Abstract
Parental involvement and communication are essential for language development in young children. However, hearing parents of deaf children face challenges in providing language input to their children. This study utilized the largest national sample of deaf children receiving cochlear implants, with the aim of identifying effective facilitative language techniques. Ninety-three deaf children (≤ 2 years) were assessed at six implant centers prior to and for three years following implantation. All parent-child interactions were videotaped, transcribed and coded at each assessment. Analyses using bivariate latent difference score modeling indicated that higher versus lower-level strategies predicted growth in expressive language and word types predicted growth in receptive language over time. These effective, higher-level strategies could be used in early intervention programs.
Ninety percent of children with sensory neural hearing losses (SNHL) are born to hearing parents (Albertini, 2010). Thus, an immediate “mismatch” between the hearing status of the child and parent presents a significant barrier to effective communication (Meadow-Orlans & Spencer, 1996; Quittner, Leibach, & Marciel, 2004). Children with moderate to profound prelingual hearing loss experience significant auditory deprivation that places them at risk for difficulties with oral language and literacy skills. These delays in the early precursors of oral language lead to deficits in vocabulary development, morphology, syntax, academic achievement, and social development (Spencer & Marschark, 2010; Svirsky, Robbins, Kirk, Pisoni, & Miyamoto, 2000). Hearing parents of children with severe to profound hearing loss, who choose oral language as their primary mode of communication, have better opportunities than in previous years to develop their children’s oral language skills with the use of a cochlear implant (Geers, Nicholas, & Sedey, 2003; Niparko et al., 2010; Svirsky et al., 2000).
Despite these encouraging results, there is significant variability in these children’s language outcomes, after accounting for child age and length of implant use (Duchesne, Sutton, & Bergeron, 2009; Niparko et al., 2010). Family variables, such as socioeconomic status (SES) and parental linguistic input, may partially account for these individual differences in the outcomes of young deaf children. To date, few studies have evaluated the impact of parental linguistic input on deaf children’s language development. In the largest, most recent multi-site study of young deaf children, cochlear implantation was associated with significant improvements in comprehension and expression of spoken language over three years of implant use (Niparko et al., 2010). After controlling for SES and child hearing characteristics (e.g., length of auditory deprivation, residual hearing), maternal sensitivity (i.e., parental warmth, positive regard, respect for child’s autonomy) was also a significant predictor of growth in oral language for deaf children. The current study extended these results by measuring specific aspects of parental linguistic input during structured (Art Gallery) and unstructured (Free Play) videotaped interactions with their children. Both the quantitative (word types, mean length of utterance [MLU]) and qualitative (facilitative language techniques; FLTs) elements of parental communication were measured in parent-child interactions over three years post cochlear implantation. Identification of these quantitative and qualitative aspects of parental linguistic input will foster the development of interventions targeted to parents of deaf children using cochlear implants.
Cochlear Implants
Prior to the introduction of cochlear implants in the 1980s, hearing aids were the only means by which children with SNHL could access auditory information. Recently, however, cochlear implant surgery has become a common practice for deaf children, particularly because those with severe to profound hearing loss receive little benefit from hearing aids (see Parment, Lynm, & Glass, 2004, for a description of cochlear implants). Following cochlear implantation, children need to learn how to decode and interpret sounds by way of parental linguistic input and follow-up intervention services with speech-language pathologists and audiologists. In general, children with cochlear implants make significant gains in speech perception, speech recognition, and oral language skills following cochlear implantation (Baldassari et al., 2009; Spencer & Marschark, 2010; Svirsky et al., 2000).
Despite these generally positive findings, most studies report substantial variability in oral language outcomes, even after accounting for child age and length of implant use. For example, in a group of 70 children using cochlear implants, over 50% of the children demonstrated severe language delays even after more than two years of experience with their cochlear implant (Svirsky et al., 2000). Further, Niparko and colleagues (2010) showed that some children’s receptive and expressive language skills were not restored to age-appropriate levels even after three years of cochlear implant use.
Child Factors Contributing to Oral Language Development
There are several factors that may impact oral language outcomes for young deaf children who receive cochlear implants. Age at implantation, extent of residual hearing, and duration of auditory deprivation appear to be important for the development of oral language (see Spencer, Marschark, & Spencer, 2011, for review). Studies have demonstrated that children implanted earlier have better language outcomes than children implanted later in life, after controlling for IQ and parent education (Geers, Moog, Biedenstein, Brenner, Hayes, 2009). For example, in a study of 106 infants and toddlers, faster rates of receptive and expressive language development were reported for children implanted during the first rather than the second year of life (Dettman et al., 2007). Another study of 96 children with congenital bilateral profound SNHL implanted before four years of age reported similar results (Holt & Svirsky, 2008). Children implanted between 13 to 24 months of age performed better than children implanted after 24 months on measures of receptive and expressive language and word recognition tasks. However, only twenty-nine percent scored within normal limits on all measures, compared to 71% that scored within the delayed range on at least one measure. Most recently, Niparko and colleagues found that both better baseline hearing thresholds and a shorter history of hearing deprivation were associated with steeper increases in receptive and expressive language over three years of implantation (Niparko et al., 2010).
Parental Contributions to Children’s Oral Language Development
For both children with and without hearing, critical parent factors have been associated with language development. Family SES, including measures of parental education and income, have predicted vocabulary size, rate of growth of expressive language and auditory comprehension in typically developing children (Hoff, 2003; Pungello et al., 2009). Similarly, higher SES and maternal education have predicted better receptive and expressive language skills in deaf children receiving cochlear implants (Geers et al., 2009; Holt & Svirsky, 2008; Niparko et al., 2010; Powers, 2003).
Studies have also shown that quality of parental involvement (Calderon, 2000; Moeller, 2000) and parent-child interactions (Niparko et al., 2010; Pressman, Pipp-Siegel, Yoshinago-Itano, & Deas, 1999) have a major impact on growth of language development in children who are deaf. Results of observational studies have indicated that mothers in “mismatched dyads” tend to be more intrusive and directive in their interactions (Spencer, Erting, & Marschark, 2000), which may negatively affect language development, attention, and parent-child attachment. Thus, language delays observed in deaf children may be due, in part, to the difficulties parents have in making adaptations to their deaf child or scaffolding the environment to facilitate their children’s gains in knowledge and communicative competence (Quittner et al., 2010; Spencer et al., 2000). In contrast, research has also shown that mothers talk to their children who are deaf in appropriate ways given their language level. Research has shown that there are no differences in input between deaf and hearing children who are at the same language level (Spencer et al., 2000).
To date, few studies have measured the quality of parent-child interactions and their effects on language learning in deaf children. Pressman and colleagues found that greater maternal “emotional availability” (Biringen & Robinson, 1991) during videotaped free play sessions was a positive and unique predictor of gains in language for children using hearing aids, after accounting for maternal education, degree of hearing loss and mode of communication. In the only study to date that has measured maternal sensitivity in deaf children using cochlear implants, higher maternal sensitivity predicted better growth of comprehension and expression in a three year longitudinal, multivariable model (Niparko et al., 2010).
The way parents talk to their young children may also impact their children’s language development. Both quantitative linguistic input (e.g., number of different types of words, MLU) (Dickinson & Tabors, 2001; Fewell & Deutscher, 2004; Hart & Risley, 1999) and qualitative linguistic elements (e.g., FLTs) (Fey, Krulik, Loeb, & Proctor-Williams, 1999; Kaiser, Hancock & Hester, 1998) are associated with better receptive and expressive language outcomes in young, hearing children. For example, young children learn words more rapidly when they frequently hear different kinds of words (Huttenlocher, Haight, Bryk, Seltzer, & Lyons, 1991; Weizman & Snow, 2001). In addition, children develop better receptive and expressive language skills when parents comment on things they are already attending to (i.e., parallel talk), rather than when adults redirect their attention and label a different referent (Girolametto et al., 1999; Kaiser & Hancock, 2003; Yoder, McCathren, Warren, & Watson, 2001).
Parental Linguistic Input and Young Children who are Deaf
Early intervention programs highlight the important role parents’ play in facilitating language development in children with hearing loss (Sandall, Hemmeter, Smith, & McLean, 2005), and specifically, children who receive cochlear implants (Estabrooks, 2007). These programs are based on the social interactionist theory of language development, which postulates that young children learn language in the context of their daily experiences and particularly through interactions with their caregivers and family (Chapman, 2000; Hoff, 2000). Within this framework, however, the parent’s role is very intentional, with emphasis on providing their children with a variety of words in varied utterance formats appropriate for the child’s developmental and language level.
Variation in the language skills of young children with cochlear implants is strongly linked to both the quantity and quality of maternal linguistic input (DesJardin & Eisenberg, 2007; Spencer 2004). Specifically, hearing parents of deaf children increase the number of word-types and provide more complex language structures during interactions with their toddlers, as their children demonstrate increased language skills (Spencer, 2004). Similar results have been found for preschool and school-age children using cochlear implants (DesJardin & Eisenberg, 2007). Specifically, the total number of word-types and sentence complexity (MLU) were strongly associated with children’s language skills. In fact, mothers’ MLU accounted for most of the variance in children’s receptive and expressive language skills.
