Abstract
How cell fate decisions of stem and progenitor cells are regulated by their microenvironment or niche is a central question in stem cell and regenerative biology. While functional analysis of hair follicle epithelial stem cells by gene targeting is well-established, the molecular and genetic characterization of the dermal counterpart during embryonic morphogenesis has been lacking due to the absence of cell type-specific drivers. Here we report that T-box transcription factor Tbx18 specifically marks dermal papilla (DP) precursor cells during embryonic hair follicle morphogenesis. With Tbx18LacZ, Tbx18H2BGFP and Tbx18Cre knock-in mouse models we demonstrate LacZ/GFP expression and Cre activity in dermal condensates of nascent first-wave hair follicles at E14.5. Since Tbx18 expression becomes more widespread throughout the dermis at later developmental stages, we utilize tamoxifen-inducible Cre expressing mice, Tbx18MerCreMer, to exclusively target DP precursor cells and their progeny. Finally, we ablate Tbx18 in full knockout mice, but find no perturbations in hair follicle formation, suggesting that Tbx18 is dispensable for normal DP function. In summary, our study establishes Tbx18 as a genetic driver to target embryonic DP precursors for labeling, isolation and gene ablation that will greatly enhance investigations into their molecular functions during hair follicle morphogenesis.
INTRODUCTION
Hair follicle formation requires a series of epithelial-mesenchymal interactions between dermal and epidermal cells that are separated by a basement membrane (Figure 1a) (Hardy, 1992; Millar, 2002). At embryonic day (E)13.5, specializing dermal cells send an unidentified first signal(s) to stem cells in the epidermis that switch from an epidermal to a hair follicle fate (Sengel, 1976). The epidermal stem cells rearrange to form hair placodes, which in return send back a signal(s) to the dermal compartment to form recognizable cell condensates of dermal papilla (DP) precursor cells (Hardy, 1992). DP precursor cells send yet another unknown signal(s) to the hair placodes that launches proliferation and downgrowth of hair germs and pegs, with DP cells at the leading edge (Figure 1a). During this process, stem cells are set aside in the upper portion of downgrowing hair follicles in the future bulge region (Nowak et al., 2008). During further downgrowth, matrix cells that are direct stem cell progeny and reside at the base of the follicle bulb, engulf DP precursor cells to form the mature DP. This basic morphogenetic sequence of hair follicle formation is repeated in three separate waves giving rise to different hair follicle types (Figure 1a) (Schlake, 2007).
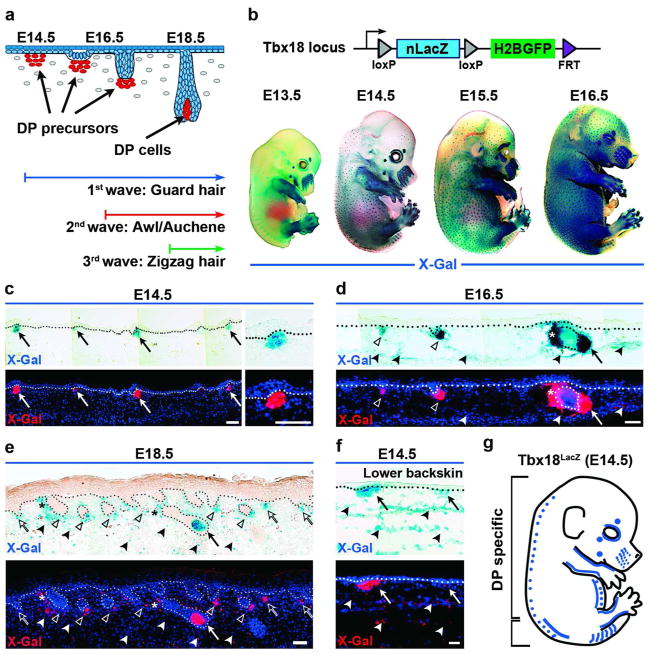
Figure 1. Tbx18LacZ expression in embryonic skin.
(a) Schematic of embryonic hair follicle development. Dermal condensates are visible at E14.5. (b) Top: Schematic of Tbx18LacZ reporter. Bottom: Whole-mount X-Gal staining in embryos. (c–f) Top: LacZ expression in skin sections at different ages. Bottom: Pseudo-colored X-Gal (red) overlaid with Dapi to highlight nuclei. The dotted line marks basement membrane. (c) LacZ in dermal condensates at E14.5 (arrows). (d) LacZ expression in DP cells of downgrowing guard hair follicles at E16.5 (arrow) and in second-wave DPs (open arrowheads). (e) At E18.5, all DPs express LacZ in downgrowing follicles (arrow, open arrowheads) and third wave dermal condensates (open arrows). (d,e) Note weak LacZ expression in dermis (filled arrowheads) and arrector pili muscle (asterisks). (f) Weak LacZ expression in dermal cells in lower backskin at E14.5 (arrowheads). (g) Schematic summarizing Tbx18 expression in embryonic skin at E14.5. Blue dots illustrate Tbx18 expression in DP precursor cells, the blue line widespread expression in the dermis (lower bracket). Bars = 50 μm.
Most of our current understanding regarding the timing of embryonic hair follicle induction is derived from classical tissue recombination assays, which established the essential role of dermal cells in driving epidermal stem cells towards a hair follicle fate (Dhouailly, 1973; Sengel, 1976). Identification of ligand/receptor expression, combined with functional analysis of ligands in bead implantation experiments, and analysis of gene knockouts and spontaneous mouse mutants (Schneider et al., 2009) revealed that Wnt, Shh, Tgf/Bmp and Eda/Edar/NFκB signaling pathways are most likely involved in the earliest steps of hair follicle morphogenesis (Botchkarev et al., 1999; Headon & Overbeek, 1999; Huelsken et al., 2001; St-Jacques et al., 1998). Compartment-specific gene ablation and transgenic overexpression of ligands/receptors in the epidermis (Vasioukhin et al., 1999; Vassar et al., 1989) further refined our knowledge of timing and requirements of many signaling pathways for hair follicle formation (Millar, 2002; Schneider et al., 2009). However, specific targeting of DP precursor cells in dermal condensates has been unavailable, thus precluding genetic analysis of signaling events in the mesenchymal counterpart during early follicle morphogenesis. Given the absence of such tools, it is not surprising that the precise order of signaling events and their epistatic hierarchy in placode stem cells and dermal condensates during hair follicle formation is still not entirely clear.
