Abstract
Resting frontal encephalographic (EEG) asymmetry, often conceptualized as a trait marker for depression, is influenced by occasion-specific factors, including time of year and the time of day of the recording session as demonstrated recently (Peterson & Harmon Jones, 2009). The current study examined the influence of seasonal and chronological variables on resting frontal asymmetry, and also assessed whether different reference montages or surface transformations were equally susceptible to these influences. In a direct replication attempt, contrary to previous findings, no simple time of year by time of day interaction was found. Time awake at recording, however, was an important moderating variable of the relationship between photoperiod and time of day. EEG asymmetry scores based on current-source density (CSD) transformed data, however, appeared less vulnerable to these influences, providing further evidence to suggest that the CSD transform may be advantageous for examining stable trait estimates of frontal EEG asymmetry.
Introduction
Over the last several decades, resting frontal alpha electroencephalographic (EEG) asymmetry has emerged as a promising trait marker of risk for types of psychopathology associated with emotion dysregulation such as depression (see Thibodeau, Jorgenson, and Kim, 2006, for a review). Relatively less left than right frontal activity – where activity is considered the inverse of alpha power – has been associated with withdrawal motivation and a history of and possible propensity towards mood disorders; conversely, relatively greater left than right frontal activity has been shown to relate to approach motivation and a possible decreased diathesis towards depression (e.g., Allen, Urry, Hitt, & Coan, 2004; Coan & Allen, 2003; Coan, Allen, & Harmon-Jones, 2001). It is estimated that approximately 60% of variance in frontal brain asymmetry can be attributed to stable trait sources, and almost 40% to occasion-specific effects (Hagemann, Naumann, Thayer, & Bartussek 2002; Hagemann, Hewig, Seifert, Naumann, & Bartussek, 2005), and very little to unreliability of measurement (Hagemann et al., 2002; Towers & Allen, 2009). Because occasion-specific influences may limit the utility of frontal alpha asymmetry as a risk indicator for depression, factors that may contribute to such variance merit closer examination.
EEG Recording as a Function of Season and Time of Day
Recently, Peterson and Harmon-Jones (2009) examined two state factors that have bearing on all frontal alpha asymmetry studies: Time of Day (TOD) and Time of Year (TOY). Their study used an averaged (“linked”)-ears reference and a between-subjects deigns where participants were allowed to self select session times. Their findings indicated that participants run on fall mornings showed less relative left frontal activity than participants run on spring mornings. Because more relative left frontal activity is typically associated with more approach type behaviors and positive affect and less relative left frontal activity is associated with withdrawal behaviors (Coan and Allen, 2004), these findings might suggest motivational differences as a function of TOD and TOY that have been largely uncontrolled and unaccounted for (Peterson & Harmon-Jones, 2009). Similarly, studies on seasonal variation of mood in community samples suggest TOY may be a very important variable to consider in research examining links between emotion and regional brain activity. In non-clinical samples, winter months are associated with the highest self-reported depressive symptom ratings, measured both prospectively and retrospectively (Murray, Allen, & Trinder, 2001; Nayyar & Cochrane, 1996), intimating that there will be a significant pattern of mood change as a function of season that might be reflected in patterns of resting frontal EEG asymmetry, and accounting for some of the non-trait-related variance in resting frontal brain asymmetry.
This possibility is further supported by studies that identify a seasonal pattern in cortisol. For example, Walker, Best, Noon, Walk, and Webb (1997) found that cortisol was significantly higher in the winter compared to summer months with mixed results in fall and spring. In contrast, King et al. (2000) found that fall and winter cortisol levels were highest compared to spring and summer, but other research employing a student sample reported that cortisol levels were highest in the spring (Malarkey, Pearl, Demers, Glaser, & Glaser, 1995). Moreover, acute cortisol administration is associated with increased relative right frontal activity (Tops, Wijers, van Staveren, Bruin, Den Boer, Meijman, & Korf, 2005) Although the present study did not assess cortisol1, collectively these findings bolster the possibility that TOD and TOY may exert uncontrolled influence on measures of frontal EEG asymmetry.
Sleep-Wake Cycles: Light Exposure and Time Awake
While the exact mechanisms of seasonal mood change are a matter of debate, the literature suggests that a key factor is fluctuations in natural light exposure as a result changes in length of sunlight due to seasonal change, often referred to as photoperiod. Interventions deriving from the photoperiod hypothesis have been designed to alter bright full-spectrum light exposure, and have shown efficacy in altering mood (Rosenthal, Sack, Skwerer, Jacobsen, & Wehr, 1988). Depressive symptoms have been shown to be reduced in both depressed and nondepressed subjects after bright light exposure (e.g., Derogatis, Lipman, & Covi, 1973; Golden et al., 2005; Partonen & Lönnqvist, 1999), with morning light exposure being more effective than light exposure at other times of day (Eastman Young, Fogg, Liu,& Meaden, 1998; Sack et al., 1990; Eastman Young, Fogg, Liu,& Meaden, 1998), although some evidence suggests that a combination of morning and evening light exposure is superior to any single time exposure (Lee, Blashko, Janzen, Paterson, & Chan, 1997). Also intriguing is the growing body of research using artificial light to simulate early sunrises in winter months. Longer, more intense artificial dawn simulations in patients with seasonal affective disorder (SAD) reduce depressive symptoms more than shorter, less intense simulations (Avery et al., 1993, Avery, Bolte, Wolfson, & Kazaras, 1994; Terman & Terman, 2006). One study also found that dawn simulation has a more efficient anti-depressant effect then later morning light exposure (Avery et al., 2001). These findings indicate that cyclical variation in sunrise time is an important factor to consider when examining the link between season, mood, and (potentially) EEG asymmetry. It should be noted that within summer months, individuals experience more morning light exposure between waking hours and noon compared to winter months, although the most significant difference is in the afternoons (Guillemette, Hèbert, Paquet, & Dumont, 1997).
Individuals have a unique biologically and exogenously-driven (e.g., by environmental and social factors) sleep wake cycle, which is often explained by the two process model of sleep (Achermann, 2004). This model explains sleep’s relationships to various physiological and behavioral variables (Achermann, 2004). It posits that there are two underlying processes -- a homeostatic and a circadian component – involved in sleep behavior that operate individually and as an non-additive interaction to regulate sleep and determine sleep propensity, timing of sleep, sleep intensity, and relate to day time to positive affect (Achermann, 2004, Murray et al., 2009). The homeostatic component is primarily defined by sleep onset, sleep offset, and time of day (Achermann, 2004, Dijk & von Schantz, 2005). As time from sleep offset increases the propensity towards sleep increases. The circadian process is an endogenous element thought to be generated in the hypothalmous (Achermann, 2004, Dijk & von Schantz, 2005). This component relates to diurnal preference and hormone peaks as well as interacts with environmental cues such as such light (Dijk & von Schantz, 2005). An underlying element of the circadian process is a pattern of endocrine secretions such as cortisol and melatonin. Research has found that photoperiod (specifically the time of sunrise) is linked to phase shifting in sleep times (Binkley, Tome, Kosher, & Mosher, 1990). Melatonin and cortisol levels have been shown to reach their peak levels later after waking in periods of less light than in periods of more light (Laakso, Porkka-Heiskanen, Alila, Stenberg, & Johansson, 1994) and there is a phase delay in sleep time in winter versus summer months (Kohsaka, Honma, & Morita, 1992). Research has also indicated that over the course of the day, time awake correlates with shifts in positive but not negative affect, and accounts for approximately 13% of daily variance in positive affect (Murray et al., 2009). Considering that the peak of the circadian hormones is signaled by an individual’s wake time and occurs in the hours after sleep offset, and considering that this peak is sensitive to changes in light, it follows that accounting for time awake before a session (TA) may be an important consideration, and that TA might interact with both photoperiod and TOD in influencing cortisol, mood, and frontal EEG asymmetry.