Qualitative elements of parental linguistic input also appear to be important for young children’s language skills who receive cochlear implants. To date, only two studies have investigated which FLTs are related to better oral language outcomes in deaf children with cochlear implants. Both studies were conducted by the same investigative group and used similar study procedures. The first study was conducted in a sample of 32 parent-child dyads with children ranging in age from two to seven (DesJardin & Eisenberg, 2007). Parents’ FLTs were coded during videotaped parent-child interactions (i.e., free play, two storybook activities) and language was measured using the Reynell Developmental Language Scales. Results suggested that the use of higher-level FLTs, such as recast, was positively associated with children’s receptive language abilities, while the use of open-ended questions was positively related to children’s expressive language skills. In contrast, lower-level techniques were negatively correlated with children’s language development.
The second study examined the relation between FLTs and children’s phonological awareness and reading skills at two points over three years (DesJardin, Ambrose, & Eisenberg, 2009). This study was an extension of the previous one, with a 50% overlap in the sample. Consistent with prior results, mothers’ higher-level FLTs were positively associated with their children’s phonological awareness and reading abilities, while lower-level FLTs were negatively correlated with these skills. Although these studies provided useful information on the effects of FLTs for children with cochlear implants, they had significant limitations, including small sample sizes, data collected at a single implant center, lack of assessment prior to implantation, and wide ranges of age and length of implant use.
The current study sought to identify the quantitative (word types and MLU) and qualitative strategies (FLTs) that are most effective in supporting language development in a large cohort of deaf children who received a cochlear implant before age two. More specifically, this study provided systematic data on how parents use and modify their FLTs over three years of cochlear implantation. This enabled us to test both unidirectional and bidirectional effects of parental language input on children’s language skills and the effects of children’s language on parents’ use of specific strategies using both latent growth curve modeling and bivariate latent difference score modeling. Strengths of our methodology included a large, national sample, use of both structured and unstructured tasks, and assessment of FLTs and language skills at baseline and each year following implantation for three years. Identifying the specific parental factors that enhance language skills is critical for tailoring early intervention programs for families and their young infants and toddlers who receive a cochlear implant. We expected that total number of word types would increase over time and be positively associated with improvements in language. It was hypothesized that higher-level FLTs would be more effective in fostering growth of language than lower-level FLTs, and parents were expected to use increasingly “higher-level” FLTs in their dyadic interactions. It was also hypothesized that “lower-level” FLTs would be used more frequently prior to cochlear implantation (prelinguistic stage of language development) and “higher-level” techniques would be used more frequently for deaf children post-implantation (postlinguistic one to two word stage of language development). A secondary objective of the study was to describe and compare the quantitative and qualitative strategies used by parents in both structured (Art Gallery) and unstructured tasks (Free Play).
Method
Participants
Participants were part of a larger study, the Childhood Development after Cochlear Implantation Study (CDaCI), a multi-center, national cohort investigation of the effectiveness of pediatric cochlear implants (Fink et al., 2007). This is the largest and youngest sample of cochlear implant candidates that has been studied longitudinally. Participants were recruited from six clinical implant centers (Los Angeles, CA; Baltimore, MD; Miami, FL; Ann Arbor, MI; Durham, NC; Dallas, TX) and two preschools (Baltimore, MD; Dallas, TX) that enrolled hearing children (Fink et al., 2007). The full CDaCI cohort consisted of 188 and 97 hearing children (for complete demographics of the CDaCI cohort see Fink et al., 2007); however, only children two years of age and younger at pre-implantation participated in the current study (N = 93)
Inclusion criteria for children in the CDaCI study were: 1) age under five years, 2) severe to profound sensorineural hearing loss, and 3) parents committed to educating the child in spoken English. Exclusion criteria included significant cognitive impairment (i.e., Bayley Mental or Motor score of less than 70 or Leiter International Performance Scale – Revised [Leiter-R] score of less than 66). Children with minor cognitive deficits were included to increase the generalizability of the findings to a broader population of deaf children receiving cochlear implants. Participants were assessed at baseline (two to four weeks prior to implantation) and every six months (from point of activation) for three years. Institutional review boards at all centers approved the study protocol.
The demographic characteristics for parents and their children appear in Table 1. At enrollment, parents were asked, “Indicate the ways your child communicates (check all that apply): Manual, Oral, Total Communication, Undecided. Although some parents reported using a combination of sign language and oral language, all parents chose to educate their child in spoken English following enrollment in this study. Upon review of video-taped parent child interactions, no families used American Sign Language to communicate, however, 33 families (36%) used sign supported speech with their children. For example, parents would sign “ball” while saying “You have a ball”. There were no incidences where parents used more than one sign per utterance. Thus, the parents did not use sign language in a complex grammatical format.
Table 1.
Demographics
| Characteristic | Deaf Children (N = 93) |
|---|---|
| Child Demographics | |
|
| |
| Age at enrollment months (SD) | 14.67 (5.76) |
| Age of onset of hearing loss (months) | 0.82 (2.59) |
| PTA4 (better ear) | 109.56 (15.42) |
| Age at diagnosis (months) | 5.14 (5.36) |
| Age at first hearing aid use (months) | 7.38 (5.63) |
| Age at CI surgery | 16.50 (4.78) |
| Age at activation | 17.27 (4.75) |
| Onset of hearing loss | |
| Sudden | 7% (6) |
| Progressive | 20% (19) |
| Congenital | 72% (67) |
| Cause of hearing loss | |
| Genetic | 32% (30) |
| Aminoglycosides | 1% (1) |
| Cytomegalovirus | 1% (1) |
| Hyperbilirubinemia | 3% (3) |
| Meningitis | 5% (5) |
| Prematurity | 1% (1) |
| Other | 1% (1) |
| Unknown | 55% (51) |
| Gender % (n) | |
| Male | 54% (50) |
| Female | 46% (43) |
| Race | |
| White | 81% (75) |
| African-American | 9% (8) |
| Asian | 3% (3) |
| Other | 8% (7) |
| Ethnicity | |
| Hispanic | 15% (14) |
| Non-Hispanic | 84% (78) |
|
| |
| Communication Mode | |
| Speech | 24% (22) |
| Sign Language | 19% (18) |
| Simultaneous/Speech Emphasis | 23% (21) |
| Simultaneous/Sign Emphasis | 2% (2) |
|
| |
| Parent Demographics | |
|
| |
| Parents’ education | |
| < High school | 2% (2) |
| High school grad | 17% (16) |
| Some college | 28% (26) |
| College | 53% (49) |
| Parents’ Income | |
| < $15,000 | 3% (3) |
| $15 – 29,999 | 13% (12) |
| $30 – 49,999 | 20% (19) |
| $50 – 74,999 | 19% (18) |
| $75 – 100,000 | 18% (17) |
| $100,000 + | 17% (16) |
Procedure
After an initial medical screening, a baseline assessment was scheduled two to four weeks prior to cochlear implant surgery, with a return visit four to six weeks later for implant activation. Assessments were conducted by a speech and language pathologist, typically over two half-day appointments to lessen fatigue for the child and family. During the first day, parents completed demographic and self-report measures of communication and behavior, and children were assessed with language measures, cognitive tests, and an audiological exam. On the second day, children participated in the videotaped free play, structured play, and problem-solving tasks with and without parents, and parents completed psychosocial questionnaires about their children.
Follow-up assessments were then conducted every six months. At each assessment point, the parent-child dyad completed the videotaped interaction tasks, along with a series of questionnaires. The yearly assessment points (Baseline, 12 months, 24 months, and 36 months post-implantation) and two videotaped tasks were analyzed for (i.e., Unstructured Free Play task, Structured Art Gallery task; Quittner et al., 2004). Only annual assessment points were included because the 6-month assessments were shorter and did not include all of the necessary measures. All measures, including those related to language, were conducted in spoken English. Parents received a $100 honorarium annually, travel stipends if required, and extended warranties for their cochlear implants as reimbursement for their time and effort. All written and videotaped materials were de-identified, replacing participant names with numbers to ensure confidentiality.
Measures: Language
Reynell Developmental Language Scales
(RDLS; Reynell & Greuber, 1990): The RDLS are commonly used, well-validated language scales for children one to seven years. They have been used with deaf and hearing children (DesJardin et al., 2009). This test also provided explicit instructions regarding adaptation of test administration for deaf children. The measure consists of a Verbal Comprehension and Expressive Language scale. Both scales have acceptable split-half reliability coefficients across age groups ranging from .74 to .93. Children’s scores can be compared to normative data to produce either standard scores or language age.
Measures: Videotaped Interactions
Free Play Task
Free play tasks are commonly used to assess a variety of developmental processes, including the quality of parent-child interactions (NICHD, 1999). Age-appropriate toys (e.g., cars, blocks, dolls) were presented to each parent-child dyad with instructions that they play “as they would at home” for 10 minutes. The first five minutes of the task were coded.
Art Gallery Task
In this more structured task, parents were asked to show the child a series of five art pictures mounted on the walls of the playroom at different heights. Parents were asked to talk about the pictures for a period of five minutes and determine which picture the child liked best and least. This task has been used in prior studies to assess parental sensitivity and communicative competence in children with atypical language development (Deckner, Adamson, & Bakerman, 2003).