In this study, we now establish a previously unreportedgenetic system to specifically target embryonic DP cell clusters for cell and gene ablation during the first wave of hair follicle morphogenesis. In screening several mouse reporter lines, we identified Tbx18 expression in dermal condensates. Using this gene locus, we show specific Cre activity in DP precursor cells of first-wave guard hair follicles in murine backskin. With tamoxifen inducible Cre, we further demonstrate spatial and temporal control of specific Cre activity. Finally, we show that gene ablation of Tbx18 itself does not cause any perturbations of hair follicle induction and growth. This suggests that Tbx18 is not required for normal DP function, which is preferable for a genetic driver in which the endogenous locus is targeted. For all these reasons, Tbx18 is a previously unreported useful genetic driver to target DP precursors for gene and cell ablation, which will help uncover their molecular functions during embryonic hair follicle formation.
RESULTS
Tbx18 is expressed in DP precursor cells during embryonic hair follicle formation
To date, the hair development field has been lacking genetic drivers for specific targeting of DP precursor cells in dermal condensates, which are thought to interact with placode stem cells for morphogenesis to proceed (Figure 1a). Here, we capitalized on our previous characterization of postnatal DP gene signatures (Rendl et al., 2005), and screened several transgenic and knock-in reporter mouse lines for specific expression in embryonic DP precursors (data not shown). We identified Tbx18LacZ reporter mice with the most specific expression in dermal condensates. In this knock-in line, LacZ is under the control of the endogenous Tbx18 promoter (Figure 1b, schematic) (Cai et al., 2008). In a series of whole-mount X-gal stained Tbx18LacZ embryos, we detected Tbx18 expression in evenly distributed foci at E14.5 (Figure 1b), reminiscent of the typical pattern of forming first-wave guard hair follicles. In some cases we observed rare stained spots as early as E14.0 (Supplementary Figure S1a online). No LacZ staining was detectable in the skin at E13.5 (Figure 1b) or earlier time points (Supplementary Figure S1a online). Tbx18 expression in other body areas besides skin was limited to the somites, limbs and whiskers (Figure 1b; Supplementary Figure S1a online), and to the meninges and epicardium (not shown), as previously described (Cai et al., 2008; Kraus et al., 2001).
To determine if Tbx18LacZ expression was confined to DP precursor cells, we next analyzed sagittal embryo sections (Figure 1c-f). At E14.5, LacZ expression was detectable in cell clusters right below the epidermis, reminiscent of early dermal condensates (Figure 1c). No expression was detectable in epidermis or hair placodes. DP precursors continued to express LacZ in downgrowing hair germs at E15.5 (Supplementary Figure S1b online) and in hair pegs at E16.5 (Figure 1d). Newly forming dermal condensates from second-wave follicles also expressed Tbx18 (Figure 1d; open arrowheads). In E18.5 first-wave follicles, mature DP cells were labeled while becoming engulfed by matrix cells (Figure 1e, arrow). Dermal condensates of nascent third-wave follicles were labeled as well (Figure 1e; open arrows). Starting at E16.5, in addition to expression in DP cells, weak LacZ labeling also became apparent more widespread in the dermis (Figure 1d,e, filled arrowheads), including cell clusters of future arrector pili muscle (Figure 1d, asterisk; Supplementary Figure S1c online). This indicates that Tbx18 expression does not remain confined to DPs at later developmental stages. We also observed more widespread LacZ expression in dermal cells in the most posterior part of the backskin at E14.5 (Figure 1f; Supplementary Figure S1d online). Taken together these data suggest that within the first two critical days of first-wave hair follicle formation Tbx18LacZ expression is specific in DP precursor cells in most of the backskin (Figure 1g, blue dots in upper bracket).
