factor or an early symptom in AD. Other researchers19,24,32 have more recently discussed the uncertainty that exists on this issue.
Previous reviews31,33,34 on the topic did not include several recent references, did not complete a systematic review of data sources, and may have included data from studies of patient groups with mixed psychiatric histories. The primary purposes of the present study were thus to systematically review studies on the relation of a history of depression to the risk for subsequent diagnosis of AD and to investigate the relations among observed risk for AD and other study variables. A secondary purpose was to investigate the relation of the interval between the diagnoses of depression and AD to observed risk for AD. We hypothesized that a negative relation (an inverse relation between the interval and increased risk for AD, implying that depression was more likely to occur near the time of AD diagnosis) would support the interpretation of depression as a prodromal symptom. A positive relation, with a larger interval between the diagnoses of depression and AD related to increased risk for AD, would then support the hypothesis that depression is an independent risk factor for AD.
METHODS
DATA SOURCES
Studies were found through a variety of methods that included computerized bibliographic searches and review of reference lists of pertinent articles. Consistent with results of a study35 that showed the importance of searching multiple databases to find the maximum number of relevant citations, we searched MEDLINE, PsychLit, EMBASE, and BIOSIS using a search strategy with maximum sensitivity for studies of etiology.36 Searches initially used the following strings: risk*(in title or abstract) OR risk* (as a Medical Subject Heading [MeSH] term, not exploded) OR cohort studies (as a MeSH term) OR group*(as a text word). Results of these searches were combined with sets created with depression OR depressive AND Alzheimer disease OR dementia OR dementing. Bibliographies of located articles were reviewed for possible data sources, as were the bibliographies of articles thus located.
STUDY SELECTION
We included studies that provided sufficient information to allow the calculation of crude odds ratios (ORs) for the risk of developing AD or AD-like dementia in persons with a history of depression compared with the same risk in persons without this history. Studies thus were required to provide data on the history of clinically diagnosed depressive disorder at some time before the clinical diagnosis of AD or dementia. Studies were included if they provided a description of diagnostic criteria that required the presence of symptoms consistent with major depressive disorder, even if they did not specifically describe use of criteria from International Classification of Diseases (ICD) or DSM.37–39 In similar fashion, studies were included if they included a description of diagnostic criteria for AD or AD-like dementia (eg, emphasized gradual progression of cognitive deficits), but they were excluded if diagnostic criteria were vague (eg, organic dementia) or included patients with vascular as well as AD-type dementias. Studies were excluded if they did not include a control group for comparison or if they provided data on continuous measures of depression (eg, number of depressive symptoms) and thus did not establish the clinical diagnosis of depression or cognitive status (eg, the Mini-Mental State Examination) and similarly did not establish the clinical diagnosis of dementia. (A list of excluded studies with explanations for exclusions is available from the authors.) Studies using continuous measures were excluded because of the diversity in use of measures and the fact that many studies only reported results adjusted for covariates such as age. Differences in measures of cognitive status and depression, as well as differences in statistical methodologies, made inclusion of these studies in the meta-analysis impossible.
DATA ABSTRACTION
Data from studies meeting inclusion criteria were extracted independently by 3 reviewers (3 from the group of us 5 authors for each extraction). In addition to the number of patients, studies were categorized as case-control or cohort, and if cohort, whether they were prospective or retrospective. Prospective cohort studies were defined as those that identified a group of participants and followed them up through time, whereas retrospective cohort studies were defined as those that identified a group of participants and used existing records to evaluate their clinical characteristics and course. The Newcastle-Ottawa Scale40 was used to assess the quality of each study. This measure assesses aspects of methodology in observational studies related to study quality, including selection of cases, comparability of populations, and ascertainment of exposure to risks. Where possible, an estimate of the interval between the diagnoses of depression and AD was extracted. In all cases, disagreements among raters were resolved through discussion so that a consensus was obtained.
DATA SYNTHESIS
Overview
Before combining studies in the meta-analysis, we evaluated the presence and possible causes of heterogeneity in risk for AD associated with a history of depression. The presence of heterogeneity in study effects was evaluated first. Because some evidence was found for the presence of heterogeneity in study outcomes, subsequent pooled analyses used random-effects estimating methods since this approach is more statistically appropriate41,42 for the question addressed in this study (ie, assuming a sample of studies, what is their likely pooled effect?). We evaluated the possible effects of dichotomous variables (such as case-control vs cohort study methodology or studies that used structured diagnostic criteria vs those that did not use structured criteria) in separate analyses stratified on the dichotomous variable. The effects of continuous variables, such as the interval between the diagnoses of depression and dementia, were evaluated via metaregression. The influence of specific study characteristics were evaluated through ratings on the Newcastle-Ottawa Scale. This scale assesses aspects of observational studies (eg, completeness of follow-up) often related to study quality, such as appropriateness of selection criteria or comparability of patient groups. Analyses of the relation of Newcastle-Ottawa Scale items to study outcomes were completed via meta-regression analyses.
Publication bias was evaluated by inspection of a funnel plot that related studies’ standard errors to their effect sizes. The underlying notion is that small studies are more likely not to have been published but would generally have had larger standard errors. If the funnel plot shows an asymmetry, it suggests that studies that might have reported negative results may not have been published.43 Because inspection of the funnel plot suggested the possibility of publication bias, we then applied a specific statistical technique that evaluated the effect of possible publication bias on estimates of pooled ORs.44 Finally, to address the question of whether a history of clinical depression was a prodrome of or a risk factor for AD, we conducted a separate metaregression analysis to test the hypothesis that the interval between the diagnoses of depression and AD might be related to the likelihood of developing AD.
Pooled Analyses
Data were subjected to meta-analyses stratified by study type (case-control vs cohort study) to obtain composite estimates of ORs separately for each study type and for all studies combined. Study heterogeneity was assessed through inspection of the funnel plot and by the Egger test.45 We used this test rather than the χ2 test for heterogeneity because of its greater sensitivity to the presence of heterogeneity.46 Odds ratios corrected for possible publication bias were calculated44 to assess the effects of this potential bias. Sensitivity analyses included comparisons of risks in studies that used structured diagnostic criteria for the diagnoses of AD and depression compared with those that used less specific criteria and for prospective vs retrospective cohort studies. Sensitivity analyses also included assessment of the influence of each study on overall estimates of risk by recalculating ORs with 1 study removed and all others included from the pooled estimate over multiple iterations.