Coding Videotaped Parent-Child Interactions
Transcription of Videotaped Language Samples
All parent and child speech, vocalizations, and sign language from the 93 videotaped dyads across four assessment points were transcribed using the Codes for the Human Analysis of Transcripts (CHAT). To ensure accurate transcription of parent and child utterances, all transcriptions (100%) were reviewed by two independent transcribers. Disagreements were then discussed among the two transcribers and a consensus transcription was developed. Reliability was calculated at the utterance level. Interrater reliability for the current study indicated good agreement, ranging from 85 to 95%.
Parental Facilitative Language Techniques (DesJardin, 2003)
Each parent’s transcribed utterance (linguistic phrase or sentence) was coded for one of 11 possible FLTs during both the Free Play and Art Gallery tasks. Some of the codes included imitation, linguistic mapping, closed and open-ended questions, and parallel talk (see Table 2 for a complete description of codes). To calculate interrater agreement, a random sample of 20% of the videotapes at every assessment point were coded by a second, independent rater. We found good interrater reliability, with Cohen’s kappa ranging from .79 to .88 (M = 0.84). Proportional scores for each FLT were calculated and used in the analyses so that less talkative parents were not penalized. Accordingly, proportional data were calculated by dividing the total number of uses of each language technique by the overall number of parental linguistic utterances, which yielded a percentage for each strategy.
Table 2.
Description and Examples of FLTs
| FLT | Description | Example |
|---|---|---|
| Lower-Level FLTs | ||
| Linguistic mapping | Putting into words or interpreting the child’s vocalization that is not recognizable as a word. | Child hands mother a toy cat and vocalizes—mother says, “kitty.” |
| Comments | Statement or phrase that signals that a message has been received or an utterance to keep conversation going. | Mother says, “yeah!” or “thank you.” |
| Imitation | Repeating verbatim the child’s preceding vocalization without adding any new words. | Child says, “cup” and mother says, “yes cup.” |
| Label | Stating the name for a toy, picture, or object. | Grandmother says, “There is a doggie.” |
| Directive | Tells or directs child to do something. | Parent says, “Look!” or “You play with this cup.” |
| Closed-ended question | Stating a question in which the child can only answer with a one-word response. | Father asks child, “Is that your favorite?” or “Do you like that picture? |
| Higher-Level FLTs | ||
| Parallel Talk | Parent talks aloud about what the child is directly doing, looking at, or referencing. | Child is looking directly at a the picture of a bee and parent says, “The bumble-bee is flying over the flowers.” |
| Open-ended question | Parent provides a phrase/question in which the child can answer using more than one word. | While looking at a picture, parent says, “What is happening in this the picture?” |
| Expansion | Parent repeats child’s verbalization providing a more grammatical and complete language model without modifying the child’s word order or intended meaning. | Child says, “baby cry” and the caregiver says, “The baby is crying.” |
| Expatiation | Same as Expansion, but parent adds new information to the child’s utterance. | While looking at the picture – child says, “swim water” and mother says, “Yes, we are going swimming at the beach. This summer we are going to the beach.” |
| Recast | Caregiver restates the child’s verbalization into a question format. | Child says, “puppy gone” and the caregiver says, “Is the puppy gone? |
Results
Statistical Procedure
A series of bivariate latent difference score (LDS) models were used to evaluate the relation between FLTs and language development (expressive and receptive) across three years post-implantation (see Ferrer & McArdle, 2010, for overview). In addition, bivariate LDS models were used to examine whether one variable predicted change in the other (McArdle & Hamagami, 2001). Prior to fitting these bivariate models, univariate latent growth curve models for each variable were completed to model the change process. Next, bivariate latent growth curve models were modeled to determine whether FLTs and language were correlated (indicating that the change processes were related). Following these models, bivariate LDS models examined whether FLTs led to later change in expressive and receptive language scores, as measured by the Reynell. Because the change process could go in either direction (change in FLTs could predict subsequent change in language, or vice versa), parameters for each direction were estimated simultaneously (i.e., with FLTs as the predictor and then with language as the predictor). The variable used to assess growth in language was “time since implantation” since this is the best reflection of the effects of the cochlear implant on language learning.
Full information maximum likelihood estimation with Mplus software (Muthen & Muthen, 2008) was used for all analyses. This procedure estimates the model parameters using all available information rather than deleting cases with incomplete data (Enders, 2001). Thus, families who did not complete all assessments were still utilized in these analyses. In the current study, 12 families (13%) missed at least one assessment point and 96% of participants completed the 36-month assessment point. Latent difference score models allow for many different types of change to be modeled. In the bivariate models, we utilized information from the univariate models (i.e., the change pattern) so that we could focus on the cross variable change pattern. Thus, the univariate change pattern was constrained to what we discovered in earlier models so that reliable parameters for each predictor could be estimated.
For descriptive purposes, goodness of fit of the models were recorded. Several goodness of fit indices were used, which can be broken down into absolute fit (how well the model reproduces the data) and predictive fit (goodness of fit in the hypothetical replication samples). Assessment of absolute model fit was based on the log-likelihood ratio chi-square. Moreover, predictive fit statistics, including the Akaike information criteria (AIC), which is a parsimony adjusted index (i.e., favors simpler models; lower values of the AIC indicate better fit), the Bayes Information criteria (BIC), which also penalizes model complexity (lower values of the BIC indicate better fit), and the root mean square error of approximation (RMSEA) were also utilized. There is no standard cutoff for an acceptable fit on the AIC and BIC, but these numbers are included to facilitate comparison of fit indices across models with higher numbers indicating better fit. For the RMSEA, a lower number indicates better fit and Browne and Cudeck (1993) indicating that .05 or less being optimal.
Descriptive Statistics
Tables 3 and 4 present descriptive statistics for the FLTs, Reynell language scores, total parent utterances, total word types, and mean length of utterance (MLU). Means and standard deviations for each variable are presented for each assessment point: baseline (pre-implantation), 12 months, 24 months, and 36 months post-implantation. For FLTs, descriptive analyses are presented for each individual strategy (e.g., imitation, close-ended question), as well as for the composite scores used in the subsequent analyses (lower-level FLTs, higher-level FLTs). The most frequently used lower-level FLTs across time were directives, comments, and close–ended questions. The most frequently used higher-level FLTs were parallel talk, open-ended questions, and recast. Overall, lower-level FLTs were used more frequently than higher-level FLTs during parent-child interactions. For all subsequent analyses, composite scores for lower-level and higher-level FLTs were used.
Table 3.
Means and Standard Deviations for FLTs & Language Measures
| Baseline | 12 Month | 24 Month | 36 Month | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| M | SD | M | SD | M | SD | M | SD | |
| Lower Level FLTs (%) | 74.60 | 9.54 | 61.85 | 9.83 | 56.12 | 10.94 | 50.47 | 10.70 |
| Linguistic Mapping (%) | 0.40 | 0.92 | 1.07 | 1.35 | 1.17 | 1.40 | 0.72 | 1.23 |
| Comments (%) | 23.53 | 7.62 | 15.91 | 5.63 | 12.61 | 6.01 | 11.88 | 6.35 |
| Imitation (%) | 0.33 | 0.58 | 2.53 | 2.27 | 2.76 | 2.30 | 2.97 | 2.20 |
| Label (%) | 6.90 | 4.79 | 4.80 | 3.77 | 2.54 | 2.71 | 2.20 | 2.56 |
| Directive (%) | 26.81 | 12.70 | 21.97 | 9.46 | 19.13 | 9.77 | 15.34 | 9.38 |
| Closed-Ended Question (%) | 16.63 | 8.02 | 15.57 | 5.95 | 17.91 | 6.47 | 17.92 | 6.63 |
| Higher Level FLTs (%) | 24.68 | 9.71 | 37.87 | 9.86 | 43.65 | 11.09 | 49.31 | 10.66 |
| Open-Ended Question (%) | 6.37 | 4.09 | 10.70 | 6.02 | 12.71 | 6.65 | 15.83 | 6.46 |
| Expansion (%) | 0.02 | 0.11 | 0.76 | 1.19 | 1.60 | 1.44 | 1.98 | 1.55 |
| Expatiation (%) | 0.01 | 0.07 | 0.53 | 0.81 | 0.72 | 0.73 | 1.03 | 1.05 |
| Recast (%) | 0.00 | 0.04 | 0.94 | 1.38 | 3.87 | 3.05 | 5.54 | 4.00 |
| Parallel Talk (%) | 18.27 | 9.43 | 24.92 | 9.03 | 24.75 | 9.32 | 24.50 | 9.32 |
| Parent Utterances | 105.99 | 42.98 | 118.50 | 32.48 | 113.12 | 33.89 | 108.10 | 30.00 |
| Number of Different Word Types | 88.60 | 30.85 | 106.54 | 30.10 | 119.60 | 32.83 | 128.55 | 33.50 |
| Mean Length of Utterance | 2.94 | 1.22 | 3.07 | 0.69 | 3.28 | 0.80 | 3.55 | 0.83 |
Table 4.