Labeling and isolation of DP precursor cells by H2BGFP in the Tbx18 locus
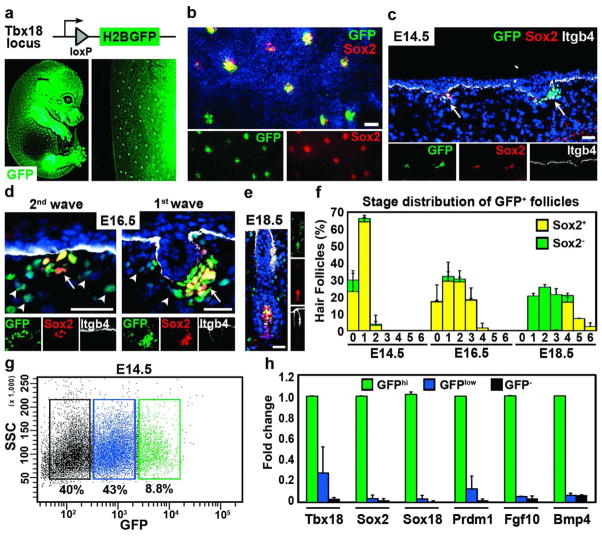
To further characterize Tbx18 expressing condensates we utilized Tbx18H2BGFP reporter mice that express a nuclear histone 2B/GFP fusion protein, H2BGFP, under the control of the endogenous Tbx18 locus (Figure 2a). We generated these mice by removing LoxP flanked LacZ in the germline (Figure 1b). Analyses of E14.5 whole-mount embryos showed GFP expression in forming hair follicles reminiscent of Tbx18LacZ embryos (Figure 2a). To verify Tbx18-driven GFP expression in DP precursors we next labeled entire skins (Figure 2b) or skin sections (Figure 2c) by immunofluorescence for Sox2, a known marker of dermal condensates at E14.5 (Driskell et al., 2009; Tsai et al., 2010). All Sox2 positive dermal condensates were also labeled for H2BGFP (Figure 2b), indicating that Tbx18-H2BGFP marks all early condensates. Sagittal sections confirmed co-labeling of nuclear GFP with nuclear Sox2 cell clusters right below the epidermis (Figure 2c; arrows). Similarly, second-wave dermal condensates at E16.5 were also double-labeled, as well as DPs in downgrowing first-wave hair pegs (Figure 2d; arrows). In addition, weaker GFP was detectable in dermal cells at E16.5 (Figure 2d; arrowheads), consistent with LacZ expression (Figure 1d). At E18.5, GFP expression was present in mature DPs that also expressed Sox2 (Figure 2e). Quantification of GFP and Sox2 positive condensates at E14.5 and E16.5 revealed double-labeling in all first- and second-wave hair follicles (Figure 2f). All DPs at later developmental stages (Paus et al., 1999) were also GFP positive, including DP precursor cells of forming third-wave zigzag hair follicles at E18.5, previously described as Sox2 negative (Driskell et al., 2009). These data demonstrated that Tbx18 is expressed in dermal condensates of all developing hair follicles. Finally we tested whether Tbx18 is also expressed in the mesenchyme during formation of other appendages, such as mammary, salivary and dental condensates. Surprisingly, Tbx18LacZ and Tbx18H2BGFP were not detectable in these appendages (Supplementary Figure S2 online), although many morphogenetic features are shared with hair follicle formation (Mikkola & Millar, 2006).
Figure 2. Labeling and isolation of dermal condensates with Tbx18H2BGFP.
(a) Schematic of Tbx18H2BGFP reporter. Below E14.5 embryo (left) showing GFP expression in hair follicles. (b–e) Sox2 co-localization with GFP in DP precursors and more mature DPs. Whole-mount skin (b) and sagittal section (c, arrows) at E14.5. (d) GFP expression in first- and second-wave DP follicles (arrows) and dermis (arrowheads) at E16.5. (e) GFP and Sox2 co-expression in mature DP. Itgb4 marks basement membrane. Dapi highlights all nuclei (blue). Bar = 50μm. (f) Quantification of Sox2 cells in GFP-positive DPs in all developmental stages (n=4). (g) FACS isolation of DP precursors at E14.5. Three cell populations were isolated: GFPhi, dermal condensates (green); GFPlow, dermal cells from lower backskin (blue); GFP−, negative cells (black). (h) Real-time PCR analysis of isolated cells. Only GFPhi cells were enriched in dermal condensate genes. Data shown are mean ± SD (n=2).
To independently confirm Tbx18H2BGFP positive cells as DP precursors, we isolated and analyzed cells from E14.5 backskin by fluorescence activated cell sorting (FACS). Single cell preparations were immunolabeled for E-cadherin, CD31, CD34 and CD45 to gate away epidermal, endothelial and hematopoietic cells, respectively (not shown). None of these markers were detectable in GFP-positive condensates in sections or in GFPhi cells in FACS (Supplementary Figure S3 online). We then sorted GFPhi, GFPlow and GFP− cells to isolate DP precursors and two populations of dermal cells, respectively (Figure 2g). Real-time PCR analysis of Tbx18, Sox2 and few other known condensate markers showed strong enrichment only in GFPhi cells (Figure 2h). This confirms that Tbx18 expression at E14.5 is confined to dermal condensates and that Tbx18H2BGFP can be used to isolate these cells. Taken together, the specific expression activity of Tbx18 in DP precursor cells during hair follicle formation suggests that Cre recombinase expression utilizing the Tbx18 locus may be suitable to specifically target dermal condensates for gene and cell ablation.
Tbx18Cre activity targets DP precursor cells during hair follicle formation
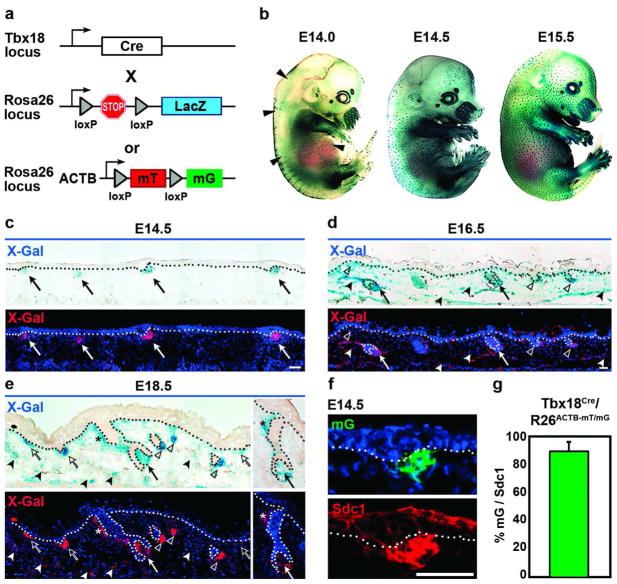
To determine whether Cre recombinase expression from the Tbx18 locus can efficiently drive LoxP recombination of floxed alleles in dermal condensates, we crossed Tbx18Cre knock-in mice (Cai et al., 2008) with R26RLacZ reporter lines (Figure 3a). R26RLacZ mice express LacZ under the ubiquitously active endogenous Rosa26 promoter (Soriano, 1999). LacZ is only expressed after excision of a loxP-flanked stop sequence by Cre-mediated recombination. In Tbx18Cre/R26RLacZ embryos, LacZ expression was detectable in few follicles at E14.0 (Figure 3b; arrowheads). By E14.5, Cre-mediated LacZ reporter expression was present in developing hair follicles throughout the backskin (Figure 3b), suggesting that Tbx18 promoter activity drives sufficient Cre levels for efficient recombination of floxed alleles during the early stages of hair follicle formation. Histological analyses of sectioned embryos revealed robust reporter expression in DP precursor cells at E14.5 (Figure 3c; arrows) and later at E16.5 and E18.5 (Figure 3d,e). Consistent with expression in Tbx18LacZ and Tbx18H2BGFP reporters, by E16.5 and E18.5 more widespread reporter activity in dermis and arrector pili muscle was also found (Figure 3d,e, filled arrowheads, asterisks).
