Abstract
Artemisinin and its derivatives, artesunate and artemether, are rapidly acting antimalarials that are used for the treatment of severe and uncomplicated multidrug-resistant falciparum malaria. To optimize treatment regimens that use this new class of antimalarials, there is a need for readily available and reproducible assays to monitor drug levels closely in patients. A sensitive and reproducible bioassay for the measurement of the concentrations of artemisinin derivatives in plasma and serum is described. By modifying the in vitro drug susceptibility test, it was found that antimalarial activity in plasma or serum containing an unknown concentration of drug could be equated to the known concentrations of dihydroartemisinin (DHA) required to inhibit parasite growth. Dose-response curves for a Plasmodium falciparum clone (clone W2) and DHA were used as a standard for each assay. Assays with plasma or serum spiked with DHA proved to be reproducible (coefficient of variation, ≤10.9%), with a lower limit of quantitation equivalent to 2.5 ng of DHA per ml. For plasma spiked with artesunate or artemether, there was good agreement of the results obtained by the bioassay and the concentrations measured by high-performance liquid chromatography (HPLC) with electrochemical detection. The bioassay for measurement of the antimalarial activities of artemisinin derivatives in body fluids requires a smaller volume of plasma or serum and is more sensitive than the presently available HPLC methods, can provide pharmacodynamic parameters for determination of activity against the parasite, and should enhance the design of more appropriate dosage regimens for artemisinin drugs.
The malaria situation is continuing to worsen in many parts of the world, and there is an urgent need for new, effective drugs for the treatment of falciparum malaria. The antimalarials derived from qinghaosu, or artemisinin, may meet this need (11). Although the artemisinin derivatives are increasingly used for the treatment of multidrug-resistant malaria, little clinical pharmacokinetic information is available to assist with the development of rational dosage regimens for this novel class of antimalarial drugs (42).
The development of selective methods for determination of the concentrations of artemisinin and its derivatives poses challenging problems since the drugs are thermally labile, lack UV or fluorescent chromophores, and do not possess functional groups for derivatization. In addition, the remarkable activities of these drugs require assays with sensitivities in the low nanogram-per-milliliter range. At present there is no one simple, selective, and sensitive method for measurement of the concentrations of these substances. The methods previously used to measure the concentrations of these drugs include gas chromatography-mass spectroscopy (30, 36), high-performance liquid chromatography (HPLC) with UV detection (3, 9, 12, 22, 33, 37, 38), HPLC with electrochemical detection (HPLC-ECD) (1, 4, 8, 15, 17, 20, 23, 28, 34, 35, 40, 43, 45), and liquid chromatography with mass spectrometry (2, 6, 13, 19, 26, 27, 31).
The HPLC-ECD technique is widely used for the measurement of artemisinin drug concentrations in biological fluids because of its selectivity, sensitivity, and ability to detect both the parent drug (artemisinin, artmether, or arteether) and a major metabolite, dihydroartemisinin (DHA), simultaneously. Unfortunately, this method requires complicated extractions, expensive instrumentation, absolute electrical stability, and highly skilled personnel. Since therapeutic drug monitoring is helpful in areas where malaria is endemic, where facilities for such procedures are not commonly available, a simple and reliable analytical method that can be easily reproduced in other laboratories is required.
A bioassay for chloroquine, artemisinin, and mefloquine that is based on the in vitro microculture technique and that was used to detect bioactivity in the serum of monkeys and humans dosed with drug has been described (16, 18, 44). This technique uses microscopic determination of parasite morphology to determine the highest serum or plasma dilution that inhibits the in vitro maturation of a parasite isolate, relates this to the MIC of the drug for that isolate, and converts the results into drug concentration equivalents in serum or plasma. Although this method provides preliminary information on drug effects and kinetics, the technique is very labor-intensive and produces semiquantitative data.
We report here on a bioassay technique for measurement of the antimalarial activities of artemisinin and its derivatives in human plasma or serum. The technique is based on the microdilution radioisotope technique used for antimalarial drug susceptibility testing (10, 41). This method proved to be quantitative, reproducible, and sensitive for detection of the antimalarial activities of artemisinin derivatives in plasma or serum.