Individual Differences in Morning/Evening Preferences
Similarly, individual differences in morning/evening preference may also play a role in how alert and high-functioning an individual is at the time of EEG assessment. Individual preferences range from a clear morning preference (larks) to clear evening preference (owls). Research suggests that larks have a more stable sleep pattern than owls, that larks wake more easily, and that larks feel more rested (Webb & Bonnet, 1978). The Horne and Östberg morningness/eveningness scale (1976) was designed to access diurnal preference, and while the scale is not a direct reflection of circadian rhythm, research suggests the two are related (Duffy, Rimmer, & Czeisler, 2001). When sleep and wake time are held constant for a group of both larks and owls, larks have earlier and higher cortisol peaks compared to owls (Bailey & Heitkemper, 2001). There also seems to be a link between diurnal preference and cortisol peaks such that larks have higher cortisol and an earlier peak then owls (Randlar & Schaal; 2010). Research suggests that those who self report to function best in the morning vary on hormonal components of circadian rhythm and sleep cycle from those who self report to function best in the evening. Some of these components, such as cortisol, can influence frontal asymmetry. It is thus possible that beyond TA, diurnal preference may interact with TOD and TOY in influencing frontal asymmetry.
Reference issues in EEG recording
Choice of EEG reference is thought to contribute to differences noted across frontal alpha asymmetry studies (Coan & Allen, 2003; Hagemann, Naumann, and Thayer, 2001; Reid, Duke, Allen, 1998; Thibodeau et al., 2006). While averaged (“linked”) mastoids (LM) and averaged reference (AR) are very commonly used montages, they are susceptible to contributions from distal signal sources and might be more influenced by variance associated with not only local but distal sources (Hagemann, Naumann, and Thayer, 2001; Stewart et al., 2010). A reference-free alternative, the current source density (CSD) transform, provides an estimate of current sources and sinks on the scalp. This transform computes the second spatial derivative of voltage between nearby electrode sites, providing a spatially-enhanced signal representation that increases the contribution of local electrical activities and attenuates those from distal volume-conducted sources. The CSD transformation for examining frontal EEG asymmetry has been suggested as an alternative to standard references, especially given the large amplitude alpha generated by distal sources in the occipital cortex (Hagemann, Naumann, and Thayer, 2001, Stewart et al, 2010). In fact, Stewart et al, found that CSD-transformed signals were less susceptible to variation due to current mood than LM and AR references (2010). Thus the impact of reference montage and the CSD transformation are important additional methodological considerations in assessing state variance effects on frontal EEG asymmetry.
The Present Study
The present investigation examined a large sample of college students without current or past psychopathology to examine the role of TOD and TOY as variables influencing frontal EEG asymmetry. Although a categorical seasonal variable was used to provide a direct replication of the TOD and TOY results of Peterson & Harmon-Jones (2009), a dimensional photoperiod variable was also included to assess the influence of light variation on frontal brain activity. In addition, the present investigation tested whether additional variables relevant to TOD and TOY, namely TA and diurnal preference, moderated the relationship between TOD, TOY, and frontal EEG asymmetry. To this end, three hypotheses were examined. First, it was predicted that longer photoperiods would be associated with relatively greater left frontal activity than shorter photoperiods similar to the results of Peterson and Harmon-Jones using seasonal coding. Second, in morning sessions, participants who recently awoke should exhibit relatively less left frontal activity compared to participants who had been awake longer and/or were assessed in other session times, as morning bright light exposure is more immediate and intense during longer photoperiods, leading to a stronger morning cortisol response (that would be expected to be associated with relatively less left frontal activity). It was further predicted that the amount of time awake would interact with photoperiod and time of year as sunlight would alter timing and intensity of the cortisol peak and frontal alpha asymmetry. Third, these patterns may also be moderated by diurnal preference, with people who report functioning best in the morning (larks) differing in their seasonal and time-of-day pattern from those who self-report as functioning best in the evening (owls). Finally, the present study examined whether EEG asymmetry assessed using two commonly employed reference montages and also a reference-free CSD transform would be similarly susceptible to the influence of such effects involving TOD, TOY, TA, and diurnal preference.
Methods
Participants
Participants for the present study were drawn from a larger study of frontal brain asymmetry and risk for depression (Stewart et al., 2010). Potential subjects were strongly right handed as indicated by a score of 36 or higher (max score = 39) on the handedness inventory of Chapman and Chapman (1986). Exclusionary criteria included past history of head injury, electroconvulsive therapy, concussion, epilepsy, a loss of consciousness exceeding 10 minutes, as well as current use of psychotropic medications. From among the 306 individuals included in the larger study of risk for depression, 163 (107 women) were identified for inclusion in the present study by virtue of being free of any current Axis I psychopathology or any past history of mood disorder as determined using the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, and Williams, 1997). Among these 163 participants used for the present study, 93 (67 female) were run in the fall/winter months and 70 (40 female) were run in the spring/summer months (see below for operational definitions of these time frames). The mean age of the sample was 19.05 with a standard deviation of 1.71years. Participants self selected session times that fit their schedule between lab operating hours of 10am and 7pm, with sessions generally being scheduled to finish by 7pm with 2 hours allotted for each session. Occasional exceptions were made for later start times, but no session started later than 6pm. Similarly, two exceptions were made throughout the course of the study for sessions earlier than 10am though no session started before 8:30am. Additional information regarding the recruitment of the sample in the larger study is provided in Stewart et al., (2010), and the number of participants run by time of day and time awake is presented in Table 1.
Table 1.
Number of participants classified by time awake (TA) and time of day (TOD)
| Morning | Afternoon | Evening | Total TA | |
|---|---|---|---|---|
| Just Awake (<= 3 hours) | 39 | 47 | na | 86 |
| Awake 4–6 hours | 22 | 88 | 28 | 138 |
| Awake 7+ hours | na | 55 | 160 | 215 |
| Total TOD | 61 | 190 | 188 |
A majority of participants also completed the Horne-Ostberg morningness/eveningness questionnaire (1976) during the intake session to assess diurnal preference. Items on the questionnaire assess participants’ time of day preferences based on a variety of questions such as what time they prefer to perform tasks, schedule appointments, go to bed, and wake up to perform tasks optimally. Higher scores indicate more of a morning preference (the higher the score, the stronger the preference) while lower scores indicate more of an evening preference. Scores were grouped into 3 general categories of morning preference (lark), neutral (no strong preference), and evening preference (owl), according to the guidelines from Horne and Östberg (1976). Because this measure was added after the start of the study, of the 163 participants in this study, 106 (65 women) provided information regarding TA and diurnal preference and could be included in testing statistical models related to TA and diurnal preference.