Figure 3. Tbx18Cre activity in DP precursor cells during hair follicle formation.
(a) Schematic of Tbx18Cre, R26RLacZ and R26ACTB-mT/mG reporter lines. (b) Whole-mount X-Gal staining of Tbx18Cre/R26RLacZ embryos at E14.5 showed Cre activity in a hair follicle pattern. Few labeled follicles were visible at E14.0 (arrowheads). (c) Cre activity in DP precursor cells at E14.5 (arrows). (d,e) At E16.5 and E18.5, Cre activity is present in all DP cells (arrows, open arrowheads) and dermal condensates (open arrows), and more widespread in the dermis and arrector pili muscle (filled arrowheads, asterisks). (f) Immunofluorescence for Sdc1 (Syndecan1) confirmed identity of GFP positive dermal condensates with the R26ACTB-mT/mG reporter. (g) Quantification of GFP and Sdc1 double-labeled dermal condensates (n=2). Bars = 50μm. Data shown are mean ± SD.
To ascertain that Cre activity is truly specific to dermal condensates at E14.5, we used R26ACTB-mT/mG as a second, independent reporter line (Figure 3a). These mice ubiquitously express the cell membrane-bound red fluorescent protein tdTomato (mT) under the control of the actin B promoter (ACTB). In the presence of Cre, loxP flanked tdTomato is deleted and cell membrane-bound GFP (mG) is expressed (Muzumdar et al., 2007). In Tbx18Cre/R26ACTB-mT/mG double-knockin E14.5 embryos, Cre-mediated GFP expression was detectable only in DP precursor cells (Figure 3f). Immunofluorescence staining for Syndecan-1 (Richardson et al., 2009) confirmed reporter GFP expression in dermal condensates (Figure 3f). Quantification of Cre recombination efficiency revealed over 90% Sdc1-positive DP precursor clusters were also GFP labeled (mG), confirming that Tbx18-driven Cre activity efficiently targets dermal condensates during first wave hair follicle formation (Figure 3g). Similarly, labeling with integrin alpha 8 (Itga8) (Fujiwara et al, 2011) and quantification of second and third wave dermal condensates and DPs revealed efficient recombination in those follicle subtypes as well (Supplementary Figure S4 online). To confirm that Tbx18 expression is absent from epidermal and hair follicle epithelial cells we double-stained E18.5 Tbx18Cre/R26ACTB-mT/mG and Tbx18H2BGFP embryos by immunofluorescence for basement membrane marker integrin-beta 4 (Itgb4) and epithelial marker keratin-14 (K14) or mesenchymal markers vimentin (Vim) and smooth muscle actin (SMA). GFP reporter positive cells were always present outside epithelial skin compartments within the dermal sheath and dermis (Supplementary Figure S5 online). Taken together, these data suggest that Tbx18-Cre activity mirrors Tbx18LacZ and Tbx18H2BGFP expression and efficiently targets DP precursor cells of first-wave hair follicles in most of the backskin in a temporally and spatially precise fashion.
Tamoxifen-inducible Cre activity in Tbx18MerCreMer mice specifically targets DP precursor cells and DP progeny of first-wave hair follicles
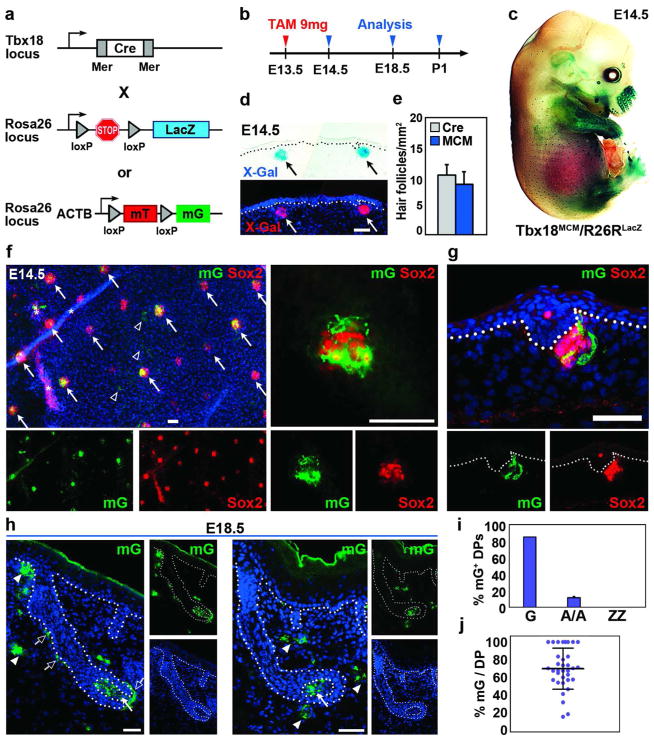
Gene ablation experiments with the constitutive Tbx18Cre line will be useful for analyzing essential gene functions in DP precursor cells during the first two days of hair follicle formation. Since Tbx18-driven Cre activity becomes more widespread in the dermis at E16.5 (Figure 3d), restricting Cre expression to E14.5 dermal condensates and their progeny will be necessary to allow interpretation of DP gene ablation phenotypes at later time points. Therefore to temporally control Cre activity, we generated Tbx18MerCreMer (Tbx18MCM) knockin mice, in which Tbx18 drives expression of drug-inducible Cre double-fusion protein MerCreMer (Zhang et al., 1996). Cre is flanked on each end with a mutated murine estrogen receptor (Mer) ligand binding domain (Figure 4a) that is only active in the presence of an estrogen analog, such as tamoxifen. We then crossed Tbx18MCM mice with R26RLacZ and R26ACTB-mT/mG reporters (Figure 4a) and activated Cre with a single intraperitoneal injection of high-dose tamoxifen in E13.5 pregnant females (Figure 4b). This dose was previously shown to optimally activate inducible Cre (Hayashi and McMahon, 2002). At E14.5, when clustering DP precursors begin to express LacZ, H2BGFP and Cre under the Tbx18 locus (Figures 1–3), we also detected robust reporter labeling in whole-mount X-Gal stained Tbx18MCM/R26RLacZ embryos (Figure 4c). Analyses of embryo sections revealed specific LacZ expression in dermal condensates (Figure 4d). Quantification of labeled hair follicles confirmed equally efficient recombination with tamoxifen-inducible Tbx18MCM compared to constitutive Tbx18Cre (Figure 4e).