Metaregression Analyses
Metaregression analyses allow the application of regression techniques to the prediction of study outcomes so that the relation of other variables of interest to outcomes can be evaluated. The technique is similar to standard linear regression except that it is usually modified to include a term, 'T,41 that estimates the magnitude of variability among studies. Because of its explicit allowance for and estimation of variability among studies, it is similar to random-effects analysis of variance and thus may be called random-effects metaregression. The effects of several variables in combination on study outcome can be evaluated and corrected for, providing estimates of the size of pooled study effects after correction for the variables of interest. In this way, metaregression analysis provides the ability to evaluate the relation of potential covariates and confounding variables to study effects.
To assess the effects of study characteristics on risk, we calculated random-effects metaregression analyses of ORs adjusted for each of the study characteristics assessed by the Newcastle-Ottawa Scale for case-control and cohort studies. Consistent with recommendations against using composite scale scores to control for study quality in meta-analyses and for the use of specific study characteristics for this purpose,47 we used the presence or absence of each study characteristic in the meta-regression. To assess the effect of the interval between depression and AD diagnoses on risk, we completed a random-effects metaregression analysis with the interval between the diagnoses of depression and AD predicting the risk of developing AD. All analyses were completed using routines available for Stata software, version 8.2 (StataCorp, College Station, Tex).
RESULTS
DATA SOURCES, ABSTRACTION, AND STUDY SELECTION
We identified 153 potentially relevant studies from results of electronic searches and reviews of bibliographies. Of these, 16 were review articles that did not include data; 53 provided data but did not allow group comparisons; 43 provided data that were not relevant to the relation of depression and AD; 17 used continuous measures; and 2 included data that appeared to overlap with another study. Twenty studies provided data that could be used in calculating crude ORs. Two additional studies provided data on AD risk in twin samples as unadjusted ORs with 95% confidence intervals (CIs) that could be used in pooling estimates48,49; these were judged potentially relevant but, because the comparison groups differed substantially from those in the other 20 studies, they were excluded from pooled data analyses. We therefore report analyses of these 2 studies separately.
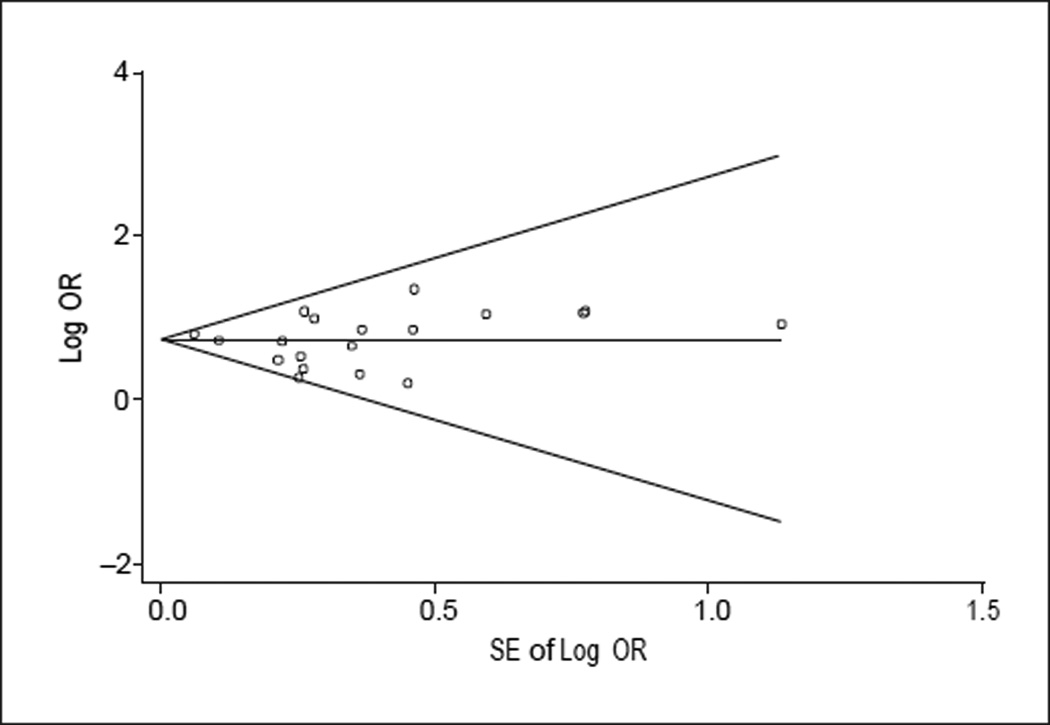
The remaining 20 studies provided data from an aggregate sample of 102 172 persons in 8 countries. Study characteristics are listed in Table 1 and ratings of study quality for each of the Newcastle-Ottawa criteria are presented in Table 2. We completed analyses of effect heterogeneity with case-control and cohort studies in combination and separately for each type of study. For all studies combined, evidence of significant heterogeneity was observed (Egger test, t=2.75; P=.01). For separate analyses by study type, evidence of heterogeneity of borderline statistical significance was found for case-control studies (P=.10), a probability larger than the generally accepted value of .05, but suggestive of the presence of heterogeneity, given the low power of these analyses stemming from the small number of studies involved. Significant heterogeneity was found for cohort studies (P=.02). Given these findings, we therefore completed subsequent meta-analyses of ORs using random-effects models stratified by study type and for all studies combined. A funnel plot was obtained (Figure 1) to visually assess the presence of publication bias. Inspection of this plot suggested the possible presence of publication bias (ie, failure to find studies with negative results). We used the “trim-and-fill” method proposed by Duval and Tweedie44 to obtain estimates of ORs corrected for possible publication bias.
Table 1.