EEG Data Collection
Individuals completed four separate EEG sessions with each session at least 24 hours apart, with all 4 sessions completed within a 14-day window.2 EEG data were collected using a 64-channel electrode cap and NeuroScan Synamps2 amplifier (Charlotte, North Carolina). To allow for blink detection, bipolar electrooculogram sites included leads on the inferior and superior orbit of the left eye; for horizontal movements, leads were placed on the outer canthii. Impedances for all sites were kept at or below 10K Ohms throughout the session. Data were amplified with a gain of 2816 and sampled at 1000 Hz. Signals were recorded using an online reference site immediately posterior to Cz, and later re-referenced off-line to averaged “linked” mastoids (LM) to be comparable to that used by Peterson and Harmon-Jones (2009), and also to an average of all EEG sites (AR), and converted to a reference-free CSD derivation. Data were passed from 0– 200Hz prior to digitization. During each session, two eight minute resting baselines were completed. Each baseline comprised four eyes-open (O) and four eyes-closed (C) periods, counterbalanced in one of two orders -- OCCOCOOC or COOCOCCO.
EEG Data Reduction
Continuous data from the online reference were visually inspected for artifacts after acquisition and epochs with muscle, movement or other problems were manually marked for removal. EEG data were then segmented into as many as 117 two second epochs for each one minute recording period (but fewer if there were artifacts identified in the continuous data), with the epochs overlapping by 1.5 seconds to allow for the minimal weight of the taper at the end of each epoch from the application of a Hamming window. Subsequently, a blink rejection algorithm removed any segments where ocular activity exceeded +/− 75 microvolts in the vertical ocular channels, and an artifact rejection algorithm then removed spikes and DC shifts that might have been missed by human inspection. Both phases of artifact detection occurred while data were referenced to the online site immediately posterior to CZ to ensure that the same epochs were present in each dataset. Data were then transformed to either the average (“linked”) mastoids reference or the average reference, or converted to the reference-free CSD transformation via the CSD Toolbox of Kayser and Tenke (Kayser & Tenke, 2006a; Kayser & Tenke, 2006b). All transforms were applied using only EEG scalp sites (thus excluding two ocular channels, and both mastoids), for a total of 60 scalp sites.
A Fast Fourier Transform (FFT) was then applied to the artifact free epochs to obtain power spectra across the 8 minutes of data for each session. Total alpha power (8–13 Hz) was extracted for each site, and natural log (ln) transformed. An asymmetry score was calculated by subtracting homologous right and left ln-transformed alpha power (i.e, ln[right]-ln[left]) for four frontal region pairs: F7 & F8, F5 & F6, F3 & F4, and F1 & F2. These scores were used in analyses because they include pairs F8-F7 and F4-F3, which are the most commonly reported throughout EEG frontal asymmetry literature, as well as pairs F2-F1 and F6-F5 to provide additional coverage in this region (see review by Coan & Allen, 2004). Higher values on this index are generally thought to reflect relatively greater left activity (i.e., relatively greater right alpha; cf. Allen et al., 2004). Each participant had a total of 8 asymmetry scores for each of these four regions of interest: 2 resting sessions by four days for each of the three surface potential transformations (LM reference, AR reference, CSD-transform).
Results
All effects were tested using mixed linear model analyses (SPSS V 18.0) that accommodate missing data. All models included EEG asymmetry scores at four frontal regions (F8-F7, F6-F5, F4-F3, and F2-F1), two resting sessions within each day, for each of four days, for each of two reference montages commonly used in the literature (average, averaged (“linked”) mastoids) as well as the current source density transformation (CSD). Separate models were run for LM-referenced, AR-referenced, and CSD –transformed data. Because trait EEG asymmetry is of primary interest, and its vulnerability to state factors, four days were included in the models, with consistency across days reflecting a trait estimate of frontal EEG asymmetry.
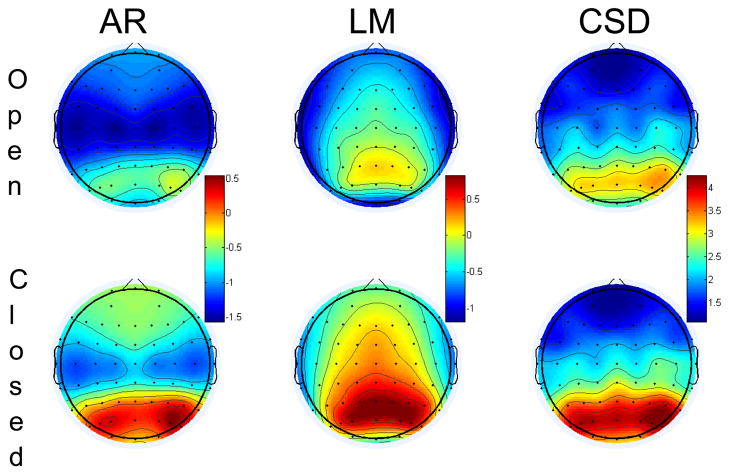
The impact of the different surface potential transformations is depicted in Figure 1. As shown in the figure, there is the expected pattern of greater occipital alpha under eyes closed compared to eyes open periods regardless of surface transformation, but the CSD transformation does a much better job of containing occipital alpha to occipital sites. The LM and AR transformations, by contrast, reveal a topography with alpha appearing at frontal sites, especially during eyes closed, when alpha power should predominate at occipital sites. The figure supports that the CSD transform attenuates the contributions of distal sources to surface leads. All subsequent analyses collapsed across eyes open and closed periods, consistent with many studies in the EEG asymmetry literature and, importantly, consistent with the study of Peterson and Harmon-Jones (2009).
Figure 1.
Topography of alpha power under eyes open (top) and eyes closed (bottom) conditions as a function transformation (AR or LM reference or CSD transformation). Power values at each site represent natural-log transformed values; thus negative number represent mean power values less than one. Each transformation is scaled independently, but within each transformation, eyes open and closed data are plotted on the same scale.
To assess whether the present study replicated the relationship between TOY and TOD found by Peterson & Harmon-Jones (2009), data were analyzed using a full factorial mixed model included TOD and TOY as between-subjects variables, whereas day (4), region (4), and resting session (2) were within subject variables. TOY was dichotomously coded, consistent with Peterson & Harmon-Jones (2009): fall/winter (September through February) and spring (March through May). Session time was grouped into morning (sessions starting before 12 pm), afternoon (12–4 pm) and evening Sessions (4–7 pm). EEG asymmetry score was the dependent variable. Effects of interest were TOY or photoperiod, TOD, TA, and their interactions. Additionally, interactions of any of these effects with region are reported and explored further.