Figure 4. Inducible Cre activity in Tbx18MerCreMer mice targets guard hair DP cells and their progeny.
(a) Schematic of tamoxifen (TAM) inducible Tbx18MerCreMer (MCM) mice crossed with R26RLacZ or R26ACTB-mT/mG reporters. (b) Timeline of Cre induction and reporter analysis. (c) Whole-mount X-gal staining of Tbx18MCM/R26RLacZ embryo showed reporter activation in hair follicle pattern at E14.5. (d) Inducible Cre activity in dermal condensates. (e) Quantification of labeled follicles in Tbx18Cre and Tbx18MCM embryos. (f) Co-expression of Sox2 and GFP in DPs of Tbx18MCM/R26ACTB-mT/mG whole-mount skin at E14.5 (arrows). Few dermal cells were labeled (arrowheads); asterisks mark autofluorescence; right: high magnification. (g) Co-localization in sagittal section. (h) GFP expression in DP (arrows) and dermal sheath cells (open arrows) of guard hair follicles at E18.5. Few dermal cells were GFP positive (arrowheads). (i) Quantification of follicles with GFP-positive DP cells (n=3). (j) Quantification of GFP-positive DP cells per DP in embryo sections. In ~25% of guard hairs 100% DP cells were labeled (n=3). Bar = 50μm. Data shown are mean ± SD.
Similar results were obtained with the R26ACTB-mT/mG reporter. In whole-mount E14.5 skin, we found GFP expression in the majority of dermal condensates that were identified by co-localization of Sox2 immunofluorescence (Figure 4f; arrows). Few dermal cells between condensates were labeled as well (Figure 4f; arrowheads). Sox2 in sagittal sections confirmed Cre-mediated reporter activation in DP precursor cells (Figure 4g). Analysis of E18.5 sections revealed that recombination activity was mostly restricted to DPs in first-wave guard hair follicles (Figure 4h; arrows), while only few dermal cells were also positive (arrowheads), as compared to the widespread labeling of dermal cells with constitutive Tbx18Cre (Figure 3e). Interestingly, GFP-positive cells were also evident in dermal sheath cells in 85% of guard hair follicles, suggesting that those are derived from early dermal condensates as well (Figure 4h, open arrows; Supplementary Figure S6a, c online). Quantification of inducible Cre-mediated recombination events showed GFP positive DP cells in >80% of first-wave guard hair follicles (Figure 4i). Some GFP expression was still found in ~10% DPs of second-wave awl/auchene hair follicles, possibly due to residual amounts of tamoxifen present at E16.5 (Figure 4i). Within each guard hair DP compartment recombination efficiency varied widely from 15–100% with an average of ~70% GFP positive DP cells (Figure 4j). Importantly, however, in >25% of all guard hair sections 100% DP cells were Cre reporter positive (Figure 4j). Tbx18MCM/R26RLacZ and Tbx18MCM/R26ACTB-mT/mG embryos from females injected only with corn oil and without tamoxifen did not show any labeling, confirming absence of any leakiness of inducible MCM (Supplementary Figure S6b online).
In summary, these data demonstrate that controlled Cre activation in Tbx18MCM mice allows specific recombination in dermal condensates and their progeny of first-wave guard hair follicles. They further suggest that temporally regulated Cre activation will be useful to ablate signature genes specifically in guard hair DP condensates to study their role during hair follicle formation.
Tbx18 is not required for hair follicle induction and growth
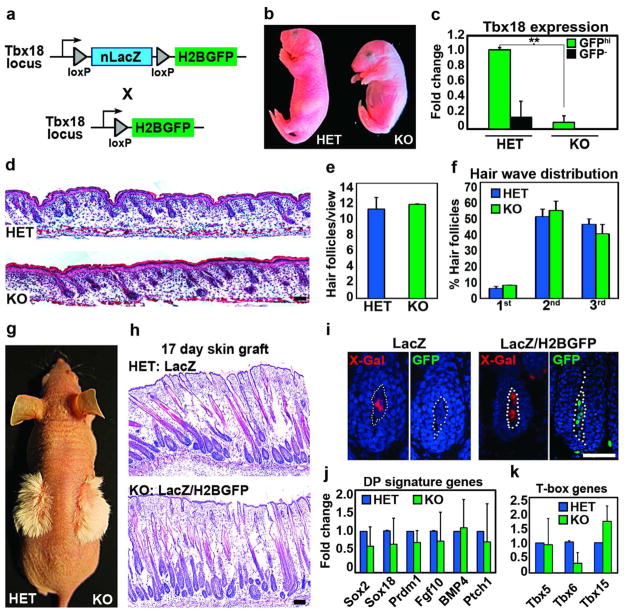
Since Tbx18 is specifically expressed in DP precursor cells at the earliest stages during hair follicle formation, we asked whether this transcription factor plays a role in follicle morphogenesis. For this purpose, we generated Tbx18LacZ/H2BGFP knockout (KO) embryos by crossing the Tbx18LacZ and Tbx18H2BGFP heterozygous (HET) knock-in lines (Figure 5a). Tbx18 KO pups had shortened body length (Figure 5b) and died soon after birth due to severe skeletal and respiratory failure, as previously described (Bussen et al., 2004). To ensure Tbx18 ablation in the absence of available Tbx18 immunostaining, we isolated HET and KO dermal condensates as GFPhi cells from Tbx18H2BGFP embryos by FACS (as in Figure 2f) and confirmed the absence of Tbx18 in the KO by real-time PCR (Figure 5c). Hematoxylin and eosin stained sections of E18.5 Tbx18 null embryos showed no apparent difference in hair follicle morphology (Figure 5d) and numbers of all follicles (Figure 5e) and follicle subtypes from all 3 waves (Figure 5f). Immunofluorescence and morphometric analysis further revealed normal matrix and DP marker expression, similar proliferation rates and comparable DP sizes and cell numbers (Supplementary Figure S7c, d online). These data suggest that Tbx18 is not essential for embryonic follicle morphogenesis.