Estimates of Oral Language Performance for Children Undergoing Cochlear Implantation
| Baselinea,b | 12 months | 24 months | 36 months | |
|---|---|---|---|---|
|
| ||||
| M (SD) | M (SD) | M (SD) | M (SD) | |
| Chronological Age (in months) | 14.67 (5.76) | 29.66 (4.78) | 41.65 (4.86) | 53.75 (6.36) |
| Receptive Language | ||||
| Raw Score | 1.15 (2.10) | 15.01(10.33) | 28.97(14.81) | 40.27(15.80) |
| Language Age | 13.00 (0.62) | 18.42 (5.37) | 26.86 (9.64) | 34.98 (12.85) |
| Months Delayed | 2.33 (5.59) | 11.09 (7.24) | 14.61 (11.10) | 18.81 (14.38) |
| Expressive Language | ||||
| Raw Score | 4.21(3.57) | 16.34(7.22) | 27.22(10.54) | 35.79(12.27) |
| Language Age | 16.07 (0.88) | 18.80 (3.26) | 25.81 (8.06) | 33.42 (11.72) |
| Months Delayed | −0.74 (5.70) | 10.83 (5.72) | 15.74 (9.68) | 20.38 (13.53) |
The RDLS is not validated for ages younger than 12 months; hence, spoken language development is numerically not ascertainable through RDLS for the youngest group at baseline.
The floor of RDLS language age measure is coded as “<13 months” for comprehension and “<16 months” for expression. Language age measures below the RDLS floor are numerically not ascertainable through RDLS.
Task Differences
Data was obtained by coding two five-minute video-taped parent-child interactions: Free Play and Art Gallery. Prior to using the composite FLTs in the final analyses, task differences were examined using a series of 2 × 4 repeated measures analysis of variance (ANOVA). The between-subjects factor was task, with two levels (Free Play and Art Gallery). Time was the within-subjects variable, with four levels (pre-implantation, 12, 24, and 36 post-implantation). Descriptive statistics by task are presented in Table 5.
Table 5.
| Baseline | 12 Month | 24 Month | 36 Month | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Free Play | Art Gallery | Free Play M | Art Gallery M | Free Play M | Art Gallery M | Free Play | Art Gallery M | |
| M (SD) | M (SD) | (SD) | (SD) | (SD) | (SD) | M (SD) | (SD) | |
| Lower-Level FLTs (%) | 74.75 (13.41) | 75.26 (11.18) | 60.86 (13.66) | 62.73 (12.91) | 56.40 (13.77) | 56.08 (13.68) | 48.07 (13.88) | 53.18 (14.80) |
| Higher-Level FLTs (%) | 24.92 (12.32) | 23.74 (11.24) | 38.94 (13.62) | 36.94 (13.14) | 43.38 (14.02) | 43.68 (13.73) | 51.85 (14.46) | 46.52 (14.60) |
| Parent Utterances | 93.41 (44.62) | 118.58 (37.48) | 107.06 (34.61) | 129.95 (25.67) | 102.52 (33.71) | 123.60 (30.83) | 97.69 (29.53) | 118.50 (26.80) |
| Different Word Types | 81.22 (31.16) | 96.75 (34.82) | 106.68 (33.64) | 107.55 (30.60) | 115.97 (35.47) | 123.22 (35.82) | 123.47 (33.43) | 134.88 (36.10) |
| Mean Length of Utterance | 2.64 (0.66) | 3.23 (1.54) | 2.96 (0.61) | 3.19 (0.75) | 3.21 (0.73) | 3.35 (0.87) | 3.49 (0.73) | 3.62 (0.91) |
FLTs & Parent Utterances by Task
The first ANOVA examined task differences for total utterances (total number of words spoken by the parent). Results indicated a significant main effect for task, F(1,128) = 34.43, p < .001, with more utterances in Art Gallery compared to Free Play. There was also a significant quadratic effect for time, F(1,158) = 28.98, p < .001. Utterances increased from pre-implantation to 12 months post-implantation, but decreased at 24 and 36 months post-implantation. A second ANOVA examined task differences for MLU. There was a main effect for task, F(1,156) = 9.22, p < .05, with longer MLUs in Art Gallery compared to Free Play. In addition, there was a significant linear time effect, F(1,156) = 97.63, p < .001, with MLUs significantly increasing over three years implantation. The third ANOVA examined task differences in parents’ use of total different word types. Although there was no significant task difference, there was a significant, quadratic interaction between time and task F(1, 163) = 10.04, p < .01. At baseline, parents used more word types in the Art Gallery than Free Play task, however at 12 months post-implantation, parents used a similar number of word types during both tasks. At 24 and 36 months post-implantation, parents resumed using more word types in Art Gallery versus Free Play. The fourth and fifth ANOVAs examined task differences in FLTs. No differences were found between the lower-level or higher-level FLTs in Art Gallery compared to Free Play, F(1,159) = 2.27, p > .05; F(1,159) = 2.55, p > .05, respectively. However, results indicated that there was a significant time effect, with lower-level FLTs decreasing, F(1,159) = 412.35, p < .001, and higher level FLTs increasing, F(1,159) = 422.56, p < .001, over three years post-implantation. No interaction effects between task and time were found; as a result, composite scores were used for all subsequent analyses.
In summary, results were consistent with our expectation that structured tasks, such as Art Gallery, would be better facilitators of oral communication between parents and children. In general, parents used more words, different word types, and longer MLUs during Art Gallery than during Free Play. However, no differences in FLTs were found by task. This suggested that although Art Gallery produces more communication between parents and children, the quality of their communication is similar in an unstructured versus structured task.
Latent Growth Curve Modeling
The first set of models were used to determine whether FLTs (lower and higher-level FLTs), parents use of different word types, and children’s oral language (expressive and receptive) changed significantly over three years post-implantation. Loadings on the factors were constrained so that each child’s trajectory would form a straight line. All variables, including lower- and higher-level FLTs, total word types, and expressive and receptive language scores demonstrated significant change. On average, parents used 91 different word types at baseline and these increased by approximately 13 words each year, p < .001. Further, estimates of lower-level FLTs at baseline occurred on average for 72.58% of the total 10-minute interaction and decreased by 7.86% over time, p < .001. Estimates of higher-level FLTs averaged 26.80% over 10 minutes and increased by 8.02% each year, p < .001. Estimates of expressive language raw scores were, on average, 5.20 and increased by 10.38 points over time, p < .001. Estimates of receptive language raw scores were on average 2.02 and increased by 12.76 points over time, p < .001.
The next set of models investigated whether changes in FLTs or parents total number of different word types and language scores were related to each other. Univariate latent growth curve models were run simultaneously and the correlation of one variable’s latent change with the other variable’s latent change was estimated. As expected, changes in total number of word types were positively related to changes in oral language. Similarly, changes in FLTs and measured language were related to one another. Specifically, lower-level FLTs were negatively related to improvements in expressive and receptive language. In contrast, higher-level FLTs were positively related to improvements in expressive and receptive language. Complete set of analyses is available upon request.
Dynamic Bivariate Latent Difference Score Modeling
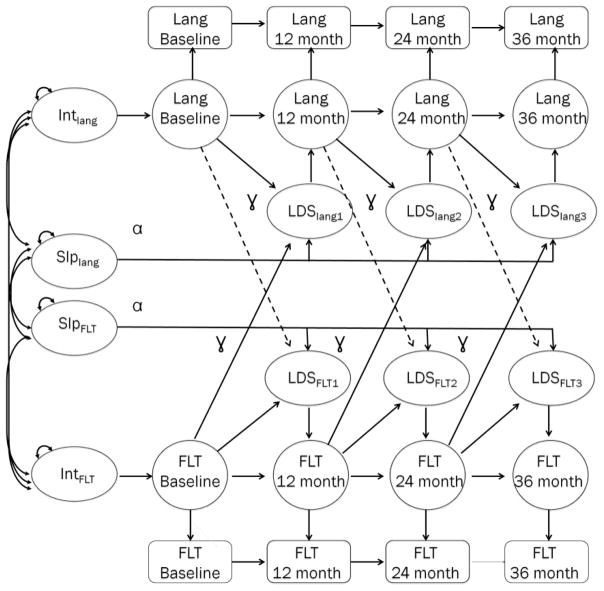
Bivariate LDS modeling provides a flexible framework for testing one variable as a predictor of change in another. Thus, it evaluates the predictive associations in a multivariate change process. It models the relation in the opposite direction, to evaluate whether change is uni- or bi-directional (Ferrer & McArdle, 2010). In this model (see Figure 1), many parameters were constrained; specifically, all of the unlabeled arrows to one were constrained. Only one alpha (α; to model straight-line growth) and gamma (γ; to model change process over time) were estimated for all time points (thus, there is an assumption that these processes do not change over time; see McArdle & Hamagami, 2001, for a detailed explanation of these procedures). The goal was to test specific hypotheses about which change process (FLTs, total word types, or oral language skills) was a leading indicator of the other. Thus, growth was constrained to be linear and the number of parameters estimated was limited in each model, allowing it to converge fairly easily.
Figure 1.
This figure shows the final bivariate latent difference score model used to test the change in FLTs and language for each assessment point over three years. SES was included as a covariate on initial scores and changes in those scores over time. Arrows in the model indicate that we tested the change process in both directions. It examined whether one variable predicted change in the other (e.g., Baseline FLTs predicting change in language at 12 month post-implantation. BL = baseline assessment (prior to cochlear implantation); Months = months post-implantation; LDS = latent difference score (change in variable); α = estimate to model straight-line growth; γ = estimate to model change process across time. These models were also tested for the effect of word types.