Characteristics of Studies Included in Meta-analyses
| Source | Country | Type | Outcome | Method of Depression Diagnosis |
Method of Dementia Diagnosis |
Sample Size |
Comments |
|---|---|---|---|---|---|---|---|
| French et al,10 1985 |
United States | Case-control | AD | Questionnaire | Nonstandard criteria | 126 | Patients with significant psychiatric history were excluded; numbers have to be inferred from data presented |
| Agbayewa,11 1986 |
Canada | Case-control | AD | Clinical diagnosis | DSM-III,38 ICD-9 | 268 | |
| Shalat et al,13 1987 |
United States | Case-control | AD | Patients, clinical records; controls, questionnaire |
DSM-III,38 NINCDS-ADRDA |
260 | All cases and controls were men |
| Broe et al,12 1990 |
Australia | Case-control | AD | Risk factor interview developed for study |
NINCDS-ADRDA | 340 | Cases drawn from dementia clinics and controls from primary care practices; numbers used in analyses were drawn from review by Jorm et al33 |
| Kokmen et al,6 1991 |
United States | Case-control | AD | Record review | NINCDS-ADRDA (no psychometric data |
830 | |
| Speck et al,7 1995 |
United States | Case-control | AD | Interview |
DSM-III-R,39 NINCDS-ADRDA |
594 | Used data for estimated history >10y to allow analysis of effect of interval between diagnoses |
| Devanand et al,16 1996 |
United States | Prospective cohort |
AD | HAM-D cutoff, empirically validated against SCID |
DSM-III 38 | 456 | Calculated estimated follow-up as weighted average of several waves of data |
| Buntinx et al,15 1996 |
The Netherlands |
Retrospective cohort |
Dementia, similar to AD |
ICHPPC, 3 of 6 listed criteria |
(ICHPPC, similar to DSM criteria for AD) |
19103 | Estimate of interval between depression and dementia onset is a weighted average calculated from information in article |
| Tsolaki et al,8 1997 |
Greece | Case-control | AD | Self-report and informant report of history |
DSM-IV,37 NINCDS-ADRDA 50 |
102 | |
| Chen et al,20 1999 |
United States | Prospective cohort |
AD | CES-D cutoff judged equivalent to DSM-III-R |
DSM-III-R,39 NINCDS-ADRDA |
803 | Calculated follow-up as weighted average across several waves of data collection |
| Palsson et al,21 1999 |
Sweden | Prospective cohort |
Dementia (unspecified type) |
DSM-III-R | Neuropsychiatric examination, no criteria specified |
267 | |
| Zalsman et al,14 2000 |
Israel | Case-control | Dementia (unspecified type) |
DSM-IV | DSM-IV 37 | 502 | Depression only late onset (after age 50 y) but “at least” 3 y between depression and onset of dementia |
| Li et al,22 2001 |
United States | Retrospective cohort |
AD | Personal report of history of depression |
DSM-III,38
DSM-IV,37 NINCDS-ADRDA |
197 | Study reported is a prospective cohort, but data on history of depression are collected retrospectively |
| Lindsay et al,23 2002 |
Canada | Prospective cohort |
AD | Self-administered questionnaire for history |
DSM-III-R39 NINCDS-ADRDA |
3316 | |
| Green et al,9 2003 |
United States | Case-control | AD | Clinical history and self-report |
NINCDS-ADRDA | 4046 | Used data from 10 y for assessment of interval |
| Zubenko et al,17 2003 |
United States | Retrospective cohort |
AD | CADD17 (developed by study authors) |
NINCDS-ADRDA | 394 | Controls came from diverse sources, including primary care practices and spouses of patients |
| Kessing and Nilsson,18 2003 |
Denmark | Retrospective cohort |
Dementia | ICD-8 | ICD-8 | 66986 | Used incidence of AD in patients with osteoarthritis as comparison group |
| Steffens et al,24 2004 |
United States | Prospective cohort |
AD | HAM-D, MADRS, Duke Depression Evaluation Scale |
DSM-IV,37 NINCDS-ADRDA |
403 | |
| Andersen et al,25 2005 |
Denmark | Retrospective cohort |
AD | Clinical diagnosis and self-report |
NINCDS-ADRDA | 1822 | Possible overlap with study by Kessing and Nilsson18 |
| Dal Forno et al,19 2005 |
United States | Prospective cohort |
AD | CES-D cutoff as equivalent of DSM diagnosis of major depression |
NINCDS-ADRDA | 1357 | |
| Total | 102 172 |
Abbreviations: AD, Alzheimer disease; CADD, Clinical Assessment of Depression in Dementia; CES-D, Centerfor Epidemiological Studies Depression Rating Scale51; HAM-D, Hamilton Depression Rating Scale52; ICD-8, International Classification of Diseases, Eighth Revision; ICD-9, International Classification of Diseases, Ninth Revision; ICHPPC, International Classification of Health Problems in Primary Care54; MADRS, Montgomery-Asberg Depression Rating Scale53; NINCDS-ADRDA, National Institute of Neurological and Communication Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria for the diagnosis of AD50; SCID, Structured Clinical Interview for DSM Disorders.55
Table 2.
Assessment of Study Quality
| Quality Indicators From Newcastle-Ottawa Scale40* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Studies | 1 | 2 | 3 | 4 | 5A | 5B | 6 | 7 | 8 |
| Case-control | |||||||||
| French et al,10 1985 | No | Yes | No | Yes | Yes | Yes | Yes | Yes | No |
| Agbayewa,11 1986 | No | Yes | No | No | Yes | Yes | No | Yes | No |
| Shalat et al,13 1987 | Yes | No | Yes | Yes | Yes | Yes | No | Yes | No |
| Broe et al,12 1990 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No |
| Kokmen et al,6 1991 | No | Yes | No | Yes | Yes | Yes | No | Yes | No |
| Speck et al,7 1995 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Tsolaki et al,8 1997 | Yes | No | Yes | Yes | No | No | No | No | No |
| Zalsman et al,14 2000 | No | Yes | No | No | No | No | No | No | No |
| Green et al,9 2003 | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
| Cohort | |||||||||
| Buntinx et al,15 1996 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Devanand et al,16 1996 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Chen et al,20 1997 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Palsson et al,21 1999 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Li et al,22 2001 | No | No | No | Yes | No | No | No | No | Yes |
| Lindsay et al,23 2002 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Zubenko et al,17 2003 | Yes | Yes | Yes | Yes | No | No | Yes | No | Yes |
| Kessing and Nilsson,18 2003 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes |
| Steffens et al,24 2004 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Dal Forno etal,19 2005 | No | Yes | No | Yes | Yes | Yes | No | Yes | No |
| Andersen et al,25 2005 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes |
For case-control studies, 1 indicates cases independently validated; 2, cases are representative of population; 3, community controls; 4, controls have no history of Alzheimer disease; 5A, study controls forage; 5B, study controls for additional factor(s); 6, ascertainment of exposure by blinded interview or record; 7, same method of ascertainment used for cases and controls; and 8, nonresponse rate the same for cases and controls. For cohort studies, 1 indicates exposed cohort truly representative; 2, nonexposed cohort drawn from the same community; 3, ascertainment of exposure; 4, outcome of interest not present at start; 5A, cohorts comparable on basis of age; 5B, cohorts comparable on other factor(s); 6, quality of outcome assessment; 7, follow-up long enough for outcomes to occur; and 8, complete accounting for cohorts.