MATERIALS AND METHODS
Treatment of plasma or serum
Fifty microliters of Affigel protein A (binding capacity of 20 mg of purified human immunoglobulin G [IgG]/mg of gel; Bio-Rad, Richmond, Calif.) was washed twice with 50 μl of phosphate-buffered saline (pH 7.2) by centrifugation in an Eppendorf centrifuge (model 5414S; Thomas Scientific, Swedesboro, N.J.) for 5 s. In a third washing step, 50 μl of RPMI 1640 medium (supplemented with 5.94 g of HEPES per liter and 2.1 g of sodium bicarbonate per liter) was used. After centrifugation the supernatant was discarded and the protein A gel was ready for incubation with non-heat-inactivated plasma. Plasma (250 μl) spiked with DHA or plasma from patients was incubated with protein A for 30 min at room temperature. Serum samples spiked with drug were treated as stated above for plasma.
Preparation of drug solutions
A stock solution of DHA (1 mg/ml) was prepared in 70% ethanol and was kept at −10°C for up to 1 month before use. Further dilutions were prepared fresh in plasma or serum to give a working concentration range of 1.25 to 100 ng/ml. The DHA used in this study was obtained from the Experimental Therapeutics Chemical Inventory of the Walter Reed Army Institute of Research (Washington, D.C.).
Bioassay
The bioassay was based in part on a microdilution radioisotope method used for antimalarial drug susceptibility testing of Plasmodium falciparum (10, 41). To prepare the 96-well microtiter plate for the assay, 100 μl of protein A-treated plasma or serum was transferred to row A of a flat-bottom plate. Heat-inactivated serum (50 μl/well) was added to rows B through G of the plate and was used as the diluent for plasma or serum containing drug. Twofold serial dilutions were then made by using an automated PRO/PETTE liquid handling system (Perkin-Elmer Cetus, Norwalk, Conn.). The result was seven concentrations of drug diluted into heat-inactivated control plasma or serum. Fifty microliters of heat-inactivated control plasma or serum was added to row H for parasitized and nonparasitized erythrocyte controls. A suspension of 175 μl of malaria parasite-infected erythrocytes (W2 clone; 0.5% parasitemia with >80% young rings at a 1.7% hematocrit) was added to all wells for rows A to G and eight wells for row H. A similar suspension of uninfected erythrocytes was added to the remaining four wells of row H for nonparasitized controls. The microtiter plates were placed into a gas-tight Plexiglas chamber; flushed with a mixture of gas consisting of 90% CO2, 5% O2, and 5% N2; and placed into an incubator (37°C). Following 24 h of incubation, the microtiter plates were removed from the chamber and pulsed with [3H]hypoxanthine (specific activity, 1 Ci/mmol) by addition of 25 μl (0.5 μCi) of isotope solution to each well. The microtiter plates were returned to the chamber and incubated for 18 to 20 h. The contents of the plates were then harvested with a Mach II harvester (TOMTEC, Orange, Conn.), in which the particulate material of the water-lysed cell suspension was filtered through glass-fiber filter paper. The filter paper was dried in an oven and placed in a plastic bag, and 10 ml of scintillation fluid was added before the plastic bag was sealed. The level of incorporation of [3H]hypoxanthine by the malaria parasites in each well was determined by counting in a liquid scintillation counter (1205 Betaplate; Pharmacia, Wallac Oy, Finland).