LM- and AR- Referenced Asymmetry Scores
TOY x TOD
A main effect of TOY for both AR and LM references showed that fall/winter sessions were associated with more relative left frontal activity than spring sessions (LM=F(1,978) = 4.10, p<.05; Fall mean ± s.d = .007 ± .005 spring = −.009 ± .005; AR= F(1,703) = 8.89, p<.01; Fall mean ± s.d = .−003 ± .006 spring = −.028 ± .006.)3 This is opposite the seasonal main effect noted by Peterson & Harmon-Jones. An interaction of region and TOY emerged for both references (LM: F(3,3654) = 3.80, p<.05; AR: F(3,3956) = 3.34, p<.05). indicating that although more relative left frontal activity is present during fall/winter sessions compared to spring across all channel pairs, the effect is stronger at more lateral sites. For the LM reference this is only significant at channel pair F8-F7, while AR referenced data shows that this pattern is present significantly across all channel pairs except F2-F1 Also, contrary to Peterson and Harmon-Jones findings, neither TOD nor the TOD x TOY interaction approached significance for either montage.
Photoperiod x TOD
The dichotomous seasonal coding, used here to replicate that used by Peterson and Harmon-Jones (2009), may confound seasonal psycho-social variables with photoperiod, and also provides a rather coarse categorization of this dimension. It is possible that a more sensitive coding of time of year might be achieved using a dimensional scale, coded relative to the summer and winter equinoxes, the longest and shortest periods of light in the year (longest and shortest photoperiods, respectively). To achieve this, photoperiod was coded on a scale where 180 represented June 22 and the number 0 was equivalent to December 22, with numbers increasing from December 22 to June 22, and decreasing from June 22 to December 22, allowing for dates far apart in time, but similar in photoperiod (e.g. September 22 and March 22) to have similar photoperiod numbers. The previous mixed model analysis was repeated examining TOD x photoperiod instead of TOY.
For the LM–referenced data, photoperiod emerged as a main effect (F(1, 983)=6.64, p<.05), demonstrating that participants run in shorter photoperiods showed more relative left frontal activity compared to those run in longer photoperiods, similar to the TOY x TOD model above (mean ± s.d. for those +1 s.d. on photoperiod = .−.0967 ± .009, and for those −1 s.d. on photoperiod = .0093 ± .008). Similar to the previous season analysis, neither TOD nor the larger photoperiod x TOD interaction approached significance. However, an interaction of region and TOD (F=(6, 3696) = 2.54, p<.05) indicated that the effect of TOD was significant only at region F6-F5, but not other regions; at F6-F5, individuals show more relative left frontal activity in morning sessions compared to afternoon session times.
For the AR-referenced data, photoperiod again emerged as a main effect (F(1, 687)=7.46, p<.01), showing the same pattern and noted with LM reference (mean ± s.d. for those +1 s.d. on photoperiod = .−026 ± .010, and for those −1 s.d. on photoperiod = −.001 ± .010. TOD also emerged as a main effect (F(2, 1105)=3.29, p<.05, Morning ± s.d = .−005 ± .009 Afternoon = −.024 ± .006 Evening = −.012 ± .006). Similar to LM reference, an interaction of region and TOD again emerged (F=(6, 3985) = 2.90, p<.01). The effect of TOD was significant only for F6-F5, with more relative left frontal activity in morning compared to afternoon sessions. As with LM reference, the photoperiod x TOD was not significant.
Effects of TA
To see if previous effects were moderated or masked by individual differences in hours awake, the previous full factorial TOD x photoperiod model was run adding TA and interactions of interest (TOD x TA, TA x photoperiod, and TA x TOD x photoperiod). Only the main effects of TA and interactions with TA are of interest and reported in this follow-up model
For LM-referenced data No significant main effect or interactions involving TA were present
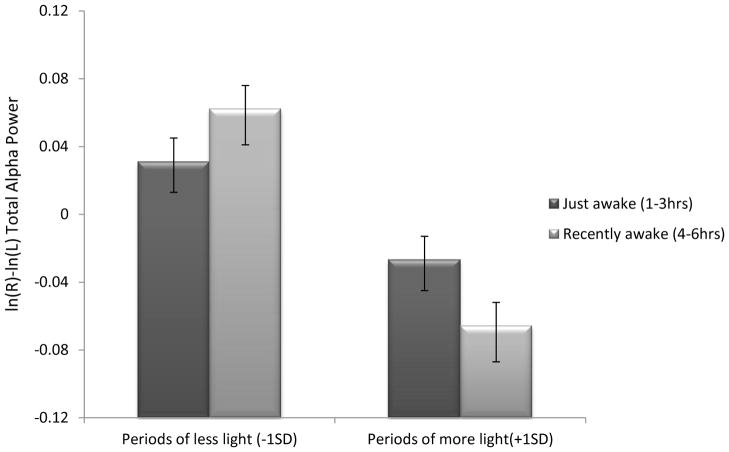
For AR-referenced data, TA interacted with TOD (F(2,936)=3.80, p<.05), but was qualified by a three way interaction of TOD x TA x photoperiod (F(3, 912)=4.65, p<.05). As depicted in Figure 2, within morning sessions, participants run in shorter photoperiods who recently awoke exhibited relatively lower left frontal activity than those who had been awake longer, whereas the pattern reversed in longer photoperiods (F(1, 76)= 11.20, p<.005,).4 No significant TA by photoperiod interactions were present within afternoon or evening sessions
Figure 2.
Time Awake by Photoperiod interaction within morning sessions for AR-referenced data. Higher scores are indicative of more relative left frontal activity. Results indicate that within morning sessions, in periods of less light, those awake fewer than 3 hours show significantly less relative left frontal activity that those awake longer. However in periods of more light the pattern is significantly reversed. Data represent collapsed scores across four frontal regions (F2-F1, F4-F3, F6-F5, & F8-F7).
Effects of diurnal preference
The moderating effects of diurnal preference were examined by rerunning the previous photoperiod by TOD model, adding diurnal preference and relevant interactions. Although previously reported effects of photoperiod were again significant in these models, no effects involving diurnal preference were significant.
EEG Asymmetry Scores based on CSD-Transformed data
The models above were computed using only asymmetry scores based on CSD transformed data. Stewart et al. (2010) found that the CSD transformed data were not influenced by current mood state to the extent the LM and AR referenced data were. The next set of analyses examined whether the CSD-transformed data were vulnerable to photoperiod, TOD, and TA effects.
TOY x TOD
A main effect of season emerged (F(2, 849)= 7.79, p<.01) indicating that participants run in the fall (.064 ± .012) exhibited less relative left frontal activity then subjects run during the spring months (.112 ± .013), similar to the results Peterson and Harmon-Jones (2009) reported for the LM reference. An interaction of TOD and region (F(6,3772) = 2.45, p<.05) revealed that medially (F2-F1), but not at other regions, those run in morning sessions exhibited less relative left frontal activity than those run in later session times. Neither TOD nor the larger TOD x TOY interaction approached significance.