Figure 5. Tbx18 in DP precursor cells is not required for hair follicle formation.
(a) Schematic of generating Tbx18 knockouts. (b) Newborn Tbx18 knockout (KO) and heterozygous (HET) pups. (c) Verification of Tbx18 ablation by Real-time PCR in FACS isolated DP precursor cells at E14.5 (n=3). (d) Hematoxylin and eosin staining of newborn backskins HET and KO. (e) Unchanged total hair follicle numbers (n=3). (f) All hair follicle subtypes are formed in KO (n=3). (g) Normal hair shaft formation in skin grafts of newborn HET (Tbx18LacZ) and KO (Tbx18LacZ/H2BGFP). (h) Hematoxylin and eosin staining showed normal follicle morphologies in KO skin grafts. (i) LacZ and GFP expression in DPs confirming donor origin of grafted skins. (j,k) Real-time PCRs of FACS-isolated DPs at E14.5 for DP signature genes (j) and T-box family members (k) (n=3). Bar = 50μm. Data shown are mean ± SD. **, p<0.01.
To determine whether Tbx18 plays a major role during postnatal hair growth, we transplanted newborn HET and KO skin onto immunocompromised nude host mice, KO pups die soon after birth. 17 days after skin grafting, well-formed hair shafts were visible in both HET and KO grafted skins (Figure 5g). Histological analyses showed comparable hair follicle numbers and morphologies in HET and Tbx18-null skins (Figure 5h). LacZ and GFP expression in grafted skins confirmed the donor origin of the transplants (Figure 5i). Gene expression analysis in HET and KO sorted DP cells revealed no significant changes of selected DP signature genes (Figure 5j). Finally, we tested expression of other T-box gene family members that could be affected by loss of Tbx18. Real-time PCRs of Tbx5, Tbx6 and Tbx15 demonstrated no significant changes in DP gene expression levels (Figure 5k). Only Tbx15 expression appeared slightly upregulated in three different experiments, suggesting mild compensatory gene regulation. In summary, these data suggest that Tbx18 expression itself is not required for DP function during hair follicle formation.
DISCUSSION
DP precursor cells in dermal condensates right beneath the epidermis exchange signals with stem cells in hair placodes to drive embryonic hair follicle formation (Driskell et al., 2011; Hardy, 1992; Yang and Cotsarelis, 2010). However, very little is currently known about how DP cells function in their interaction with the stem cells to promote hair follicle formation. This has been largely due to the absence of genetic tools to target these cells for specific cell isolation and functional analysis in gene ablation assays. With this study, we have addressed this need and established genetic mouse models for studying the molecular properties and interrogating gene functions of DP precursor cells in dermal condensates during hair follicle formation.
First, with Tbx18LacZ and Tbx18H2BGFP knock-in mice we demonstrated specific expression in E14.5 DP precursor cells at the earliest stages of hair follicle formation. Besides marker co-staining in embryo sections, we verified the identity of Tbx18 expressing DP condensates in FACS isolated GFP-positive cells by high enrichment of known DP signature genes. Future genome-wide interrogation of gene expression in dermal condensates, combined with rigorous comparison of their transcriptomes with those from regular fibroblasts and epidermal stem cells should reveal specific molecular signature features that could play a role in DP precursor functions. In our FACS analysis we noticed weak GFP expression matching lower dermal GFP levels in the most posterior backskin. This would not pose a problem for gene profiling efforts, since GFPlow cells do not express DP signature genes and can be clearly separated from GFP-high cells (Figure 2). It should be also noted that as development proceeds, Tbx18H2BGFP expression expands more widely throughout the entire dermis by E16.5-E18.5, precluding isolation of pure second- and third-wave condensates, thereby limiting clean cell isolations to first-wave guard hair condensates. While it is possible that first-wave guard hair-specific genes that play a role in hair-type specification will be identified using such methods, it is equally conceivable that genes involved in the general follicle formation paradigm will be uncovered as well.
Second, with Tbx18Cre mice we demonstrate robust Cre activity in dermal condensates at E14.5, which is essential for successful conditional gene ablation to interrogate the functional role of DP genes during early follicle formation. As with Tbx18 gene expression, more widespread dermal Cre reporter activity was found throughout the entire backskin, starting at E16.5. However, this should not hinder conclusive interpretation of gene knockout effects on hair follicle formation during the initial two-day time window in which Tbx18 is restricted to dermal condensates. Additionally, effects of ablating genes that are specifically expressed in DP precursor cells, such as Bmp4, Blimp1, Sox2 and others should be interpretable throughout later hair follicle development, as long as gene expression remains confined to DP.