Dose-response curve
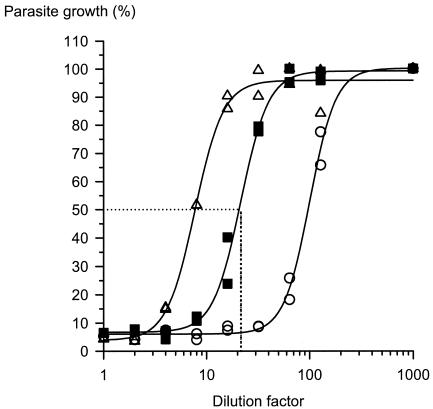
A dose-response curve for each plasma sample was obtained by plotting on the y axis the percent parasite growth calculated from the level of [3H]hypoxanthine uptake (in counts per minute) as a measure of parasite nucleic acid synthesis against the corresponding dilution factor on the x axis (Fig. 1). After logarithmic transformation of the dilution factors, a nonlinear regression analysis was performed by use of a four-parameter logistic regression equation (logistic dose-response transition equation in Table Curve Automated Curve Fitting software; Jandel Scientific, San Rafael, Calif.). The function used was y = (a + b)/[1 + (x/c)d], where y is the level of incorporation of [3H]hypoxanthine (in counts per minute), x is the dilution factor, a is the lower asymptote of the function (or the mean for the nonparasitized erythrocyte controls), b is the difference between the upper asymptote (mean level of incorporation of [3H]hypoxanthine by control parasitized erythrocytes) and the lower asymptote, c is the dilution factor of DHA that inhibits incorporation of the isotope by 50% (DF50), and d is the slope of the linear portion of the dose-response curve. DF50 was determined for each plasma sample.
FIG. 1.
Dose-response curves for plasma spiked with standard DHA showing the dilution factor values at various known concentrations: ○, 50.0 ng/ml and DF50 of 100.77; ▪, 12.50 ng/ml and DF50 of 21.62; ▵, 5.00 ng/ml and DF50 of 7.74.
Standard curve and calculation
A linear standard curve was constructed by plotting the log DF50 on the y axis versus the log DHA concentration on the x axis (Fig. 2). The antimalarial activity of the unknown plasma sample was then read from the plot by using the DF50 (obtained from the dose-response curve described above) and was expressed as DHA concentration equivalents. Thus, the antimalarial activity of the unknown plasma sample was reported as the corresponding DHA concentration that produced the same activity on the standard curve.
FIG. 2.
Relationship between observed DF50 and DHA concentration: comparison of plasma and serum spiked with DHA. ▪, plasma (log y = 0.9705 log x + 0.1222; r2 = 0.9919); ▵, serum (log y = 0.9121 log x + 0.1979; r2 = 0.9884).
Preparation of hemolysate
Fresh whole blood was collected and placed in heparinized tubes, and the plasma and buffy coat were removed. The erythrocytes were lysed and serially diluted (2- to 64-fold) with sterile water. Aliquots (50 μl) of these hemolysates were diluted in plasma or medium (250 μl) spiked with DHA to give a final concentration of 50 ng/ml and a range of hemoglobin concentrations from 1.5 to 0.05 g%. The effect of hemolysate in both plasma and culture medium (RPMI 1640 medium with 15% heat-inactivated human serum) was then examined to determine if the hemolysate inhibited the detection of DHA activity by the bioassay. For the bioassay format, the final concentration of plasma in each well was 30%, whereas for serum it was 15%, which is similar to that used in the normal drug susceptibility testing format.
In addition to the testing of hemolysates of normal erythrocytes, plasma samples collected from two patients with severe falciparum malaria were spiked with 50 ng of DHA per ml and subjected to the same bioassay. The plasma samples were obtained from both patients prior to treatment with any antimalarial drugs.
RESULTS
Effect of heat inactivation on DHA activity
Plasma or serum from healthy humans can cause lysis of P. falciparum-infected erythrocytes in vitro via complement-mediated lysis of cells bound with immunoglobulin. In standard in vitro drug susceptibility testing, plasma or serum is heat inactivated to destroy complement, thus reducing the growth-inhibitory activity of normal plasma. Since the serum or plasma used in the bioassay would contain various drug concentrations, the effect of heat inactivation on DHA activity was evaluated. To assess this effect, the following solutions were heated at 56°C for 30 min: DHA in RPMI 1640 medium with no plasma, DHA in medium with 20% plasma, and plasma to which no DHA was added (until after heat inactivation). The 50% inhibitory concentration (IC50) of heat-inactivated DHA in RPMI 1640 medium was 0.36 ng/ml, whereas the IC50 of DHA spiked into medium after heat inactivation was 0.31 ng/ml. In contrast, the IC50 of DHA treated with heat in the presence of 20% plasma was 1.17 ng/ml, a fourfold decrease in activity. The results suggest that DHA activity is relatively stable after heat treatment, but only in the absence of excess protein.