Photoperiod x TOD
No significant effects of photoperiod, TOD, nor the photoperiod x TOD interaction were found.
Effects of TA and diurnal preference
No significant main effect or interactions involving TA were found. For the model involving diurnal preference, however, a three way interaction of TOD x diurnal preference x photo period emerged as significant (F(4,767)=3.02, p<.05) such that within evening sessions- only in periods of more light- those who report preferring evening times show greater relative left frontal activity compared to both those who prefer morning times and those who report no strong preference No main effect of diurnal preference or other interactions involving diurnal preference were significant for this model.
Discussion
Resting frontal EEG asymmetry has been proposed to serve as a trait-like index of risk for depression and related psychopathology (e.g., Allen et al., 2004; Stewart et al., 2010), yet findings by Peterson and Harmon-Jones (2009) suggest that uncontrolled effects related to time of day and time of year may contribute variance that might attenuate or obscure the ability to establish trait relationships between frontal asymmetry and risk for depression or other psychopathology, at least when using reference montages such as averaged-mastoids (LM). The present study examined these same factors as Peterson and Harmon-Jones, and additionally examined individual difference variables of time awake and diurnal preference. A tabular synopsis of the present findings and those of Peterson and Harmon-Jones (2009) are presented in Table 2. The present study failed to replicated Peterson and Harmon Jones (2009) using seasonal coding. A photoperiod variable, designed to address problems that may be inherent in a categorical variable yielded non-significant results as well. However, time awake proved an important moderating variable and findings were consistent with our first hypothesis, but contradictory to that of Peterson & Harmon-Jones (2009). Diurnal preference did not moderate the TODxPhotoperiod interaction as hypothesized.
Table 2.
Comparison of Peterson and Harmon-Jones (2009) results to findings of the present study
| Effect | Peterson & Harmon-Jones: LM | Present Study: AR/LM | Present Study: CSD |
|---|---|---|---|
| TOY | ↓ midline and lateral LFA in fall in Study 1, laterally in study 2 | significant ↑ LFA in fall compared to spring | sig ↓LFA in fall sessions compared to spring |
| TOD | Study 1:↓ midline LFA in mornings; Study 2: ns | ns | ns |
| TOY x TOD | Study 1: Sig laterally/mid- frontal; Study 2: Sig laterally | ns | ns |
| Photoperiod | Not examined | significant ↑ LFA in periods of less light compared to periods of more light (LM d = .07, AR d = .07) | ns (d = .015) |
| Photoperiod x TOD | Not examined | ns | ns |
| Photoperiod x TOD x TA | Not examined | LM =ns. AR=w/in morning sessions in less light ↓ LFA compared to those awake longer in periods of less light and the opposite pattern in periods of more light (Less light:, LM d =.15 AR d =−.25; More light: LM d = −.26, AR d =.35) | ns (Less light: d =−.10; More light: d=−.15) |
Note: ns = nonsignificant effect; d= Cohen’s d.
Perhaps most apparent from Table 2 is that references widely used in the literature (LM, AR) may be susceptible to influences such as photoperiod, time of year, or time of day, that are seldom monitored or assessed in this literature. EEG asymmetry scores from CSD-transformed data, however, appear less susceptible to these influences. This conclusion derives from finding significant effects for LM-referenced and AR-referenced data, and not for CSD-transformed data. This argument, which depends in part on nonsignificant findings, would be bolstered by an examination of the effect sizes for these key comparisons where CSD effects were nonsignificant but LM or AR effects were significant (see Table 2). For the effect of photoperiod, the effect size (Cohen’s d, comparing plus and minus 1 s.d. on photoperiod) was .07 for LM-referenced data, .07 for AR referenced data, and .015 for CSD-referenced data. For the Photoperiod by TOD by TA interaction, which found only significant effects for morning sessions using AR-referenced, the effect sizes during periods of less light (Cohen’s d, comparing just awake to recently awake participants) were .15 for LM-referenced and −.25 for AR -referenced and −.10 for CSD-transformed data; the effect sizes during periods of greater light (Cohen’s d, comparing just awake to recently awake participants) were .−26 for LM-referenced and .35 for AR-referenced and −.15 for CSD-transformed data. These effect sizes for CSD-transformed data are small, and thus such effects would appear unlikely to exert a major influence on frontal asymmetry scores using CSD-transformed data. Although such an argument must be regarded as provisional, this finding that the CSD-based asymmetry scores appear least influenced by extraneous state factors is also consistent with our recent findings (Stewart et al., 2010) that whereas asymmetry scores derived from the LM and AR references were influenced to various degrees by current emotional state, only the CSD-based asymmetry scores robustly differentiated individuals with any history of depression independent of current emotional state. Nonetheless, monitoring and assessing these influences remains a reasonable practice until the extent of the effects can be replicated in other studies.
The findings of the present study suggest that extraneous factors such as season/photoperiod and time of day may need to be monitored or controlled when examining frontal asymmetry scores from standard reference montages. This is, in a very broad sense, consistent with implications of the study of Peterson and Harmon-Jones (2009), despite the fact that the direction of some of the present findings conflict with their results. Despite recruiting a sample that would appear comparable in age and college student status to that of Peterson and Harmon-Jones (2009), the present findings did not replicate their findings of a time of day by time of year interaction, and the findings concerning time of year are in a direction opposite to what they found. Several other unmonitored and uncontrolled factors may have varied between the present study and their study, such as seasonally-varying stressors characteristic of student samples, including adjustment to starting school and transitioning to college as well as midterms and finals.
The present findings also extend the list of potential influences on asymmetry scores recorded using conventional reference montages (LM and AR): time of day and photoperiod are important factors in influencing frontal EEG asymmetry when considered jointly with time awake at assessment and diurnal preference. During morning sessions in periods of less light, individuals who woke up closest to their session time showed less relative left frontal activity than those who just woke up during periods of more light. In contrast, individuals who were awake between 4–6 hours before their session showed more relative left frontal activity during periods of less light than those during periods of more light. Regardless of photoperiod, those who just awoke showed less relative left frontal activity then those who had been up longer. Although the session start time of Peterson and Harmon-Jones (2009) was not specified, the present sample had very few (n = 2) sessions that were run before 10am; this relatively late start time may have resulted in participants being up longer before the assessment in the current study compared to the former study. The current study did not directly measure the circadian component of sleep regulation, but sleep-related circadian processes might plausibly underlie these effects involving the time awake, and a more detailed circadian assessment might be profitably used in future work.
Homeostatic and Circadian Processes
The sleep wake cycle is affected by photoperiod, and in many parts the country it is additionally affected by the twice a year observance of daylight savings time (DST). Although the current study and that of Peterson & Harmon-Jones (2009) were conducted in highly similar latitudes (30.6 at Texas A&M versus 32.2 at the University of Arizona), Arizona does not observe daylight savings time. Interestingly, there are few studies examining the effects of DST on the processes affecting sleep, but results to date indicate it takes most people up to a week to adjust to the time change, with spring being more of a transition period than the fall (Kantermann, Juda, Merrow, & Roenneberg, 2007; Monk & Aplin, 1980). This would not likely entirely account for the discrepant findings but could contribute to variance between the samples.