Figure 1.
Funnel plot assessing potential publication bias. The horizontal line indicates the average effect size as the log of the odds ratio (OR); diagonal lines, approximate 95% confidence intervals for estimates.
DATA SYNTHESIS
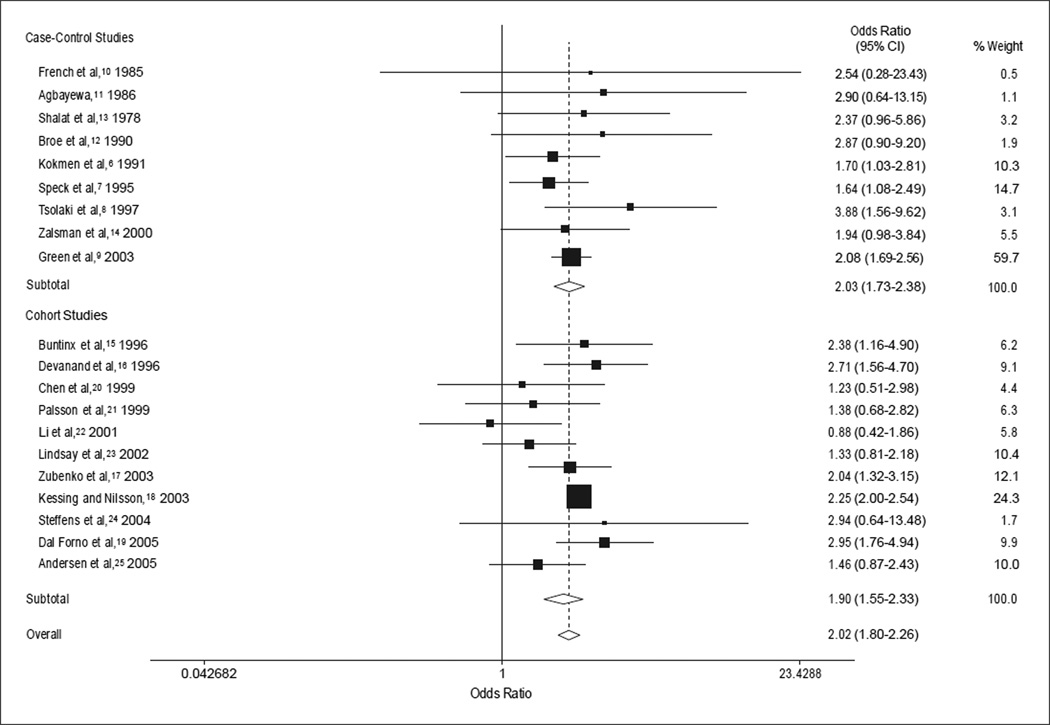
Forest plots for random-effects meta-analysis stratified by study type and for all studies combined are presented in Figure 2. The OR for case-control studies was 2.03 (95% CI, 1.73–2.38; z = 8.65; P<.001) and for cohort studies, 1.90 (95% CI, 1.55–2.33; z = 6.15; P<.001). The estimate for all studies combining both case-control and cohort studies was 2.02 (95% CI, 1.80–2.26; z = 12.07; P<.001). Stratified analyses for prospective vs retrospective cohort studies yielded pooled OR estimates of 1.78 (95% CI, 1.16–2.73; z = 2.64; P = .008) for 6 prospective cohort studies and 2.11 (95% CI, 1.82–2.45; z = 9.73; P<.001) for 5 retrospective studies.
Figure 2.
Forest plots for random effects meta-analyses. CI indicates confidence interval.
Stratified analyses for use of structured criteria (DSM or the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association) for AD diagnosis yielded pooled ORs of 2.22 (95% CI, 1.98–2.49; z = 13.67; P<.001) for studies not using structured criteria and 1.91 (95% CI, 1.62–2.26; z = 7.54; P<.001) for studies using structured criteria. Evaluation of studies that did not use specific diagnostic criteria for depression (DSM or ICD) yielded an OR of 1.85 (95% CI, 1.58–2.17; z = 7.68; P<.001) whereas that for studies that did use specific criteria was 2.23 (95% CI, 2.00–2.48; z = 14.68; P<.001). The pooled OR for the 4 studies that used structured or specific diagnostic criteria for both depression and AD diagnoses was 2.30 (95% CI, 1.71–3.09; z = 5.53; P<.001). Only studies that found sex differences in ORs reported data on sex differences in AD risk, so that pooled comparisons of risk by sex might have yielded misleading results because of reporting bias.
Evaluation of possible publication bias suggested its presence, and we used a technique that estimates the number and magnitude of missing studies and then calculates a corrected OR to further evaluate the importance of this possible bias.44 Random-effects ORs corrected in this way for publication bias were 1.96 (95% CI, 1.68–2.30; z = 8.50; P<.001) for case-control studies, 1.90 (95% CI, 1.55–2.33; z = 6.16; P<.001) for cohort studies, and 1.98 (95% CI, 1.76–2.24; z = 11.19; P<.001) for all studies combined. Correction for possible publication bias thus did not substantially change estimates of ORs.
We recognized that some of the 6 prospective cohort studies might have included persons with a history of depression in their comparison groups. The reports of 2 of the prospective cohort studies explicitly controlled for this possibility, in one instance by excluding persons with a history of depression from their comparison group24 and in the other by characterizing patients as having early-or late-onset depression.21 Reports of the remaining 4 studies did not explicitly state how this issue had been dealt with. The OR for the 2 prospective cohort studies that accounted for personal history of depression was 1.58 (95% CI, 0.83–3.02) whereas the OR for the prospective studies that did not state how they accounted for a history of depression was 1.92 (95% CI, 1.34–2.74).