Effect of Affigel protein A on parasite growth
Since heat inactivation cannot be used to reduce the growth-inhibitory activity of plasma or serum from healthy individuals, removal of IgG from plasma and serum by incubation with Affigel protein A was evaluated. The effect of protein A treatment of serum was compared with that of heat inactivation by measuring the level of parasite growth inhibition by the [3H]hypoxanthine method. When heat-inactivated serum was used as the control (control growth, 100% ± 12.5%; mean ± standard deviation [SD]), serum from healthy individuals inhibited growth by 25% (growth, 75% ± 3.9%). In contrast, serum treated with protein A produced growth which was similar to that of the control (97% ± 12.2%).
The effect of protein A treatment on the activity of DHA-spiked serum was evaluated by comparing standard curves prepared with serum prior to and after protein A treatment. The standard curve of DF50 versus concentration obtained with DHA-spiked serum treated with Affigel protein A gave a slope close to unity (−0.944), whereas the slope for untreated spiked serum was −0.347. Thus, Affigel protein A-treated serum gave the optimum standard curve for determination of unknown concentrations of DHA in serum and removed most of the growth-inhibitory activity from normal serum.
Intra-assay and interassay variations and accuracy
To validate the accuracy of the P. falciparum-based bioassay, a series of standard curves were compared (Table 1). The coefficient of variation (CV) was ≤10.9% for assays conducted on the same day; in addition, the interassay variation was remarkably low (CV, <8.5%) for DHA concentrations ≥12.5 ng/ml. The assay proved to be more variable with lower concentrations in the interassay variation analysis.
TABLE 1.
Validation data for standard curves for DHA spiked in plasma used in the bioassay
| Actual concn (ng/ml) | Intra-assay (n = 5)
|
Interassay (n = 6)
|
||||
|---|---|---|---|---|---|---|
| Concn found (mean ± SD) | % CVa | % Error | Concn found (mean ± SD) | % CV | % Error | |
| 1.25 | 1.50 ± 0.04 | 2.46 | 20.19 | NDb | ||
| 2.50 | 2.19 ± 0.24 | 10.86 | (12.56)c | ND | ||
| 5.00 | 5.12 ± 0.32 | 6.26 | 2.50 | 4.89 ± 0.81 | 16.47 | (2.18) |
| 12.50 | 12.46 ± 0.87 | 6.96 | (0.35) | 12.20 ± 1.10 | 8.29 | (2.44) |
| 25.00 | 25.13 ± 2.20 | 8.76 | 0.52 | 24.08 ± 2.03 | 8.45 | (3.69) |
| 50.00 | 50.23 ± 5.19 | 10.34 | 0.46 | 53.02 ± 3.70 | 6.97 | 6.04 |
| 100.00 | 111.30 ± 4.36 | 3.92 | 11.30 | ND | ||
CV is a measure of relative variation which expresses the SD as a percentage of the mean.
ND, not determined.
Negative values are in parentheses.
To confirm the accuracy of the bioassay, solutions of DHA were prepared by a technician not involved in the study and a blinded analysis was performed. The concentrations of DHA in unknowns were determined from standard curves prepared at the same time (Table 2). The between-day CV was <11.5%, while the within-day CVs were ≤10% for both concentrations of drug tested (12.5 and 25 ng/ml).
TABLE 2.
Validation data for DHA spiked in plasma treated as unknowns in the bioassay
| Actual concn (ng/ml) | Intra-assay (n = 5)
|
Interassay (n = 6)
|
||||
|---|---|---|---|---|---|---|
| Concn found (mean ± SD) | % CV | % Error | Concn found (mean ± SD) | % CV | % Error | |
| 12.50 | 12.42 ± 0.87 | 6.97 | (0.64)a | 12.08 ± 1.39 | 11.50 | (3.35) |
| 25.00 | 25.13 ± 2.20 | 8.76 | 0.52 | 23.72 ± 2.69 | 11.34 | (5.11) |
Negative values are in parentheses.