Cortisol, Photoperiod, and EEG Asymmetry
Peterson and Harmon-Jones (2009) speculated that cortisol may underlie the time of year effects on frontal EEG asymmetry, noting that cortisol levels are higher in the fall/winter than spring months, consistent with their findings of relatively less left frontal activity in fall mornings compared to spring mornings. Cortisol is a well-documented component of the circadian rhythm and has been shown to be affected by changes in day length (Laakso et al., 1994). Higher cortisol levels have been linked to relatively less left frontal activity (Tops et al. (2005); Tops, van Peer, Wester, Wijers, Korf, 2006). In addition to its influence on mood, early morning bright light exposure results in an immediate and substantial increase in cortisol levels (Leproult, Colecchia, L’Hermite-Balèriaux, & van Cauter, 2001). Furthermore, higher cortisol levels in infants are associated with less relative left frontal activity as well as task-related withdrawal behaviors (Buss et al., 2003). Given cortisol’s relationship to bright light and its apparent effect on frontal EEG asymmetry, it is possible that frontal EEG asymmetry may be affected by changes in bright light exposure (sunrise times via alterations in the morning cortisol spike). Significant cortisol spikes have been shown to be triggered by morning bright light exposure, whereas afternoon light exposure does not measurably change cortisol levels (Leproult et al., 2001). Work by Lewy et al. (1998) demonstrates that morning bright light exposure has a larger effect on cortisol levels than evening bright light exposure. Importantly, within summer months; there is more morning light exposure between waking hours and noon compared to winter months, although the most significant difference is in the afternoons (Guillemette, et al., 1997). This research suggests that the cortisol component of the circadian process may relate not only to seasonal changes, but also help explain the discrepancy between the present findings and those of Peterson and Harmon-Jones (2009), and also help explain the moderating role of time awake found in the current study.
As with TOY, Peterson and Harmon-Jones (2009) attributed their TOD results to cortisol levels, which are highest upon waking and decrease throughout the day, with the lowest amounts present at night, the substantial early morning cortisol spike might plausibly overshadow other effects (including seasonal effects) for subjects who have most recently awakened. The pattern of findings in the present study, however, were not entirely consistent with the hypothesis that the morning spike in cortisol would be associated with relatively less left frontal activity, particularly in conditions likely to be characterized by the most pronounced spike: longer photoperiods, in those individuals most recently awake. This is perhaps due to the fact the cortisol spike upon waking is observed within the first hour (Edwards, Evans, Hucklebridge, Clow, 2001) but only a small number of participants (n = 2) were assessed that close to wake time for this present study. In addition, while cortisol administration does result in relatively less left then right frontal activity, EEG in the Tops et al. (2005) experiment was not recorded until 1–3 hours after administration. How long it takes cortisol -- both naturally occurring spikes and experimentally administered --to affect frontal asymmetry is unknown but an important factor in interpreting results. Overall, the present results suggest that while processes involved in sleep regulation affect frontal asymmetry, the findings are not obviously or unequivocally driven by cortisol. Alternatively, college students have a rather variable sleep schedule and it is possible that this contributes to phase shifting in unpredictable ways within this sample.
While exposure to artificial bright light has been shown to shift mood, the duration and intensity of light exposure needed to shift frontal asymmetry is not definitively established. In a small study of treatment of patients with Seasonal Affective Disorder, bright light treatment reduced depressive symptoms but did not alter EEG asymmetry assessed at a two-week interval (Allen, Iacono, Depue, & Arbisi, 1993), but this does not address whether there would be acute effects of light exposure within each day. Assuming bright light exposure might produce acute shifts in frontal asymmetry within individuals, perhaps its biggest boost would be immediately after exposure, explaining why those who had been awake longer in this sample show less relative frontal activity than those who had been awake shorter periods of time, regardless of photoperiod. Those run in periods of more light, but awake longer show more relative left frontal activity then those run in periods of less light awake for comparable periods of time. While the research on light’s effect on mood is robust, there are few studies looking at the effect as a function of frontal asymmetry.
Conclusions, Limitations, and Future Directions
The present study, like that of Peterson and Harmon-Jones (2009), found that generally uncontrolled and unassessed factors contribute to variance in frontal EEG asymmetry scores when recorded using reference montages that are common in the literature (LM and AR) but perhaps less so when asymmetry scores are based on CSD transformed data. Because the CSD-transformation attenuates the impact of distal and widely distributed brain electrical sources, the resultant signals at frontal EEG leads are a spatially-enhanced representation of the direction, location, and intensity of current generators underlying each signal (Kayser & Tenke, 2006; Nunez, 1981), and are thus more likely to reflect activity that is predominantly of frontal cortical origin. This might suggest that the circadian and seasonal effects on EEG asymmetry derive not so much from an impact on frontal sources, but rather from distal sources that project to frontal sites and are picked up using conventional reference montages but not CSD transformed data. The present study suggests that future studies examining resting frontal EEG asymmetry and its relationships to psychopathology or other traits should consider using the CSD transformation, and also statistically account for or experimentally control for time of year, time of day, and importantly, time awake. These steps would likely increase the extent to which the frontal asymmetry metrics reflect trait variance rather than the influence of extraneous factors.
The current examination and that of Peterson and Harmon-Jones (2009) were run using college student samples in a narrow range of geographical latitude. Examination of the effects including more extreme latitudes would provide a better range of photoperiod to assess its impact alone, and in combination with time of day in influencing frontal EEG asymmetry. Also, given age-related sleep changes (Van Cauter, Leproult, & Plat, 2000) and time of day preference (Drennan, Klauber, Kripke, & Goyette, 1990), future studies might investigate populations with a wider age range to determine whether effects persist, strengthen, or weaken with age. In addition, future examinations may wish to measure cortisol to elucidate its role on frontal asymmetry as a function of TOD and photoperiod. Finally, similar to the Peterson & Harmon-Jones (2009) study, the results must be regarded as correlational, as participants were not assigned to session times or seasons. The present findings reflect some degree of self-selection for session times on the part of the participants although diurnal preference did not seem to account for variance associated with TOD. Moreover, participants could not select the season during which they were assessed. Either random assignment of individuals to session times or a controlled assignment to several session times across days and seasons would provide a more robust test of the impact of recording time and season on frontal EEG asymmetry. However, the current between-subjects design may allow for generalization to studies that utilize subject self selection of session times.
The present findings raise the possibility that some occasion-specific non-trait variance in frontal EEG asymmetry may be accounted for by factors that can be assessed in any study: the time and season of the assessment session, and the time the participants have been awake. Studies that examine frontal EEG alpha asymmetry as markers of risk for psychopathology such as major depression might assess these factors in an effort to reduce non-trait sources of variance that could obscure relationships between frontal asymmetry and other indices of risk.