We evaluated the relation of study quality and observed risk through metaregression of study characteristic ratings and log ORs. This analysis was undertaken to further assess the possibility that the observed increase in risk of developing AD in depressed patients might be related to specific study characteristics not accounted for in stratified analyses. The OR for case-control studies adjusted in a metaregression analysis for the presence of quality characteristics from the Newcastle-Ottawa Scale (Table 2) was 4.14 (95% CI, 1.14–15.11; z = 2.16; P = .03). The similarly adjusted OR for cohort studies was 3.85 (95% CI, 1.88–7.89; z = 3.69; P<.001). In the regression model for case-control studies, none of the individual study characteristics was independently significantly related to the OR. In the combined regression of all cohort study variables on the OR, 1 study characteristic was significantly and negatively related to the predicted OR. This characteristic was the completeness with which study cohorts were followed up (eg, whether all participants who began the study were accounted for at the study’s end; coefficient, −1.48; SE, −0.53; z = −2.79; P = .005). Correction of ORs for case-control and cohort studies for characteristics assessed by the Newcastle-Ottawa Scale, whether or not the characteristics were significant predictors of the log OR, did not change the inference that a history of depression may confer an increased risk for developing AD.
We completed influence analyses by recalculating pooled ORs for the sample on multiple occasions with 1 of the studies removed at each iteration. These analyses were especially important because several studies15,18 included samples that were substantially larger than most of the other studies and thus may have exerted large effects on overall effect estimates. For all studies, these analyses yielded ORs ranging from 1.93 (95% CI, 1.69–2.20) to 2.12 (95% CI, 1.94–2.31). Influence analyses stratified by study type yielded a range of ORs from 1.96 (95% CI, 1.52–2.52) to 2.07 (95% CI, 1.75–2.45) for case-control studies and from 1.81 (95% CI, 1.45–2.24) to 2.03 (95% CI, 1.71–2.41) for cohort studies.
As noted, 2 studies48,49 reported results from samples of twins in the form of ORs and CIs so that pooled estimates could be calculated. These results were judged potentially relevant but difficult to compare with other studies. The pooled OR for AD with a history of depression for these 2 studies was 2.53 (95% CI, 1.00–6.39; z = 1.97; P = .05).
Thirteen studies, including 2 case-control and 11 cohort studies, provided data on the interval between the diagnoses of depression and AD. Metaregression of the log OR for each study on the interval in years showed a positive and statistically significant relation (Table 3). Because whether a study was carried out retrospectively or prospectively might have been related to the length of the observed interval between the diagnoses of depression and AD, a second metaregression analysis was completed. This analysis showed that even after correction for this element of the study design, the relation between interval length and risk for AD continued to be significant and positive (coefficient, 0.003; SE, 0.001; z=2.01; P=.05). The interval between the diagnoses of depression and AD was thus positively related to increased risk for developing AD. We explored the possibility of a nonlinear relation between interval and risk in the metaregression via regression models that included fractional polynomial coefficients.56 In no case did adding coefficient terms of higher powers significantly improve fit, as assessed by change in the deviance statistic, of regression models when compared with the more parsimonious baseline linear coefficients model.
Table 3.
Metaregression Analyses
| Variable | OR (95% CI)* | z Score | P Value | χ2 Statistic† | Coefficient | z Score | P Value |
|---|---|---|---|---|---|---|---|
| Unadjusted | 2.03 (1.81–2.28) | 12.00 | <.001 | 0.0053 | … | … | … |
| Adjusted for interval (in years) between depression and AD diagnoses |
1.53 (1.11–2.11) | 2.58 | .01 | 0.00 | 0.04‡ | 2.01 | .04 |
Abbreviations: AD, Alzheimer disease; CI, confidence interval; OR, odds ratio; ellipses, not applicable.
Indicates OR for developing AD in individuals with a history of depression without and with adjustment for interval between diagnoses of depression and AD.
Indicates the estimate of between-study variability.41
Indicates the regression coefficient for the variable representing the interval between the two diagnoses.
COMMENT
The purpose of this study was to investigate the relation of a history of depression to risk for subsequent development of AD. Results of this meta-analysis show that persons with a history of depression were more likely to be diagnosed as having AD later in life. This finding was robust across analyses stratified by study type, retrospective vs prospective data collection, and strictness of diagnostic criteria used for AD and depression. The ORs were still significantly greater than 1 when adjusted in separate metaregression analyses for case-control and cohort studies for 8 quality indicators from the Newcastle-Ottawa Scale. The ORs were also significantly greater than 1 in metaregression analyses that controlled for the interval between the diagnoses of depression and AD.
Influence analysis showed no substantial difference in pooled ORs when any single study was excluded. This was important because 1 cohort study18 contributed more than 65 000 individuals to the total sample of more than 100 000. Although heterogeneity was found across studies, all but 1 study examined found an increased (although sometimes nonsignificant) risk for AD in persons with a history of depression. Although significant heterogeneity was observed in effect sizes, it should be noted that 19 of the 20 studies used in these analyses yielded a positive relation of history of depression to risk for developing AD. Previous reports of negative findings may thus be attributable to failure to find statistical significance rather than reduced or no change in risk for AD in individuals with a history of depression.
A secondary purpose of this study was to evaluate whether observed risk for developing AD was related to the interval between diagnoses of depression and AD. This interval was positively and significantly related to the odds of developing AD in a metaregression analysis. This finding is consistent with the interpretation that occurrence of depression is a risk factor for AD rather than a prodrome of the disease, and it is also consistent with the results of 1 large study that specifically examined the issue.9 Given the small number of studies included in the metaregression, however, this interpretation must be tentative, but it further strengthens the observation that depression is a distinct risk factor for AD. It should be recognized as well, however, that these findings do not rule out the possibility that depression is both a remote risk factor for AD and a proximal prodromal feature of it.57
Limitations of this study should be acknowledged. These data relate only the occurrence of at least 1 episode of diagnosable depression to later risk for AD and neglect the fact that both depression and AD are heterogeneous entities that may reflect multiple underlying pathologies.58 Several studies used data on hospitalizations due to depression as an indicator of clinical depression whereas others recruited nonpatients and then examined them for depression on the basis of current symptoms. Groups of persons who were so severely depressed as to require hospitalization may not be equivalent to outpatients whose depression was discovered during study evaluations. Data used in the meta-analysis do not distinguish among the risk for AD in persons who may have had 1 episode of depression and recovered compared with those who have had multiple episodes or who may have had chronic minor depression. In this connection, 1 study59 found that risk for dementia increased with multiple episodes of depression. By choosing to include studies that allowed us to calculate crude ORs, we excluded studies that provided estimates of the relation between depression and AD risk in the form of adjusted ORs or on the basis of continuous measures. Exclusion of these studies may have biased our results.