Model 1: Total Number of Different Words and Expressive Language
This model examined the association between the total number of different word types used by parents and growth of expressive language (fit indices: Log-likelihood = −2704.28, df = 23, AIC = 5466.57, BIC = 5539.70, RMSEA = .03). SES was a significant predictor of both initial level and change in expressive language over time, p < .01. Similarly, SES significantly affected initial level and change in the total number of different word types used by parents, p < .01. Controlling for SES, the number of total different word types did not significantly predict changes in expressive language over time (12 months t = −1.20, p > .05, 24 months t = −1.02, p > .05, 36 months t = −0.57, p > .05). In contrast, expressive language at baseline predicted changes in number of word types used at 12 months post-implantation (t = −2.75, p < .01). However, this association was no longer present at 24 and 36 months post-implantation, p > .05.
Model 2. Total Number of Different Words and Receptive Language
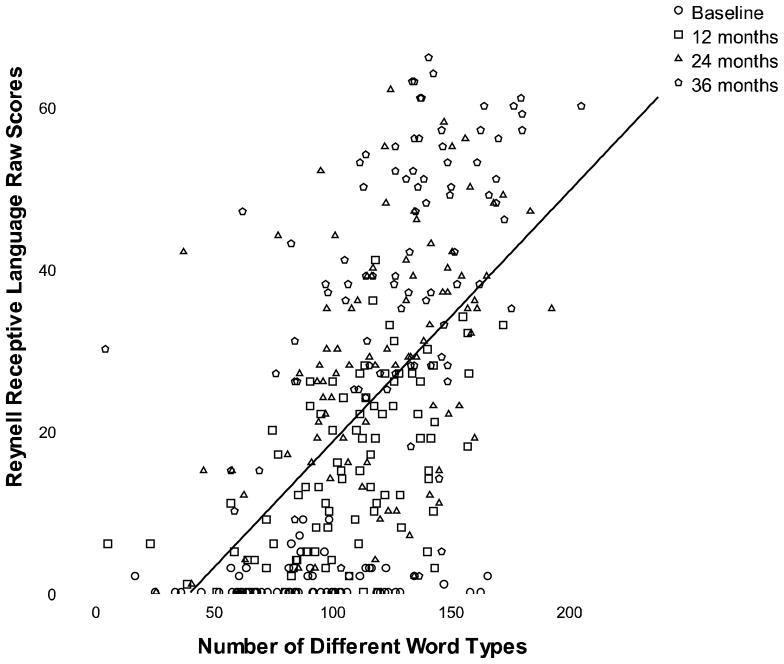
This model examined the association between the total number of different word types used by parents and growth of receptive language (fit indices: Log-likelihood = −2748.03, df = 23, AIC = 5554.06, BIC = 5627.19, RMSEA = .12). In this model, SES was only a significant predictor of initial level of receptive language and total number of word types; SES was not a significant predictor of change in language or number of word types over three years post-implantation. In contrast to Model 1, total number of word types significantly predicted growth in receptive language skills over time (12 months t = 2.74, p < .01, 24 months t = 2.83, p > .01, 36 months t = 3.00, p > .01; see Figure 2). Receptive language, however, did not predict changes in the use of different words over time, p > .05.
Figure 2.
This figure shows the relation between number of different word types used by parents and improvements in receptive language raw scores over three years of cochlear implantation.
Model 3: Lower-Level Language Techniques and Expressive Language
Model 3 attempted to determine whether lower-level FLTs predicted later change in expressive language scores (fit indices: Log-likelihood = −2385.24, df = 23, AIC = 4828.47, BIC = 4901.60, RMSEA = 0.10). SES significantly affected initial expressive language scores (t = −3.04, p < .01) and change in expressive language over time (t = 2.81, p < .01). SES did not significantly influence initial level or change in lower-level FLTs, p > .05. Controlling for SES, lower-level FLTs did not significantly predict improvements in expressive language over three years of implantation. Specifically, they did not predict change in expressive language at 12 months (t = 1.88, p > .05), 24 months (t = 1.88, p > .05), or 36 months post-implantation (t = 1.98, p > .05). Similarly, expressive language scores did not predict change in lower-level FLTs at 12 months (t = 1.17, p > .05), 24 months (t = 1.00, p > .05), or 36 months post-implantation (t = 0.89, p > .05).
Model 4: Lower-Level Language Techniques and Receptive Language
Similar to Model 3, Model 4 attempted to determine whether lower-level FLTs predicted change in receptive language (fit indices: Log-likelihood = −2416.50, df = 23, AIC = 4891.00, BIC = 4964.13, RMSEA = .10). SES predicted initial receptive language scores (p < .001); however, SES did not predict initial level of lower-level techniques or changes in receptive language or strategies over time, p < .05. As expected, lower-level FLTs did not predict improvements in receptive language over three years of cochlear implantation (12 months, t = 1.03, p < .05, 24 months, t = 1.03, p < .05, 36 months, t = 1.04, p < .05). Similar to Model 3, receptive language scores did not predict change in lower-level FLTs at 12 months (t = 0.50, p >.05), 24 months (t = −0.58, p > .05), or 36 months post-implantation (t = −1.50, p > .05).
Model 5: Higher-Level Language Techniques and Expressive Language
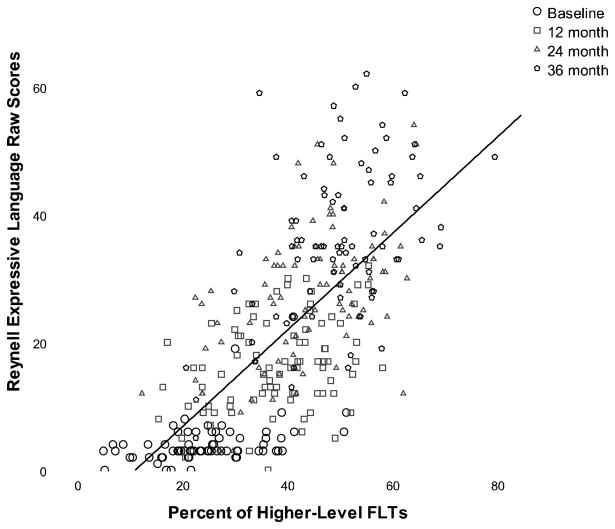
Model 5 attempted to determine whether higher-level FLTs predicted change in expressive language scores (fit indices: Log-likelihood = −2371.81, df = 23, AIC = 4801.62, BIC = 4874.75, RMSEA = .00). SES significantly affected initial level of expressive language (t = −4.68, p < .001), therefore, SES was included as a covariate in the model. SES did not significantly affect initial levels of higher-level FLTs or changes in expressive language or strategies over time. As hypothesized, higher-level FLTs significantly predicted improvements in expressive language over time (see Figure 3). Increases in higher-level FLTs predicted improvements in expressive language at 12 months (t = 3.00, p < .01), 24 months (t = 2.86, p < .01), and 36 months post-implantation (t = 2.79, p < .01). Contrary to prior models, expressive language at baseline did predict an increase in the use of higher-level FLTs at 12 months post-implantation (t = 2.51, p < .01). However, this association was no longer significant at 24 months and 36 months post-implantation.
Figure 3.
This figure shows the relation between higher-level facilitative language techniques (FLTs) and improvements in expressive language raw scores over three years of cochlear implantation.
Model 6: Higher-Level Language Techniques and Receptive Language
Model 6 attempted to determine whether higher-level FLTs predicted change in receptive language (fit indices: Log-likelihood = −2410.81, df = 25, AIC = 4875.62, BIC = 4943.71, RMSEA = .06). Similar to Model 5, SES significantly influenced initial levels of receptive language (t = −4.43, p < .001), but did not predict initial level of higher-level FLTs or change in language or strategies over three years. Controlling for SES, the relation between higher-level FLTs and change in receptive language trended toward significance at 12 months (t = 1.74, p < .08), 24 months (t = 1.82, p < .08), and 36 months post-implantation (t = 1.85, p < .08). Similar to previous models, receptive language scores did not predict change in higher-level FLTs over three years of post-implantation, p > .05.
In summary, total number of different word types significantly affected growth in receptive language, while higher-level FLTs significantly impacted growth in expressive language skills over time. Number of word types did not affect expressive language and lower-level FLTs did not significantly affect change in oral language skills over time. In terms of model fit, Models 1, 5, and 6 had the best model fit according to criteria established by Browne and Cudeck (1993). These models had an RMSEA of .05 or less, indicating optimal levels of model fit. Note that the other models were able to converge but did not yield optimal levels of fit.
Discussion
Although cochlear implantation in young, deaf children has been associated with significant improvements in oral language, there is a great deal of variability in these children’s outcomes (Holt & Svirsky, 2008; Niparko et al., 2010; Spencer et al., 2011). This study examined whether the quantity and quality of parental input affects growth of oral language drawing upon the largest, youngest and national sample of deaf children receiving cochlear implants. The major aim was to evaluate the effects of parental FLTs and word types on young deaf children’s oral language development over three years post-implantation. Secondary aims included identifying the most frequent FLTs used by parents and comparing the types of FLTs used in a structured (Art Gallery) vs. unstructured task (Free Play). We also investigated how parents’ use of total words, different word types, and MLUs in two different tasks changed over time.