Highlights.
Seasonal and chronolobiological influences on resting frontal asymmetry were examined.
Time awake, photoperiod, and time of day interacted to affect frontal asymmetry.
Current source density (CSD) transformed data were not vulnerable to these influences.
CSD transformed data may provide a better trait estimate of resting frontal asymmetry.
Acknowledgments
The authors wish to thank Dara Halpern, Eliza Ferguson, Craig Santerre, Eynav Elgavish Accortt, Andrew Bismark, Jay Hegde, and myriad research assistants for their efforts in recruiting and testing participants, and to thank Jim Coan for his invaluable contributions leading to the receipt of NIMH R01-MH066902, which funded portions of the collection of these data. This work was also funded, in part, by a grant from the NARSAD foundation.
Footnotes
The current study did not test for cortisol. However, the literature informing this study as well as that of Peterson and Harmon-Jones (2009), was based, in part, on diurnal cortisol rhythms.
Eleven participants completed their 4 sessions beyond the 2 week window. Moreover, 4 participants dropped out before completing all 4 sessions.
A model seeking using just participants’ first EEG session and linked mastoid reference to approximate Peterson and Harmon-Jones study was also not significant (F(2, 192)=1.8, p<.500).
Those who had been awake over 7 hours in morning sessions as well as those who just awoke before their evening sessions were removed from analysis as there were very few participants in those groupings
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jamie R. Velo, Email: jamievelo@gmail.com.
Jennifer L. Stewart, Email: j6stewart@ucsd.edu.
Brant P. Hasler, Email: haslerbp@upmc.edu.
David N. Towers, Email: dtowers@uis.edu.
John J.B. Allen, Email: John.JB.Allen@arizona.edu.
References
- Allen JJ, Iacono WG, Depue RA, Arbisi P. Regional electroencephalographic asymmetries in bipolar seasonal affective disorder before and after exposure to bright light. Biol Psychiatry. 1993;33(8–9):642–646. doi: 10.1016/0006-3223(93)90104-l. [DOI] [PubMed] [Google Scholar]
- Allen JJ, Urry HL, Hitt SK, Coan JA. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology. 2004;41(2):269–280. doi: 10.1111/j.1469-8986.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- Achermann P. The two-process model of sleep regulation revisited. Aviat Space Environ Med. 2004;75(3 Suppl):A37–43. [PubMed] [Google Scholar]
- Avery DH, Bolte MA, Dager SR, Wilson LG, Weyer M, Cox GB, et al. Dawn simulation treatment of winter depression: a controlled study. Am J Psychiatry. 1993;150(1):113–117. doi: 10.1176/ajp.150.1.113. [DOI] [PubMed] [Google Scholar]
- Avery DH, Bolte MA, Wolfson JK, Kazaras AL. Dawn simulation compared with a dim red signal in the treatment of winter depression. Biol Psychiatry. 1994;36(3):180–188. doi: 10.1016/0006-3223(94)91223-8. [DOI] [PubMed] [Google Scholar]
- Avery DH, Eder DN, Bolte MA, Hellekson CJ, Dunner DL, Vitiello MV, et al. Dawn simulation and bright light in the treatment of SAD: a controlled study. Biol Psychiatry. 2001;50(3):205–216. doi: 10.1016/s0006-3223(01)01200-8. [DOI] [PubMed] [Google Scholar]
- Bailey SL, Heitkemper MM. Circadian rhythmicity of cortisol and body temperature: morningness-eveningness effects. Chronobiol Int. 2001;18(2):249–261. doi: 10.1081/cbi-100103189. [DOI] [PubMed] [Google Scholar]
- Binkley S, Tome MB, Crawford D, Mosher K. Human daily rhythms measured for one year. Physiol Behav. 1990;48(2):293–298. doi: 10.1016/0031-9384(90)90316-v. [DOI] [PubMed] [Google Scholar]
- Buss KA, Schumacher JR, Dolski I, Kalin NH, Goldsmith HH, Davidson RJ. Right frontal brain activity, cortisol, and withdrawal behavior in 6-month-old infants. Behav Neurosci. 2003;117(1):11–20. doi: 10.1037//0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of handedness. Brain and Cognition. 1987;6:175–183. doi: 10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJ. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003;40(1):106–114. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJ, Harmon-Jones E. Voluntary facial expression and hemispheric asymmetry over the frontal cortex. Psychophysiology. 2001;38(6):912–925. doi: 10.1111/1469-8986.3860912. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry as amoderator and mediator of emotion. Biological Psychology. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale--preliminary report. Psychopharmacol Bull. 1973;9(1):13–28. [PubMed] [Google Scholar]
- Dijk DJ, von Schantz M. Timing and consolidation of human sleep, wakefulness, and performance by a symphony of oscillators. J Biol Rhythms. 2005;20(4):279–290. doi: 10.1177/0748730405278292. [DOI] [PubMed] [Google Scholar]
- Drennan MD, Klauber MR, Kripke DF, Goyette LM. The effects of depression and age on the Horne-Ostberg morningness-eveningness score. Journal of Affective Disoders. 1991;23(2):93–98. doi: 10.1016/0165-0327(91)90096-b. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115(4):895–899. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Young MA, Fogg LF, Liu L, Meaden PM. Bright light treatment of winter depression: a placebo-controlled trial. Arch Gen Psychiatry. 1998;55(10):883–889. doi: 10.1001/archpsyc.55.10.883. [DOI] [PubMed] [Google Scholar]
- Edwards S, Evans P, Hucklebridge F, Clow A. Association between time of awakening and diurnal cortisol secretory activity. Psychneuroendocrinology. 2001;26:613–622. doi: 10.1016/s0306-4530(01)00015-4. [DOI] [PubMed] [Google Scholar]
- First MG, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV Axis I disorder—Clinical version, administration booklet. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1997. [Google Scholar]
- Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry. 2005;162(4):656–662. doi: 10.1176/appi.ajp.162.4.656. [DOI] [PubMed] [Google Scholar]
- Guillemette J, Hebert M, Paquet J, Dumont M. Natural bright light exposure in the summer and winter in subjects with and without complaints of seasonal mood variations. Biol Psychiatry. 1998;44(7):622–628. doi: 10.1016/s0006-3223(97)00543-x. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Hewig J, Seifert J, Naumann E, Bartussek D. The latent state-trait structure of resting EEG asymmetry: replication and extension. Psychophysiology. 2005;42(6):740–752. doi: 10.1111/j.1469-8986.2005.00367.x. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Thayer JF. The quest for the EEG reference revisited: A glance from brain asymmetry research. Psychophysiology. 2001;38:847–857. [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Thayer JF, Bartussek D. Does resting electroencephalograph asymmetry reflect a trait? an application of latent state-trait theory. J Pers Soc Psychol. 2002;82(4):619–641. [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- Kantermann T, Juda M, Merrow M, Roenneberg T. The human circadian clock’s seasonal adjustment is disrupted by daylight saving time. Curr Biol. 2007;17(22):1996–2000. doi: 10.1016/j.cub.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin Neurophysiol. 2006a;117:348–368. doi: 10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: II. Adequacy of low-density estimates. Clin Neurophysiol. 2006b;117:369–380. doi: 10.1016/j.clinph.2005.08.033. [DOI] [PubMed] [Google Scholar]