Comparison of bioassay with HPLC-ECD method
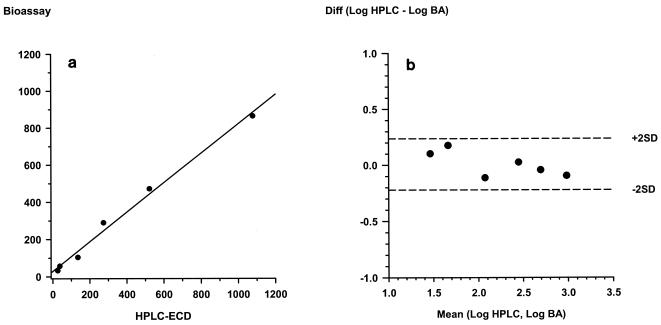
The method preferred at present for specific measurement of the concentrations of artemisinin derivatives is reductive HPLC-ECD; therefore, plasma samples spiked with artesunate were analyzed by both methods in an open comparison, and the results were compared by the limit-of-agreement test (7). The HPLC-ECD method was conducted as described previously (17, 20). As shown in Fig. 3, the correlation and the agreement of results obtained by the two methods for the measurement of artesunate concentrations were good. After log transformation, the mean difference in the artesunate concentration was 0.008, with the limits of agreement being between −0.22 and 0.24.
FIG. 3.
Comparison of results for plasma samples spiked with various concentrations (n = 6) of artesunate determined by the bioassay and HPLC-ECD. The results of the two methods showed excellent correlations and agreement. (a) Scatterplot of bioassay versus HPLC-ECD (r2 = 0.992); (b) Bland-Altman plot of the difference (Diff) in log values determined by bioassay (BA) and HPLC-ECD versus the mean values (mean difference = 0.008; limits of the agreement = −0.221 and 0.236, respectively).
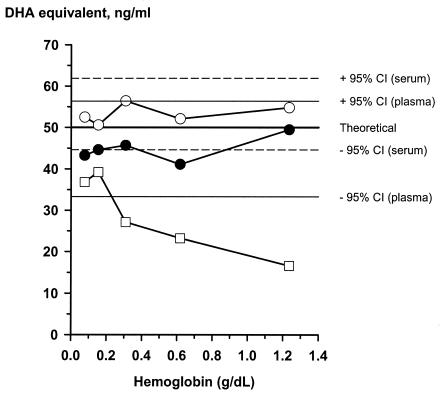
Effect of hemolysate on DHA activity
Hemolysate from normal erythrocytes was used to spike plasma or serum samples containing 50 ng of DHA per ml (Fig. 4). The bioassay results demonstrated that hemolysate does not significantly affect the antimalarial activity of DHA in protein A-treated plasma or serum if the final concentration of plasma or serum is 30% in each well. In contrast, high concentrations of hemolysate significantly decreased the activity of DHA in serum if the final concentration of serum was 15% in each well and protein A was not added.
FIG. 4.
Effect of hemolysate on the activity of DHA in culture medium containing 30% serum (○) or plasma (•) in the bioassay format and culture medium containing 15% serum in the drug susceptibility test format (□).
Plasma samples from two patients with severe falciparum malaria with visible hemolysis in each sample were spiked with 50 ng of DHA per ml, and the DHA activity equivalents were measured by the bioassay. The DHA activity equivalents obtained by the bioassay in samples from both patients were greater than or equal to 45 ng of DHA per ml.