These data do not provide information about why depression and AD may be linked. There is increasing awareness of the possible role of vascular disease in the expression of the clinical symptoms of AD, and it has been reported that AD and depression may share risk factors for vascular disease.60,61 One possible link may be long-term occurrence of inflammatory processes that may underlie depression and AD.62 Several proinflammatory cytokines (eg, tumor necrosis factor a) have been linked to both vascular disease and depression,63–65 and the same cytokines may have direct effects on cognitive status.66 Possible genetic links between the 2 disorders have also been explored, most notably between apolipoprotein E ε4 and the disorders, because the ε4 allele is an established risk factor for AD. 67 Research on the co-occurrence of apolipoprotein E in depression and AD has been inconclusive, with at least 1 study68 finding a cross-sectional relation between the ε4 genotype and depressive symptoms whereas other studies48,69 have not found a relation. Other authors61,70,71 have speculated on potential mechanisms for the link between mood disorder and AD. The findings of this meta-analysis and meta-regression thus may have implications for those investigating the biochemical etiology of AD.
The clinical significance of the findings of the meta-analysis and metaregression should also be recognized. From one perspective, the absolute risk for AD conferred by a history of depression is small so that the importance of having a history of depression should not be overly emphasized in work with patients. On the other hand, however, depression may be viewed as a modifiable risk factor for AD if, in fact, depression is part of the same disease process that produces clinical AD, and its treatment will affect cognitive outcome. This possibility is intriguing in light of evidence that antidepressants can modify levels of inflammatory cytokines,72 but the possibility that treatment with antidepressants might reduce the risk for AD remains speculative without additional studies.
This meta-analysis and metaregression thus help clarify the relation between a history of depression and the subsequent risk of developing AD by drawing together data from numerous sources and exploring several possible reasons for the observed heterogeneity among studies. Results were found to be robust in several analyses stratified on study characteristics and in meta-analyses that controlled for study quality and explored the relation of the interval between the two diagnoses on risk for AD. These findings thus underscore the possible relation between the two disorders and the importance of continued research on the common and disparate factors in the etiology of depression and AD.
Acknowledgment
We thank Carmen Bou-Crick, MSLS, for her assistance in completing the electronic bibliographic searches reported herein.
Funding/Support: This study was supported by grant K23 AG 19745 from the National Institute on Aging (Dr Ownby).
REFERENCES
- 1.Alexopoulos GS, Chester JG. Outcomes of geriatric depression. Clin Geriatr Med. 1992;8:363–376. [PubMed] [Google Scholar]
- 2.Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry. 1999;56:425–430. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]
- 3.Callahan CM, Kroenke K, Counsell SR, Hendrie HC, Perkins AJ, Katon W, Noel PH, Harpole L, Hunkeler EM, Unutzer J IMPACT Investigators. Treatment of depression improves physical functioning in older adults. J Am Geriatr Soc. 2005;53:367–373. doi: 10.1111/j.1532-5415.2005.53151.x. [DOI] [PubMed] [Google Scholar]
- 4.Wragg RE, Jeste DV. Overview of depression and psychosis in Alzheimer’s disease. Am J Psychiatry. 1989;146:577–587. doi: 10.1176/ajp.146.5.577. [DOI] [PubMed] [Google Scholar]
- 5.Espiritu DAV, Rashid H, Mast BT, Fitzgerald J, Steinberg J, Lichetnberg PA. Depression, cognitive impairment and function in Alzheimer’s disease. Int J Geriatr Psychiatry. 2001;16:1098–1103. doi: 10.1002/gps.476. [DOI] [PubMed] [Google Scholar]
- 6.Kokmen E, Beard CM, Chandra V, Offord KP, Schoenberg BS, Ballard DJ. Clinical risk factors for Alzheimer’s disease: a population-based case-control study. Neurology. 1991;41:1393–1397. doi: 10.1212/wnl.41.9.1393. [DOI] [PubMed] [Google Scholar]
- 7.Speck CE, Kukull WA, Brenner DE, Bowen JD, McCormick WC, Teri L, Pfanschmidt ML, Thompson JD, Larson EB. History of depression as a risk factor for Alzheimer’s disease. Epidemiology. 1995;6:366–369. doi: 10.1097/00001648-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Tsolaki M, Fountoulakis K, Chantzi E, Kazis A. Risk factors for clinically diagnosed Alzheimer’s disease: a case-control study of a Greek population. Int Psychogeriatr. 1997;9:327–341. doi: 10.1017/s104161029700447x. [DOI] [PubMed] [Google Scholar]
- 9.Green RC, Cupples LA, Kurz A, Auerbach S, Go R, Sadovnick D, Duara R, Kukull WA, Chui H, Edeki T, Griffith PA, Friedland RP, Bachman D, Farrer L. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol. 2003;60:753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- 10.French LR, Schuman LM, Mortimer JA, Hutton JT, Boatman RW, Christians B. A case-control study of dementia of the Alzheimer type. Am J Epidemiol. 1985;121:414–421. doi: 10.1093/oxfordjournals.aje.a114013. [DOI] [PubMed] [Google Scholar]
- 11.Agbayewa MO. Earlier psychiatric morbidity in patients with Alzheimer’s disease. J Am Geriatr Soc. 1986;34:561–564. doi: 10.1111/j.1532-5415.1986.tb05759.x. [DOI] [PubMed] [Google Scholar]