Third, to temporally control Cre activity we established a Tbx18MerCreMer line to drive tamoxifen-inducible Cre. With this previously unreported mouse line, we were able to efficiently and specifically target reporter activity to early DP condensates. Following a single high-dose tamoxifen injection one day prior to hair formation at E13.5, mostly selective labeling of DP cells from first-wave guard hair follicles was achieved. No DP cells of third-wave zigzag hair follicles were labeled and only few DP cells of ~10% second-wave follicles were labeled. Importantly, widespread Cre activity, which ensues after E16.5 with constitutive Tbx18Cre, was prevented with the inducible line. This will allow unequivocal attribution of future gene ablation phenotypes to interference with DP function in first-wave guard hair follicles. We noticed reduced recombination efficiency compared to constitutive Cre activation, since several DP compartments showed only partial reporter expression, although >80% DPs were labeled. Importantly, in over 25% of all guard hair sections 100% DP cells were Cre reporter positive suggesting efficient recombination and successful future gene ablation in entire DPs of several hair follicle units. Identification of 100% recombined DPs with Cre reporters followed by phenotype analysis of such hair follicle units is reminiscent of the successful analysis strategy in the Drosophila clone marking and gene knockout system (del Valle Rodriguez et al., 2012). Since in whole-mount skin hundreds of follicles can be analyzed simultaneously, individually affected hair follicle units with 100% recombined DPs can be detected and analyzed as clones.
In several hair follicles around birth we noticed labeling of dermal sheaths as well, indicating that at least many, and possibly all of these cells are derived from DP condensates rather than having joined hair follicles from the dermis during follicle downgrowth. The concept of cellular mobility between the two dermal hair follicle components was previously proposed during postnatal hair growth (Tobin et al., 2003), and more recently during the hair cycle (Chi et al., 2010). Future lineage tracing experiments with low-dose tamoxifen induced Cre activity in single DP precursor cells and clonal cell fate analysis should be useful to further decipher the relationship between DP cells and adjacent dermal sheath cells during hair follicle morphogenesis.
Recent work introduced Corin-Cre as the first in vivo driver to target mature DP cells (Enshell-Seijffers et al., 2010), limiting its use to studying DP gene functions during postnatal hair follicle growth. At that stage, DP cells are thought to instruct surrounding proliferating and differentiating matrix cells to give rise to the outgrowing hair shaft. Ablation of Wnt/β-catenin signaling in DP cells by postnatal day 7 caused reduced hair shaft outgrow and a failure of hair re-growth during the cycle (Enshell-Seijffers et al., 2010). While broad dermal inhibition of Wnt/β-catenin signaling during embryogenesis demonstrated an important role for dermal development and hair follicle initiation (Chen et al., 2012), it should be interesting to interrogate with the Tbx18 lines the role of Wnt/β-catenin signaling in dermal condensates for interaction with placodes during further embryonic follicle formation.
Finally, despite its remarkably specific expression in DP condensates at E14.5, ablating Tbx18 in full knockout mice did not interfere with normal hair follicle morphogenesis, demonstrating that Tbx18 is not required for hair follicle formation. One possible explanation could be genetic redundancy with closely related family members that may be expressed within the DP compartment and that functionally could compensate for the loss of Tbx18. We found that Tbx subfamily members Tbx5, 6 and 15 were expressed in isolated DP precursor cells. Tbx15 is the closest related T-box protein by phylogenetic, structural and sequence conservation analysis (Naiche et al., 2005), suggesting a potential for functional redundancy. Tbx22, another highly related T-box protein family member, was not expressed in our analysis (not shown). Indeed, recent biochemical analysis of Tbx15 and Tbx18 showed identical DNA binding properties, suggesting that both proteins could regulate similar target genes (Farin et al., 2007) and contribute to functional redundancy. Future analysis of Tbx18/Tbx15 double-knockouts should clarify the role of these T-box family members in embryonic DP precursor cell function during hair follicle formation. The fact that Tbx18 knockout and heterozygous mice showed no detriment to normal hair follicle morphogenesis is important for the successful use of our Tbx18 reporter and Cre expressing knockin mice. This is especially critical, since several other T-box proteins show dosage sensitivity with phenotypes even in heterozygous conditions (Naiche et al., 2005).
In summary, we have established a previously unreported GFP reporter and Cre recombinase knock-in lines using the Tbx18 locus to genetically target embryonic DP precursor cells. GFP-mediated purification of dermal condensates from nascent first-wave hair follicles will provide a unique resource for studying the molecular and functional properties that sets these cells apart from regular fibroblasts. Constitutive and inducible Cre-mediated genetic recombination in DP precursor cells should provide a valuable tool to decipher specific gene functions and their critical role for interaction with placode stem cells during embryonic hair follicle formation.
MATERIALS AND METHODS
Animals
Tbx18LacZ and Tbx18Cre mice were described previously (Cai et al., 2008). Tbx18MerCreMer knock-in mice were generated with the same targeting strategy as described previously (Cai et al., 2008). Here, tamoxifen-inducible MerCreMer (Zhang et al., 1996) was targeted into the Tbx18 locus instead of Cre recombinase. R26RLacZ (129S-Gt(ROSA) 26Sortm1Sor/J; (Soriano, 1999) and R26ACTB-mT/mG (Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J; (Muzumdar et al., 2007) reporter mice were obtained from Jackson Laboratories (Bar Harbor, ME).
Real-Time PCR
Total RNAs from FACS sorted cells were purified using the Absolutely RNA Nanoprep kit (Stratagene, Santa Clara, CA), quantified with the NanoDrop spectrophotometer (Thermo Scientific, Asheville, NC) and reverse transcribed using oligo(dT) primers (Superscript III First-Strand Synthesis System, Invitrogen, Grand Island, NY). Real-time PCR was performed with a LightCycler 480 (Roche, Indianapolis, IN) instrument with Lightcycler DNA master SYBR Green I reagents. Differences between samples and controls were calculated based on the 2-DDCT method and normalized to Gapdh. Measurements were performed in duplicate or triplicate as indicated in legends. Primer sequences are listed in Supplementary Table 1.