- King JA, Rosal MC, Ma Y, Reed G, Kelly TA, Stanek EJ, 3rd, et al. Sequence and seasonal effects of salivary cortisol. Behav Med. 2000;26(2):67–73. doi: 10.1080/08964280009595753. [DOI] [PubMed] [Google Scholar]
- Kohsaka M, Fukuda N, Honma K, Honma S, Morita N. Seasonality in human sleep. Experientia. 1992;48(3):231–233. doi: 10.1007/BF01930461. [DOI] [PubMed] [Google Scholar]
- Laakso ML, Porkka-Heiskanen T, Alila A, Stenberg D, Johansson G. Twenty-four-hour rhythms in relation to the natural photoperiod: a field study in humans. J Biol Rhythms. 1994;9(3–4):283–293. doi: 10.1177/074873049400900309. [DOI] [PubMed] [Google Scholar]
- Lee TM, Blashko CA, Janzen HL, Paterson JG, Chan CC. Pathophysiological mechanism of seasonal affective disorder. J Affect Disord. 1997;46(1):25– 38. doi: 10.1016/s0165-0327(97)00076-1. [DOI] [PubMed] [Google Scholar]
- Leproult R, Colecchia EF, L’Hermite-Baleriaux M, Van Cauter E. Transition from dim to bright light in the morning induces an immediate elevation of cortisol levels. J Clin Endocrinol Metab. 2001;86(1):151–157. doi: 10.1210/jcem.86.1.7102. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Bauer VK, Cutler NL, Sack RL, Ahmed S, Thomas KH, et al. Morning vs evening light treatment of patients with winter depression. Arch Gen Psychiatry. 1998;55(10):890–896. doi: 10.1001/archpsyc.55.10.890. [DOI] [PubMed] [Google Scholar]
- Malarkey WB, Pearl DK, Demers LM, Kiecolt-Glaser JK, Glaser R. Influence of academic stress and season on 24-hour mean concentrations of ACTH, cortisol, and beta-endorphin. Psychoneuroendocrinology. 1995;20(5):499–508. doi: 10.1016/0306-4530(94)00077-n. [DOI] [PubMed] [Google Scholar]
- Monk TH, Aplin LC. Spring and autumn daylight saving time changes: studies of adjustment in sleep timings, mood, and efficiency. Ergonomics. 1980;23(2):167–178. doi: 10.1080/00140138008924730. [DOI] [PubMed] [Google Scholar]
- Murray G, Allen NB, Trinder J. A longitudinal investigation of seasonal variation in mood. Chronobiol Int. 2001;18(5):875–891. doi: 10.1081/cbi-100107522. [DOI] [PubMed] [Google Scholar]
- Murray G, Allen NB, Trinder J. Mood and the circadian system: investigation of a circadian component in positive affect. Chronobiol Int. 2002;19(6):1151–1169. doi: 10.1081/cbi-120015956. [DOI] [PubMed] [Google Scholar]
- Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, et al. Nature’s clocks and human mood: the circadian system modulates reward motivation. Emotion. 2009;9(5):705–716. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- Nayyar K, Cochrane R. Seasonal changes in affective state measured prospectively and retrospectively. Br J Psychiatry. 1996;168(5):627–632. doi: 10.1192/bjp.168.5.627. [DOI] [PubMed] [Google Scholar]
- Nunez PL. Electric fields of the brain: the neurophysics of EEG. New York: Oxford University Press; 1981. [Google Scholar]
- Partonen T. Bright light and high-density negative air ionization reduces symptoms of seasonal affective disorder. West J Med. 1999;171(5–6):315–316. [PMC free article] [PubMed] [Google Scholar]
- Peterson CK, Harmon-Jones E. Circadian and seasonal variability of resting frontal EEG asymmetry. Biol Psychol. 2009;80(3):315–320. doi: 10.1016/j.biopsycho.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Randler C, Schaal S. Morningness-eveningness, habitual sleep-wake variables and cortisol level. Biol Psychol. 85(1):14–18. doi: 10.1016/j.biopsycho.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Reid SA, Duke LM, Allen JJB. Resting frontal electroencephalographic asymmetry in depression: Inconsistencies suggest the need to identify mediating factors. Psychophysiology. 1998;35:389– 404. [PubMed] [Google Scholar]
- Rosenthal NE, Sack DA, Skwerer RG, Jacobsen FM, Wehr TA. Phototherapy for seasonal affective disorder. J Biol Rhythms. 1988;3(2):101–120. doi: 10.1177/074873048800300202. [DOI] [PubMed] [Google Scholar]
- Sack RL, Lewy AJ, White DM, Singer CM, Fireman MJ, Vandiver R. Morning vs evening light treatment for winter depression. Evidence that the therapeutic effects of light are mediated by circadian phase shifts. Arch Gen Psychiatry. 1990;47(4):343–351. doi: 10.1001/archpsyc.1990.01810160043008. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJB. Resting frontal EEG asymmetry as an endophenotype for depression risk: sex-specific patterns of frontal brain asymmetry. J Abnorm Psychol. 2010;119(3):502–512. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman M. Review: light therapy is an effective treatment for seasonal affective disorder. Evid Based Ment Health. 2006;9(1):21. doi: 10.1136/ebmh.9.1.21. [DOI] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. J Abnorm Psychol. 2006;115(4):715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Tops M, Wijers AA, van Staveren AS, Bruin KJ, Den Boer JA, Meijman TF, Korf J. Acute cortisol administration modulates EEG alpha asymmetry in volunteers: relevance to depressions. Biological Psychology. 2005;69(2):181–193. doi: 10.1016/j.biopsycho.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Tops M, van Peer JM, Wester AE, Wijers AA, Korf J. State-dependent regulation of cortical activity by cortisol: an EEG study. Neurosci Lett. 2006;404(1–2):39–43. doi: 10.1016/j.neulet.2006.05.038. [DOI] [PubMed] [Google Scholar]
- Towers DN, Allen JJ. A better estimate of the internal consistency reliability of frontal EEG asymmetry scores. Psychophysiology. 2009;46(1):132–142. doi: 10.1111/j.1469-8986.2008.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BR, Best R, Noon JP, Watt GC, Webb DJ. Seasonal variation in glucocorticoid activity in healthy men. J Clin Endocrinol Metab. 1997;82(12):4015–4019. doi: 10.1210/jcem.82.12.4430. [DOI] [PubMed] [Google Scholar]
- Webb WB, Bonnet MH. The sleep of ‘morning’ and ‘evening’ types. Biol Psychol. 1978;7(1–2):29–35. doi: 10.1016/0301-0511(78)90040-6. [DOI] [PubMed] [Google Scholar]