- 12.Broe GA, Henderson AS, Creasey H, McCusker E, Korten AE, Jorm AF, Longley W, Anthony JC. A case-control study of Alzheimer’s disease in Australia. Neurology. 1990;40:1698–1707. doi: 10.1212/wnl.40.11.1698. [DOI] [PubMed] [Google Scholar]
- 13.Shalat SL, Seltzer B, Pidcock C, Baker EL., Jr Risk factors for Alzheimer’s disease: a case-control study. Neurology. 1987;37:1630–1633. doi: 10.1212/wnl.37.10.1630. [DOI] [PubMed] [Google Scholar]
- 14.Zalsman G, Aizenberg D, Sigler M, Nahshony E, Karp L, Weizman A. Increased risk for dementia in elderly psychiatric inpatients with late-onset major depression. J Nerv Ment Dis. 2000;188:242–243. doi: 10.1097/00005053-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Buntinx F, Kester A, Bergers J, Knottnerus JA. Is depression in elderly people followed by dementia? a retrospective cohort study based in general practice. Age Ageing. 1996;25:231–233. doi: 10.1093/ageing/25.3.231. [DOI] [PubMed] [Google Scholar]
- 16.Devanand DP, Sano M, Tang MX, Taylor S, Gurland BJ, Wilder D, Stern Y, Mayeux R. Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- 17.Zubenko GS, Zubenko WN, McPherson S, Spoor E, Marin DB, Farlow MR, Smith GE, Geda YE, Cummings JL, Petersen RC, Sunderland T. A collaborative study of the emergence and clinical features of the major depressive syndrome of Alzheimer’s disease. Am J Psychiatry. 2003;160:857–866. doi: 10.1176/appi.ajp.160.5.857. [DOI] [PubMed] [Google Scholar]
- 18.Kessing LV, Nilsson FM. Increased risk of developing dementia in patients with major affective disorders compared to patients with other medical illnesses. J Affect Disord. 2003;73:261–269. doi: 10.1016/s0165-0327(02)00004-6. [DOI] [PubMed] [Google Scholar]
- 19.Dal Forno G, Palermo MT, Donohue JE, Karagiozis H, Zonderman AB, Kawas CH. Depressive symptoms, sex, and risk for Alzheimer’s disease. Ann Neurol. 2005;57:381–387. doi: 10.1002/ana.20405. [DOI] [PubMed] [Google Scholar]
- 20.Chen P, Ganguli M, Mulsant BH, DeKosky ST. The temporal relationship between depressive symptoms and dementia: a community-based prospective study. Arch Gen Psychiatry. 1999;56:261–266. doi: 10.1001/archpsyc.56.3.261. [DOI] [PubMed] [Google Scholar]
- 21.Palsson S, Aevarsson O, Skoog I. Depression, cerebral atrophy, cognitive performance and incidence of dementia. Br J Psychiatry. 1999;174:249–253. doi: 10.1192/bjp.174.3.249. [DOI] [PubMed] [Google Scholar]
- 22.Li YS, Meyer JS, Thornby J. Longitudinal follow-up of depressive symptoms among normal versus cognitively impaired elderly. Int J Geriatr Psychiatry. 2001;16:718–727. doi: 10.1002/gps.423. [DOI] [PubMed] [Google Scholar]
- 23.Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 24.Steffens DC, Welsh-Bohmer KA, Burke JR, Plassman BL, Beyer JL, Gersing KR, Potter GG. Methodology and preliminary results from the neurocognitive outcomes of depression in the elderly study. J Geriatr Psychiatry Neurol. 2004;17:202–211. doi: 10.1177/0891988704269819. [DOI] [PubMed] [Google Scholar]
- 25.Andersen K, Lolk A, Kragh-Sorensen P, Petersen NE, Green A. Depression and the risk of Alzheimer disease. Epidemiology. 2005;16:233–238. doi: 10.1097/01.ede.0000152116.32580.24. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Meyer JS, Thornby J. Depressive symptoms among cognitively normal versus cognitively impaired elderly subjects. Int J Geriatr Psychiatry. 2001;16:455–461. doi: 10.1002/gps.360. [DOI] [PubMed] [Google Scholar]
- 27.Wilson RS, Schneider JA, Bienias JL, Arnold SE, Evans DA, Bennett DA. Depressive symptoms, clinical AD, and cortical plaques and tangles in older persons. Neurology. 2003;61:1102–1107. doi: 10.1212/01.wnl.0000092914.04345.97. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Fuhrer R, Dufouil C, Dartigues JF PAQUID Study. Exploring sex differences in the relationship between depressive symptoms and dementia incidence: prospective results from the PAQUID Study. J Am Geriatr Soc. 2003;51:1055–1063. doi: 10.1046/j.1532-5415.2003.51352.x. [DOI] [PubMed] [Google Scholar]
- 30.Fuhrer R, Antonucci TC, Gagnon M, Dartigues JF, Barberger-Gateau P, Alperovitch A. Depressive symptomatology and cognitive functioning: an epidemiological survey in an elderly community sample in France. Psychol Med. 1992;22:159–172. doi: 10.1017/s0033291700032815. [DOI] [PubMed] [Google Scholar]
- 31.Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- 32.Stewart R. Depressive symptoms and cognitive decline—disentangling the effect of affect. J Neurol Neurosurg Psychiatry. 2004;75:5. doi: 10.1136/jnnp.2003.028399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jorm AF, van Duijn CM, Chandra V, Fratilioni L, Graves AB, Heyman A, Kokmen E, Kondo K, Mortimer JA, Rocca WA, Shalat SL, Soininen H, Hofman A EURODEM Risk Factors Research Group. Psychiatric history and related exposures as risk factors for Alzheimer’s disease: a collaborative re-analysis of case-control studies. Int J Epidemiol. 1991;20(suppl 2):S43–S47. doi: 10.1093/ije/20.supplement_2.s43. [DOI] [PubMed] [Google Scholar]
- 34.Jorm AF. Is depression a risk factor for dementia or cognitive decline? a review. Gerontology. 2000;46:219–227. doi: 10.1159/000022163. [DOI] [PubMed] [Google Scholar]
- 35.McDonald S, Taylor L, Adams C. Searching the right database: a comparison of four databases for psychiatry journals. Health Libr Rev. 1999;16:151–156. doi: 10.1046/j.1365-2532.1999.00222.x. [DOI] [PubMed] [Google Scholar]
- 36.Health Information Research Unit. [Accessed April 13, 2004];The “hedges” project. http://hiru.mcmaster.ca/hedges/indexhiru.htm.