DISCUSSION
A bioassay method was developed for measurement of the antimalarial activities of artemisinin (qinghaosu) and derivatives in either the sera or plasma of patients. Antimalarial activity in plasma in vitro was expressed in terms of the equivalent activity of DHA, the common bioactive metabolite for many of the artemisinin derivatives. The method is simple, reproducible, and sensitive compared with the other analytical methods available. Either serum or plasma samples can be used since the standard dose-response curves for DHA spiked into serum and plasma were similar, with a correlation coefficient >0.99. An added advantage is that small sample volumes are required. Only 250 μl of sample is sufficient for duplicate measurements in the bioassay, whereas 1 to 2 ml of plasma is needed for HPLC. Since numerous laboratories worldwide already conduct in vitro antimalarial drug susceptibility testing, this method could easily be adapted by other research centers (14, 39).
A critical component of this bioassay method is the standardized approach used to construct the dose-response curves necessary to derive DHA equivalents. To obtain an accurate estimate of the DF50s for unknown samples, the DHA concentration range must span the full sigmoidal dose-response curve and cover both the upper and the lower asymptotes of the function. In this study the optimal concentration was found to be 12.5 ng/ml with a working concentration range of 2.5 to 100.0 ng/ml. When the concentration is too low to give a full sigmoidal curve (activity equivalent to <5 ng of DHA per ml), accurate estimation of DF50 for the sample is not possible with a four-parameter fit. In this situation, the level of incorporation of hypoxanthine by control nonparasitized erythrocytes can be used as a weighted lower asymptote of the function. This method is applicable as long as at least one concentration of unknown drug in plasma along the linear portion of the curve provides inhibition. When the concentration of drug in plasma is too high (>50 ng/ml), the level of hypoxanthine incorporation of the parasitized erythrocyte controls may be used to weight the upper asymptote. For these samples the plasma must be diluted with heat-inactivated plasma before incubation with protein A, and the assay is then repeated.
Serum or plasma containing DHA could not be heat inactivated to remove any growth-inhibitory activity encountered in plasma from healthy individuals because this also reduced antimalarial activity. Since heat treatment had no effect on DHA in medium without protein, heat treatment may have increased the protein binding sites for the drug, thus reducing the free concentration and diminishing the inhibitory effects on the parasite. Most of the inhibitory activity of serum from healthy individuals could be eliminated by preincubation of samples with a protein A-gel matrix. Presumably, this treatment reduces the nonspecific binding of IgG to infected erythrocytes, which can then activate complement-mediated lysis. It remains to be determined if such treatment will be sufficient to reduce any growth-inhibitory activity seen in serum or plasma from semi-immune individuals. Even though plasma or serum samples containing drug from patients cannot be heat treated, plasma collected prior to the start of drug treatment could be used as an internal control for activity in the bioassay.
As has been shown for the microculture technique (16, 18, 29, 44), this P. falciparum-based bioassay should be applicable to other antimalarial drugs. The sensitivity of the bioassay is directly related to the inherent activity of the drug against the parasites to be tested. Drugs with in vitro activities in the low nanogram-per-milliliter range (e.g., artemisinin) are ideal for this type of assay, whereas measurement of the activities of less active antimalarial drugs (e.g., tetracycline) is not practical. At present we are using similar methods to measure the levels of halofantrine and metabolites in plasma samples from phase I and II clinical trials (D. E. Kyle et al., unpublished data).
Part of the pathology associated with malaria is the lysis of erythrocytes, with the resultant hemolysate being present in the plasma of patients. A previous report (21) indicated that ferriprotoporphyrin inactivates artemisinin and may reduce its antimalarial activity. In this study we demonstrated that hemolysate from lysed red cells (in vitro) or from patients with severe malaria did not significantly inhibit the activity of DHA in 30% plasma or serum in the bioassay format. However, a significant loss of DHA activity was seen in medium containing only 15% serum (without protein A treatment) plus hemoglobin concentrations >0.1 g/dl in the in vitro drug susceptibility format. The reason for the different results with 30% serum and 15% serum is unknown but may be related to the protein binding of DHA. Regardless of the mechanism, the ability to accurately measure drug activity in hemolysates in serum or plasma obtained either from patients with severe malaria or by the in vitro lysis of erythrocytes demonstrates that hemolysis should not affect the use of the bioassay for clinical studies.