Based on prior research on hearing children with language delays (Fey et al., 1999; Kaiser & Hancock, 2003; McCauley & Fey, 2006) and deaf preschool children with cochlear implants (DesJardin et al., 2009; DesJardin & Eisenberg, 2007), it was hypothesized that higher-level FLTs would be more effective in fostering the growth of language skills than lower-level FLTs in this sample. Moderate support was found for this hypothesis. Higher-level FLTs, such as recasts and open-ended questions, significantly predicted growth in expressive language but had no significant effect on the growth of receptive language. Similar positive results were found for parental use of dialogic reading, which is based on higher-level FLTs and produced higher vocabulary development in deaf children (Fung, Chow, McBride-Chang, 2005). Note that we enrolled children who varied in their cause of deafness. Although the majority of children had sensorineural hearing losses (92%), a small number had auditory neuropathy, which may introduce more variability in the oral language development of children post-implantation. Thus, our results are a conservative test of the effects of these language techniques. Further, our results showed that, regardless of child or family characteristics (e.g., length of implant use, SES), higher-level FLTs facilitated improvements in expressive language. Interestingly, although SES was related to initial levels of expressive and receptive language, it was not related to parental use of FLTs.
In contrast, lower-level FLTs had no effect on either expressive or receptive language scores. Although lower-level FLTs, such as linguistic mapping, imitation, and labeling may enhance language learning in young hearing children at the prelinguistic stage of development (Girolametto et al., 1999; McCauley & Fey, 2006; Weitzman, Wiigs, & Pearce, 1999), they did not appear to affect language learning in this sample of infants and toddlers with cochlear implants. In contrast to the literature with preschool deaf children with cochlear implants, which showed a negative association between lower-level FLTs and language (DesJardin e al., 2009; DesJardin & Eisenberg, 2007), no effects were found for lower-level FLTs.
The positive effects of higher-level FLTs may be partly explained by the parents’ level of involvement and differing demands for the child’s attention. For example, parents who are asking open-versus closed—ended questions are likely to provide more complex language, along with a demand for the child to attend and respond with two or more words. In contrast, closed-ended questions tend to be simpler and can be answered with a one-word or non-verbal response (nodding “yes”). Thus, higher-level FLTs are providing an enriched language environment. Further, prior studies have documented deficits in attention and behavioral control in young, deaf children with and without cochlear implants, suggesting that communication which requires some level of “joint attention” will produce better responses from children (Barker et al., 2009; Quittner et al., 2007; Smith, Quittner, Osberger, & Miyamoto, 1998). Thus, higher-level FLTs are more facilitative of language development in this population than lower level FLTs.
Our hypothesis that number of word types would positively affect the growth of language received partial support. Total number of different word types predicted greater improvements in receptive language; however, word types did not predict growth in expressive language. In contrast to the results for FLTs, SES was significantly related to number of word types at baseline and to change in number of word types predicting expressive language, but not word types related to receptive language. These results are similar to the hearing literature showing that quantitative linguistic input is associated with better receptive and expressive language outcomes (Dickinson & Tabors, 2001; Fewell & Deutscher, 2004; Hart & Risley, 1999). This positive association of word types with both expressive and receptive language has also been identified in preschool deaf children using cochlear implants (DesJardin et al., 2009; DesJardin & Eisenberg, 2007). One explanation for our lack of findings in relation to expressive language is the young age of the children in our sample, who were predominantly in the prelinguistic stage of development (mean age of 15 months). At this stage, research suggests that children’s receptive language skills are advancing more quickly than their expressive language abilities (Puckett, Black, Wittmer, & Peterson, 2009), and in our case, parental use of word types may have only affected their development of receptive skills. Note that in general, our results showed there were greater gaps in children’s receptive than expressive language skills and receptive language was also the skills that evidenced the greatest gains across time (see Table 4).
A secondary aim was to assess the FLTs used by parents of young deaf children at baseline (prior to implantation) and for three years post-implantation. It was hypothesized that lower-level techniques would be used more frequently with deaf children prior to cochlear implantation and higher-level techniques would be used more frequently post-implantation. Findings showed that parents used a combination of lower and higher-level FLTs during both videotaped parent-child interactions. Directives, comments, and close-ended questions were the most frequently used lower-level strategies and parallel talk, open-ended questions, and recast were the most frequently used higher-level strategies.
These results also strongly supported the hypothesis that, over time, parents would increase their use of higher-level FLTs in their dyadic interactions. Over three years, higher-level FLTs increased significantly from 25% to 50%, while lower-level FLTs decreased significantly from 75% to 50%. To date, only two studies have investigated FLTs in deaf children with cochlear implants and only one study used a longitudinal design. The longitudinal study, which assessed phonological awareness and reading skills, showed that higher-level FLTs positively contributed to children’s literacy abilities (DesJardin et al., 2009). However, these studies used small, convenience samples from a single implant center and neither study reported descriptive information about the distribution of parents’ use of these strategies in a dyadic context.
As expected, the structured Art Gallery task was more effective in fostering communication than the unstructured Free Play task, facilitating more and longer parental utterances and different word types. However, no differences were found in the use of FLTs. These findings are similar to those reported by Deckner and colleagues (2003), who used a modified version of the Art Gallery task in typically developing hearing children. The use of pictorial materials, such as Art Gallery, may be optimal for assessing the quality of parental language input and teaching parents how to facilitate their child’s communication skills (DesJardin & Eisenberg, 2007; Fung et al., 2005). These results have important clinical implications because they suggest that parental linguistic input has important effects on the growth of language skills in young deaf children with cochlear implants. Use of higher-level FLTs could potentially be taught to parents soon after the diagnosis and once a decision is made to implant a cochlear device.
Further, although this study could not determine cause and effect, analyses were conducted to test both unidirectional and bidirectional effects. Specifically, we examined whether parents’ use of higher-level FLTs led to increases in oral language and simultaneously, whether children’s expressive or receptive language skills led to increases in parents’ use of higher-level FLTs. As previously described, parents use of higher-level FLTs predicted improvements in expressive language over three years post-implantation. Similarly, at baseline, level of expressive language, predicted changes in parents’ use of different word types and higher-level FLTs at 12 months. However, this was not found at subsequent assessment points or for receptive language. This suggests that there is a bidirectional association between higher-level FLTs and expressive language within the first year of cochlear implantation. Parents of children with more oral language prior to implantation may have been reinforced in dyadic interactions with their child to use higher-level strategies post-implantation. In contrast, for receptive language there is a unidirectional effect between number of different word types used by parents and growth in receptive language.
Limitations
First, although there was strong evidence that parents’ use of higher-level FLTs increased children’s language skills over time, it is difficult to conclude that this association was causal without randomizing parents to different levels of parental language input. A future study is planned to test the effects of these language strategies in a randomized, controlled trial.
Second, the sample was limited to deaf children ages 2 and younger receiving cochlear implants. Thus, we could not determine whether these FLTs would be effective in deaf children implanted after the age of 2. However, DesJardin and Eisenberg (2007) included older, implanted children and found positive effects for parents’ FLTs.
Third, we relied on parental reports of their children’s educational and rehabilitation strategies and thus, have limited data on the types of school or rehabilitation programs these children attended. Due to the young age of the sample, few of these children were enrolled in any formal preschool program. Further, these six implant centers drew from a large geographical area, with children attending a variety of different, local (private and public) programs. A requirement of the study, however, was a commitment by parents to educate their children in oral language. Thus, parents and children did not use any formal sign language.
Finally, in the first year of the study, many children had little to no formal oral language and thus, had limited variability in their language scores. A different language measure, designed for younger children might have been more sensitive to changes in language early in the study.
Clinical Implications and Future Directions
These results have several implications for early intervention for families and their young deaf children who receive cochlear implants. Results suggest that parents play an important role in facilitating their child’s oral language development post-implantation by using higher-level strategies and more word types. Thus, high quality parent-child interactions, combining higher-levels FLTs and a variety of word choices appear to be optimal for children’s oral language development in the first three years post-implantation.
Similar to the classic study by Hart and Risely (1999), SES was associated with parents’ use of different word types and children’s initial levels of expressive and receptive language. Note, however, that SES did not predict initial use of higher or lower FLTs or change in the use of these strategies over time. Thus, parents with more limited education and family income appeared to be equally capable of using higher-level FLTs when compared to those from a higher SES group. Thus, training parents in the use of these strategies is likely to be effective across socioeconomic strata. The categorization and identification of effective parental language strategies could serve as a guide for early interventionists, preschool teachers, and professionals who work with children and their parents following cochlear implantation.
Given that newborn screening for childhood deafness has been implemented nationally, children with severe to profound hearing loss will be identified earlier and thus, may receive a cochlear implant in the first year of life. Development of an evidence-based, family-focused intervention to promote oral language development could potentially be incorporated into existing early intervention programs for young deaf children and their families. A “coaching model,” in which parents receive hands-on training and practice in using FLTs is one way to empower parents in supporting their children’s communication skills (DesJardin, 2009). In sum, family-focused early intervention programs could serve as the vehicle to disseminate this intervention more broadly, ultimately increasing communicative competence in young deaf children who receive cochlear implants.
Acknowledgments
The Childhood Development after Cochlear Implantation (CDaCI) Study was supported by grant RO1 DC004797 from the National Institute on Deafness and Other Communication Disorders, the CityBridge Foundation, and the Sidgmore Family Foundation. Warranties on the implant devices used by children with implants in this study were discounted by 50% by the Advanced Bionics Corporation, Cochlear Corporation, and the MEDEL Corporation.