- 37.Amercian Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 38.Amercian Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- 39.Amercian Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Revised. 3rd ed. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 40.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. [Accessed April 29, 2004];The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- 41.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Smith GD, Altman DG, editors. Systematic Reviews in Health Care: Meta-analysis in Context. 2nd ed. London, England: BMJ Publishing; 2001. pp. 285–312. [Google Scholar]
- 42.Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychol Methods. 1998;3:486–504. [Google Scholar]
- 43.Sterne JAC, Egger M, Smith GD. Investigating and dealing with publication and other biases. In: Egger M, Smith GD, Altman DG, editors. Systematic Reviews in Health Care: Meta-analysis in Context. London, England: BMJ Publishing; 2001. pp. 189–208. [Google Scholar]
- 44.Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 45.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med. 1998;17:841–856. doi: 10.1002/(sici)1097-0258(19980430)17:8<841::aid-sim781>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 47.Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054–1060. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- 48.Steffens DC, Plassman BL, Helms MJ, Welsh-Bohmer KA, Saunders AM, Breitner JC. A twin study of late-onset depression and apolipoprotein E ε4 as risk factors for Alzheimer’s disease. Biol Psychiatry. 1997;41:851–856. doi: 10.1016/S0006-3223(96)00247-8. [DOI] [PubMed] [Google Scholar]
- 49.Wetherell JL, Gatz M, Johansson B, Pedersen NL. History of depression and other psychiatric illness as risk factors for Alzheimer disease in a twin sample. Alzheimer Dis Assoc Disord. 1999;13:47–52. doi: 10.1097/00002093-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 50.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 51.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 52.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montgomery SA, Asberg M. A new depression rating scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 54.ICHPPC-2 Defined: International Classification of Health Problems in Primary Care. New York, NY: Oxford University Press Inc; 1987. Classification Committee of the World Organization of National Colleges and Academies. [Google Scholar]
- 55.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version. Washington, DC: American Psychiatric Press Inc; 1997. [Google Scholar]
- 56.Bagnardi V, Zambon A, Quatto P, Corrao G. Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. Am J Epidemiol. 2004;159:1077–1086. doi: 10.1093/aje/kwh142. [DOI] [PubMed] [Google Scholar]
- 57.Alexopoulos GS, Buckwalter K, Olin J, Martinez R, Wainscott C, Krishnan KRR. Comorbidity of late life depression: an opportunity for research on mechanisms and treatment. Biol Psychiatry. 2002;52:543–558. doi: 10.1016/s0006-3223(02)01468-3. [DOI] [PubMed] [Google Scholar]
- 58.Lyketsos CG, Lee H. Insulin resistance as a link between affective disorder and Alzheimer’s disease: a hypothesis in need of refinement. J Gerontol A Biol Med Sci. 2004;59:M185–M187. [Google Scholar]
- 59.Kessing LV, Andersen PK. Does the risk of developing dementia increase with the number of episodes in patients with depressive disorder and in patients with bipolar disorder? J Neurol Neurosurg Psychiatry. 2004;75:1662–1666. doi: 10.1136/jnnp.2003.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iadecola C, Gorelick PB. Converging pathogenic mechanisms in vascular and neurodegenerative dementia. Stroke. 2003;34:335–337. doi: 10.1161/01.str.0000054050.51530.76. [DOI] [PubMed] [Google Scholar]
- 61.Rasgon N, Jarvik L. Insulin resistance, affective disorders, and Alzheimer’s disease: review and hypothesis. J Gerontol A Biol Med Sci. 2004;59:M178–M183. doi: 10.1093/gerona/59.2.m178. [DOI] [PubMed] [Google Scholar]
- 62.Baldwin RC. Is vascular depression a distinct subtype of depressive disorder? a review of causal evidence. Int J Geriatr Psychiatry. 2005;20:1–11. doi: 10.1002/gps.1255. [DOI] [PubMed] [Google Scholar]
- 63.Parissis JT, Adamopoulos S, Rigas A, Kostakis G, Karatzas D, Venetsanu K, Kremastinos DT. Comparison of circulating proinflammatory cytokines and soluble apoptosis mediators in patients with chronic heart failure with versus without symptoms of depression. Am J Cardiol. 2004;94:1326–1328. doi: 10.1016/j.amjcard.2004.07.127. [DOI] [PubMed] [Google Scholar]
- 64.Dantzer R, Wollman E, Vitkovic L, Yirmiya R. Cytokines and depression: fortuitous or causative association? Mol Psychiatry. 1999;4:328–332. doi: 10.1038/sj.mp.4000572. [DOI] [PubMed] [Google Scholar]
- 65.Suarez EC, Lewis JG, Krishnan RR, Young KH. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29:1119–1128. doi: 10.1016/j.psyneuen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 66.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 67.St George-Hyslop PH, Petit A. Molecular biology and genetics of Alzheimer’s disease. CR Biol. 2005;328:119–130. doi: 10.1016/j.crvi.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 68.Lavretsky H, Ercoli L, Siddarth P, Bookheimer S, Miller K, Small G. Apolipoprotein ε4 allele status, depressive symptoms, and cognitive decline in middle-aged and elderly persons without dementia. Am J Geriatr Psychiatry. 2003;11:667–673. doi: 10.1176/appi.ajgp.11.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Butters MA, Sweet RA, Mulsant BH, Ilyas Kamboh M, Pollock BG, Begley AE, Reynolds CF, III, DeKosky ST. APOE is associated with age-of-onset, but not cognitive functioning, in late-life depression. Int J Geriatr Psychiatry. 2003;18:1075–1081. doi: 10.1002/gps.1006. [DOI] [PubMed] [Google Scholar]
- 70.Lee HB, Lyketsos CG. Depression in Alzheimer’s disease: heterogeneity and related issues. Biol Psychiatry. 2003;54:353–362. doi: 10.1016/s0006-3223(03)00543-2. [DOI] [PubMed] [Google Scholar]
- 71.Heininger K. A unifying hypothesis of Alzheimer’s disease, III: risk factors. Hum Psychopharmacol. 2000;15:1–70. doi: 10.1002/(SICI)1099-1077(200001)15:1<1::AID-HUP153>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 72.Castanon N, Leonard BE, Neveu PJ, Yirmiya R. Effects of antidepressants on cytokine production and actions. Brain Behav Immun. 2002;16:569–574. doi: 10.1016/s0889-1591(02)00008-9. [DOI] [PubMed] [Google Scholar]