Engraftment Experiments
Full thickness grafts from newborn P0 mice were performed as described (Nowak et al., 2008). Briefly, tissues from Tbx18LacZ (HET) and Tbx18GFP/LacZ (KO) pups of identical size were grafted on a single Nude recipient mouse. Bandages were removed 14 days after grafting and mice sacrificed 17 days after grafting. Graft areas were embedded in OCT. For histological examination, skins were cryosectioned and stained with Hematoxylin and Eosin.
Supplementary Material
Acknowledgments
We thank Valerie Horsley, Robert Krauss, Ihor Lemischka, Hoang Nguyen and Phil Soriano for general discussions and advice and for valuable comments on the manuscript. We also thank Chen Wei for technical assistance and the personnel of the Flow Cytometry Core Facility for excellent cell-sorting service. M.R. was supported by a Dermatology Foundation Research Career Development Award. This work was supported by a grant to M.R. from the NIH/NIAMS (1R01AR059143) and by grants to C.L.C. from the NIH/NHLBI (1R01HL095810 and 1K02HL094688), the American Heart Association (0855808D), and NYSTEM (C026426).
Abbreviations
- DP
dermal papilla
- MCM
MerCreMer
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–53. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol. 1999;1:158–64. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- Bussen M, Petry M, Schuster-Gossler K, Leitges M, Gossler A, Kispert A. The T-box transcription factor Tbx18 maintains the separation of anterior and posterior somite compartments. Genes Dev. 2004;18:1209–21. doi: 10.1101/gad.300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–8. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Jarrell A, Guo C, Lang R, Atit R. Dermal β-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development. 2012;139:1522–33. doi: 10.1242/dev.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi WY, Enshell-Seijffers D, Morgan BA. De novo production of dermal papilla cells during the anagen phase of the hair cycle. J Invest Dermatol. 2010;130:2664–6. doi: 10.1038/jid.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Valle Rodriguez A, Didiano D, Desplan C. Power tools for gene expression and clonal analysis in Drosophila. Nat Methods. 2012;9:47–55. doi: 10.1038/nmeth.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhouailly D. Dermo-epidermal interactions between birds and mammals: differentiation of cutaneous appendages. J Embryol Exp Morphol. 1973;30:587–603. [PubMed] [Google Scholar]
- Driskell RR, Clavel C, Rendl M, Watt FM. Hair follicle dermal papilla cells at a glance. J Cell Sci. 2011;124:1179–82. doi: 10.1242/jcs.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–23. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010;18:633–42. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin HF, Bussen M, Schmidt MK, Singh MK, Schuster-Gossler K, Kispert A. Transcriptional repression by the T-box proteins Tbx18 and Tbx15 depends on Groucho corepressors. J Biol Chem. 2007;282:25748–59. doi: 10.1074/jbc.M703724200. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Hartner A, Sekiguchi K, Reichardt LF, Watt FM. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577–89. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy MH. The secret life of the hair follicle. Trends Genet. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–18. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Headon DJ, Overbeek PA. Involvement of a novel Tnf receptor homologue in hair follicle induction. Nat Genetics. 1999;4:370–4. doi: 10.1038/11943. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–45. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Kraus F, Haenig B, Kispert A. Cloning and expression analysis of the mouse T-box gene tbx20. Mech Dev. 2001;100:87–91. doi: 10.1016/s0925-4773(00)00499-8. [DOI] [PubMed] [Google Scholar]
- Mikkola ML, Millar SE. The mammary bud as a skin appendage: unique and shared aspects of development. J Mammary Gland Biol Neoplasia. 2006;3–4:187–203. doi: 10.1007/s10911-006-9029-x. [DOI] [PubMed] [Google Scholar]
- Millar SE. Molecular mechanisms regulating hair follicle development. Journal of Investigative Dermatology. 2002;118:216–25. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Naiche LA, Harrelson Z, Kelly RG, Papaioannou VE. T-box genes in vertebrate development. Annu Rev Genet. 2005;39:219–39. doi: 10.1146/annurev.genet.39.073003.105925. [DOI] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus R, Muller-Rover S, Van Der Veen C, Maurer M, Eichmuller S, Ling G, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–32. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. Plos Biology. 2005;3:1910–24. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GD, Fantauzzo KA, Bazzi H, Maatta A, Jahoda CA. Dynamic expression of Syndecan-1 during hair follicle morphogenesis. Gene Expr Patterns. 2009;9:454–60. doi: 10.1016/j.gep.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Schlake T. Determination of hair structure and shape. Semin Cell Dev Biol. 2007;18:267–73. doi: 10.1016/j.semcdb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19:R132–42. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Sengel P. Morphogenesis of the skin. In: Abercrombie M, Neweth DR, Torrey JG, editors. Developmental and Cell Biology. Vol. 1. Cambridge: Cambridge University Press; 1976. p. 277. [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–68. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- Tobin DJ, Gunin A, Magerl M, Handijski B, Paus R. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme: implications for hair growth control. J Invest Dermatol. 2003;120:895–904. doi: 10.1046/j.1523-1747.2003.12237.x. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Clavel C, Kim S, Ang YS, Grisanti L, Lee DF, et al. Oct4 and klf4 reprogram dermal papilla cells into induced pluripotent stem cells. Stem Cells. 2010;28:221–8. doi: 10.1002/stem.281. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci U S A. 1999;96:8551–6. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Rosenberg M, Ross S, Tyner A, Fuchs E. Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc Natl Acad Sci U S A. 1989;86:1563–7. doi: 10.1073/pnas.86.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CC, Cotsarelis G. Review of hair follicle dermal cells. J Dermatol Sci. 2010;57:2–11. doi: 10.1016/j.jdermsci.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Riesterer C, Ayrall AM, Sablitzky F, Littlewood TD, Reth M. Inducible site-directed recombination in mouse embryonic stem cells. Nucleic Acids Res. 1996;24:543–8. doi: 10.1093/nar/24.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.