CDaCI Investigative Team
House Research Institute, Los Angeles: Laurie S. Eisenberg, PhD, CCC-A (PI); Karen Johnson, PhD, CCCA (coordinator); William Luxford, MD (surgeon); Leslie Visser-Dumont, MA, CCC-A (data collection); Amy Martinez, MA, CCC-A (data collection); Dianne Hammes Ganguly, MA (data collection); Jennifer Still, MHS (data collection); Carren J. Stika, PhD (data collection).
Johns Hopkins University, Listening Center, Baltimore: John K. Niparko, MD (PI); Steve Bowditch, MS, CCC-A (data collection); Jill Chinnici, MA, CCC-A (data collection); James Clark, MD (data assembly); Howard Francis, MD (surgeon); Jennifer Mertes, AuD, CCC-A (coordinator); Rick Ostrander, EDD (data collection); Jennifer Yeagle, MEd, CCC-A (data collection).
Johns Hopkins University, The River School, Washington, DC: Nancy Mellon (administration); Meredith Dougherty (data collection); Mary O’Leary Kane, MA, CCC-SLP (former coordinator, data assembly); Meredith Ouellette (coordinator); Julie Verhoff, AuD, CCC-A (data collection); Dawn Marsiglia, MA, CCC-A/SLP (data collection).
University of Miami, Miami: Annelle Hodges, PhD (PI); Thomas Balkany, MD (surgeon); Alina Lopez, MA, CCC-SLP/A (coordinator); Leslie Goodwin, MSN, CCRC (data collection).
University of Michigan, Ann Arbor, MI: Teresa Zwolan, PhD, CCC-A (Principal Investigator); Caroline Arnedt, MA, CCC-A (clinic coordinator); Hussam El-Kashlam, MD (surgeon); Kelly Starr, MA, CCC-SLP (data collection); Ellen Thomas, MA, CCC-SLP, Cert AVT.
University of North Carolina, Carolina Children’s Communicative Disorders Program, Chapel Hill: Holly F.B. Teagle, AuD, (PI); Craig A. Buchman, MD (surgeon); Carlton Zdanski, MD (surgeon); Hannah Eskridge, MSP (data collection); Harold C. Pillsbury, MD (surgeon); Jennifer Woodard (coordinator).
University of Texas at Dallas, Dallas Cochlear Implant Program, Callier Advanced Hearing Research Center, Dallas: Emily A. Tobey, PhD, CCC-SLP (PI); Lana Britt, AUD, (Co-coordinator); Janet Lane, MS, CCC-SLP (data collection); Peter Roland, MD (surgeon); Sujin Shin, MA (data collection); Madhu Sundarrajan, MS, CCC-SLP (data collection); Andrea Warner-Czyz Ph.D. CCC-AUD (co-coordinator).
Resource Centers
Data Coordinating Center, Johns Hopkins University, Welch Center for Prevention, Epidemiology & Clinical Research, Baltimore: Nae-Yuh Wang, PhD (PI, biostatistician); Patricia Bayton (data assembly); Enrico Belarmino (data assembly); Christine Carson, ScM (study manager, data analysis); Nancy E. Fink, MPH (Former PI); Thelma Grace (data assembly).
Psychometrics Center, University of Miami, Department of Psychology, Coral Gables: Alexandra Quittner, PhD (PI); David Barker, PhD (data analysis); Ivette Cruz, PhD (data analysis); Cara Kimberg (data assembly); Sandy Romero (data assembly); Mary Beth Grimley (data assembly).
Study Oversight Committees
Executive Committee: John K. Niparko, MD (chair); Laurie S. Eisenberg, PhD; Nancy E. Fink, MPH (former member); Alexandra L. Quittner, PhD; Donna Thal, PhD; Emily A. Tobey, PhD; Nae-Yuh Wang, PhD.
External Advisors: Noel Cohen, MD; Julia Evans, PhD; Ann Geers, PhD; Karen Iler Kirk, PhD.
Contributor Information
Ivette Cruz, Email: icruz@med.miami.edu, Department of Otolaryngology, University of Miami, 1120 NW 14th Street, CRB 5th Floor, Miami, FL, 33136.
Alexandra L. Quittner, Department of Psychology, University of Miami.
Craig Marker, Department of Psychology, University of Miami.
Jean L. DesJardin, Moravian College.
References
- Albertini JA. Deafness and hearing loss. In: Weiner IB, Craighead WE, editors. The Corsini Encyclopedia of Psychology. 4. Vol. 2. Hoboken, NJ: John Wiley & Sons, Inc; 2010. p. 461. [Google Scholar]
- Baldassari CM, Schmidt CS, Schubert CM, Srinivasan P, Dodson KM, Sismanis A. Receptive language outcomes in children after cochlear implantation. Otolaryngology-Head and Neck Surgery. 2009;140:114–119. doi: 10.1016/j.otohns.2008.09.008. doi:0.1016/j.otohns.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Barker DH, Quittner AL, Fink NE, Eisenberg LS, Tobey EA, Niparko JK CDaCI Investigative Team. Predicting behaviors problems in deaf and hearing children: The influences of language, attention, and parent-child communication. Development and Psychopathology. 2009;21:373–392. doi: 10.1017/S0954579409000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biringen Z, Robinson J. Emotional availability in mother-child interactions: A conceptualization for research. American Journal of Orthopsychiatry. 1991;61:258–271. doi: 10.1037/h0079238. [DOI] [PubMed] [Google Scholar]
- Bornstein M. Infant into conversant: Language and non-language processes indeveloping early communication. In: Budwig N, Uzgiris IC, Wersch JV, James V, editors. Communication: An arena of development. Westport, CT: Ablex Publishing; 2000. pp. 109–129. [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Calderon R. Parental involvement in deaf children’s education programs as a predictor of child’s language, early reading, and social-emotional development. Journal of Deaf Studies and Deaf Education. 2000;5:140–155. doi: 10.1093/deafed/5.2.140. [DOI] [PubMed] [Google Scholar]
- Catts H, Fey M, Tomblin J, Zhang X. A longitudinal investigation of reading outcomes in children with language impairments. Journal of Speech, Language, and Hearing Research. 2002;45:1142–1157. doi: 10.1044/1092–4388. [DOI] [PubMed] [Google Scholar]
- Chapman RS. Children’s language learning: An interactionist perspective. Journal of Child Psychology and Psychiatry: Annual Research Reviews. 2000;41:33–54. doi: 10.1111/1469–7610.00548. [DOI] [PubMed] [Google Scholar]
- Deckner DF, Adamson LB, Bakerman R. Rhythm in mother-infant interactions. Infancy. 2003;4:201–217. doi: 10.1207/S15327078IN0402_03. [DOI] [Google Scholar]
- DesJardin JL. Coding Ttranscriptions of mother-child interactions. (Unpublished manuscript) House Ear Institute, Children’s Auditory Research and Evaluation Center; California: 2003. [Google Scholar]
- DesJardin JL. Empowering families of young children with cochlear implants: Implications for early intervention and language development. In: Eisenberg LS, editor. Clinical management of children with cochlear implants. San Diego, CA: Plural Publishing Inc; 2009. pp. 513–553. [Google Scholar]
- DesJardin JL, Ambrose SE, Eisenberg LS. Literacy skills in children with cochlear implants: The importance of early oral language and joint storybook reading. Journal of Deaf Studies and Deaf Education. 2009;14:22–43. doi: 10.1093/deafed/enn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesJardin JL, Eisenberg LS. Maternal contributions: Supporting language development in young children with cochlear implants. Ear & Hearing. 2007;28:456–469. doi: 10.1097/AUD.0b013e31806dc1ab. [DOI] [PubMed] [Google Scholar]
- Dettman SJ, Pinder D, Briggs RJ, Dowell RC, Leigh JR. Communication development in children who receive the cochlear implant younger than 12 months: Risks versus benefits. Ear & Hearing. 2007;28(Suppl):11S–18S. doi: 10.1097/AUD.0b013e31803153f8. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Tabors P, editors. Beginning literacy with language: Young children learning at home and school. Baltimore, MD: Paul H Brookes Publishing; 2001. [Google Scholar]
- Enders CK. The performance of the full information maximum likelihood estimator in multiple regression models with missing data. Educational and Psychological Measurement. 2001;61:713–740. doi: 10.1177/0013164401615001. [DOI] [Google Scholar]
- Estabrooks W. The auditory-verbal approach: A professional point of view. In: Schwartz S, editor. Choices in deafness. 3. Bethesda, MD: Woodbine House; 2007. pp. 53–88. [Google Scholar]
- Ferrer E, McArdle JJ. Longitudinal modeling of developmental changes in psychological research. Current Directions in Psychological Science. 2010;19:149–154. doi: 10.1177/0963721410370300. [DOI] [Google Scholar]
- Fewell RR, Deutscher B. Contributions of early language and maternal facilitation variables to later language and reading abilities. Journal of Early Intervention. 2004;26:132–145. doi: 10.1177/105381510402600205. [DOI] [Google Scholar]
- Fey ME, Krulik T, Loeb DF, Proctor-Williams K. Sentence recast use by parents of children with typical language and children with specific language impairment. American Journal of Speech-Language Pathology. 1999;8:273–286. [Google Scholar]
- Fink NE, Wang NY, Quittner AL, Eisenberg ES, Tobey EA, Niparko JK the CDaCI Investigative Team . Childhood Development after Cochlear Implantation (CDaCI): Design and baseline characteristics. Cochlear Implants International. 2007;8:92–116. doi: 10.1002/cii.333. [DOI] [PubMed] [Google Scholar]
- Fung P, Chow BW, McBride-Chang C. The impact of a dialogic reading program on deaf and hard-of-hearing kindergarten and early primary school-aged students in Hong Kong. Journal of Deaf Studies and Deaf Education. 2005;10:82–95. doi: 10.1093/deafed/eni005. [DOI] [PubMed] [Google Scholar]
- Geers AE, Moog JS, Biedenstein J, Brenner C, Hayes H. Spoken language scores of children using cochlear implants compared to hearing age-mates at school entry. Journal of Deaf Studies and Deaf Education. 2009;14:371–385. doi: 10.1093/deafed/enn046. [DOI] [PubMed] [Google Scholar]
- Geers AE, Nicholas JG, Sedey AL. Language skills of children with early cochlear implantation. Ear & Hearing. 2003;24:46S–58S. doi: 10.1097/01.AUD.0000051689.57380.1B. [DOI] [PubMed] [Google Scholar]
- Girolametto L, Weitzman E, Pearce P, Wiigs M. The relationship between maternal language measures and language development in toddlers with expressive vocabulary delays. American Journal of Speech-Language Pathology. 1999;8:364–374. [Google Scholar]
- Hart B, Risley T. Observing children and families talking. In: Hart B, Riley TR, editors. The social world of children learning to talk. Baltimore, MD: Paul H. Brookes Publishing Co., Inc; 1999. pp. 7–29. [Google Scholar]
- Hoff E. Language development. Pacific Grove, CA: Brooks/Cole; 2000. [Google Scholar]
- Hoff E. The specificity of environmental influence: Socioeconomic status affects early vocabulary development via maternal speech. Child Development. 2003;74:1368–1378. doi: 10.1111/1467–8624.00612. [DOI] [PubMed] [Google Scholar]
- Holt RF, Svirsky MA. An exploratory look at pediatric cochlear implantation: Is earliest always better? Ear & Hearing. 2008;29:492–511. doi: 10.1097/AUD.0b013e31816c409f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulit LM, Howard MR. Born to talk: An introduction to speech and language development. Boston: Allyn & Bacon; 1997. [Google Scholar]
- Huttenlocher J, Haight W, Bryk A, Seltzer M, Lyons T. Early vocabulary growth: Relation to language input and gender. Developmental Psychology. 1991;27:236–248. doi: 10.1037//0012-1649.27.2.236. [DOI] [Google Scholar]
- Kaiser AP, Hancock TB. Teaching parents new skills to support their young children’s development. Infants and Young Children. 2003;16:9–21. [Google Scholar]
- Kaiser AP, Hancock TB, Hester PP. Parents as co-interventionists: Research on applications of naturalistic language teaching procedures. Infants and Young Children. 1998;10:46–55. [Google Scholar]
- Kaiser AP, Hemmeter M, Ostrosky MM, Fischer R. The effects of teaching parents to use responsive interaction strategies. Topics in Early Childhood Special Education. 1996;16:375–406. doi: 10.1177/027112149601600307. [DOI] [Google Scholar]
- Lederberg AR, Everhart VS. Conversations between deaf children and their hearing mothers: Pragmatic and dialogic characteristics. Journal of Deaf Studies and Deaf Education. 2000;5:303–322. doi: 10.1093/deafed/5.4.303. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F. Latent difference score structural models for linear dynamic analyses with incomplete longitudinal data. In: Collins L, Sayer A, editors. New methods for the analysis of change. Washington, DC: APA Press; 2001. pp. 137–176. [Google Scholar]
- McCauley RJ, Fey ME. Treatment of language disorders in children. Baltimore, MD: Paul H. Brookes; 2006. [Google Scholar]
- Meadow-Orlans K, Spencer P. Maternal sensitivity and the visual attentiveness of children who are deaf. Early Development and Parenting. 1996;5:213–223. doi: 10.1002/(SICI)1099-0917. [DOI] [Google Scholar]
- Moeller MP. Early intervention and language development in children who are deaf and hard of hearing. Pediatrics. 2000;106:1–9. doi: 10.1542/peds.106.3.e43. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. User’s guide. 5. Los Angeles: Muthen & Muthen; 2008. Mplus: The comprehensive modeling program for applied researchers. [Google Scholar]
- NICHD Early Child Care Research Network. Child care and mother-child interactions in the first three years of life. Developmental Psychology. 1999;35:1399–1413. doi: 10.1037/0012-1649.35.6.1399. [DOI] [PubMed] [Google Scholar]
- Niparko JK, Tobey EA, Thal DJ, Eisenberg LS, Wang NY, Quittner AL CDaCI Investigative Team. Spoken language development in children following cochlear implantation. JAMA. 2010;303:1498–1506. doi: 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parment S, Lynm C, Glass R. Cochlear Implants. Journal of the American Medical Association. 2004;291:2398. doi: 10.1001/jama.291.19.2398. [DOI] [PubMed] [Google Scholar]
- Pressman LJ, Pipp-Siegel S, Yoshinaga-Itano C, Deas A. Maternal sensitivity predicts language gain in preschool children who are deaf and hard of hearing. Journal of Deaf Studies and Deaf Education. 1999;4:294–304. doi: 10.1093/deafed/4.4.294. [DOI] [PubMed] [Google Scholar]
- Puckett MB, Black JK, Wittmer DS, Peterson SH. The young child: Development from prebirth through age eight. Upper Saddle River, NJ: Pearson; 2009. [Google Scholar]
- Pungello EP, Iruka IU, Dotterer AM, Mills-Koonce R, Reznick J. The effects of socioeconomic status, race, and parenting on language development in early childhood. Developmental Psychology. 2009;45:544–557. doi: 10.1037/a0013917. [DOI] [PubMed] [Google Scholar]
- Quittner AL, Barker DH, Cruz I, Snell C, Grimley ME, Botteri M. Parenting stress among parents of deaf and hearing children: associations with language delays and behavior problems. Parenting: Science and Practice. 2010;10:136–155. doi: 10.1080/15295190903212851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quittner AL, Barker DH, Snell CJ, Cruz I, McDonald L, Grimley MB, Marciel K. Improvements in visual attention in deaf infants and toddlers after cochlear implantation. Audiological Medicine. 2007;5:242–249. doi: 10.1080/16513860701745401. [DOI] [Google Scholar]
- Quittner AL, Leibach PL, Marciel K. The impact of cochlear implants on young, deaf children: New methods to assess cognitive and behavioral development. Archives of Otolaryngology, Head and Neck Surgery. 2004;130:547–554. doi: 10.1001/archotol.130.5.547. [DOI] [PubMed] [Google Scholar]
- Reynell JK, Greuber CP. Reynell Developmental Language Scales. Los Angeles: Western Psychological Services; 1990. [Google Scholar]
- Sandall S, Hemmeter ML, Smith BJ, McLean ME. DEC recommended practices: A comprehensive guide for practical application in early intervention/early childhood special education. Longmont, CO: Sopris West; 2005. [Google Scholar]
- Smith L, Quittner AL, Osberger M, Miyamoto R. Audition and visual attention: The developmental trajectory in deaf and hearing populations. Developmental Psychology. 1998;34:840–850. doi: 10.1037/0012-1649.34.5.840. [DOI] [PubMed] [Google Scholar]
- Spencer PE. Individual differences in language performance after cochlear implantation at one to three years of age: Child, family, and linguistic factors. Journal of Deaf Studies and Deaf Education. 2004;9:395–412. doi: 10.1093/deafed/enh033. [DOI] [PubMed] [Google Scholar]
- Spencer PE, Erting CJ, Marschark M. The deaf child in the family and at school: Essays in honor of Kathryn P Meadow-Orlans. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; [Google Scholar]
- Spencer PE, Marschark M. Evidence-based practice in educating deaf and hard of hearing. New York, NY: Oxford University Press; 2010. [Google Scholar]
- Spencer PE, Marschark M, Spencer LJ. Cochlear implants: Advances, issues, and implications. In: Marschark M, Spencer PE, editors. Oxford Handbook of Deaf Studies, Language, and Education. New York: Oxford University Press; 2011. pp. 452–471. [Google Scholar]
- Svirsky MA, Robbins AM, Kirk KI, Pisoni DB, Miyamoto RT. Language development in profoundly deaf children with cochlear implants. Psychological Science. 2000;11:153–158. doi: 10.1111/1467-9280.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weizman Z, Snow C. Lexical input as related to children’s vocabulary acquisition: effects of sophisticated exposure and support for meaning. Developmental Psychology. 2001;37:265–279. doi: 10.1037/0012-1649.37.2.265. [DOI] [PubMed] [Google Scholar]
- Yoder PJ, McCathren RB, Warren SF, Watson AL. Important distinctions in measuring maternal responses to communication in prelinguistic children with disabilities. Communication Disorders Quarterly. 2001;22:135–147. doi: 10.1177/152574010102200303. [DOI] [Google Scholar]