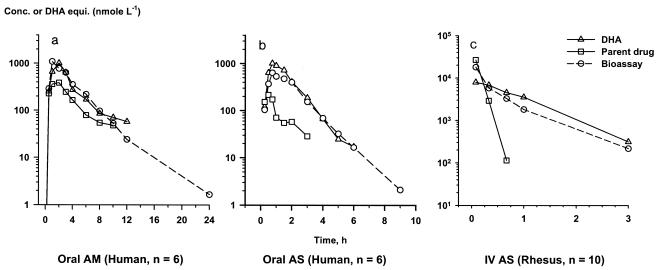
Shortcomings of the bioassay include the inability to distinguish between activity due to parent drug and that due to metabolites and the inherent difficulties in measuring activity in patients who have received more than one antimalarial drug. Despite this lack of specificity, the bioassay has much to offer for the assessment of a critical pharmacodynamic component: activity against the parasite. Application of this bioassay method can be found in clinical studies of artesunate, DHA, and artemether with patients with uncomplicated and complicated falciparum malaria (5, 24, 25, 32, 34, 35). Although the kinetics of discrete drug are important, the added pharmacodynamic data derived from the bioassay can be equally valuable for devising new dosage regimens for artemisinin derivatives. Ideally, the bioassay and HPLC-ECD methods could be used simultaneously to obtain both kinetic and dynamic parameters. This approach has recently been used to assess the comparative bioavailabilities of artemether in healthy adult volunteers (Fig. 5a) and the pharmacokinetics of artesunate in adults (Fig. 5b) with falciparum malaria (34, 35). This bioassay method has been modified to measure bioactivity in rhesus monkey plasma and has been used for the study of intravenous artemisinin derivatives administered to rhesus monkeys (Fig. 5c) in a severe malaria model.
FIG. 5.
Parent drugs, their metabolites, and total antimalarial activity in plasma after oral administration to healthy volunteers. (a) Artemether (AM) at 5 mg/kg (n = 8); (b) artesunate (AS) at 100 mg (n = 6); (c) intravenous artesunate (IV AS) administered at a dose of 8 mg/kg to healthy rhesus monkeys (n = 10). Conc., concentration; DHA equiv., DHA concentration equivalents. Panels a and b are reprinted with permission (see references 34 and 35, respectively).
Acknowledgments
We thank Prasit Sookto, Theera Wimonwattrawatee, Kelly Masonic, and Anthony Aguilar for technical assistance.
This work was supported in part by the U.S. Army Medical Research and Materiel Command and a grant from the UNDP/World Bank/WHO Special Programme for Research in Tropical Diseases. N. J. White is supported by the Wellcome Trust.
REFERENCES
- 1.Acton, N., D. L. Klayman, and I. J. Rollman. 1985. Reductive electrochemical HPLC assay for artemisinin (qinghaosu). Planta Med. 4:445-446. [DOI] [PubMed] [Google Scholar]
- 2.Baker, J. K., R. H. Yarber, C. D. Hufford, I. S. Lee, H. N. ElSohly, and J. D. McChesney. 1989. Thermospray mass spectroscopy/high performance liquid chromatographic identification of the metabolites formed from arteether using a rat liver microsome preparation. Biomed. Environ. Mass Spectrom. 18:337-351. [DOI] [PubMed] [Google Scholar]
- 3.Batty, K. T., T. M. E. Davis, L. T. A. Thu, T. Q. Binh, T. K. Anh, and K. F. Ilett. 1996. Selective high-performance liquid chromatographic determination of artesunate and alpha- and beta-dihydroartemisinin in patients with falciparum malaria. J. Chromatogr. B Biomed. Appl. 677:345-350. [DOI] [PubMed] [Google Scholar]
- 4.Benakis, A., C. Schopfer, M. Paris, C. T. Plessas, P. E. Karayannakos, I. Dondas, D. Kotsarelis, S. T. Plessas, and G. Skalkeas. 1991. Pharmacokinetics of arteether in dog. Eur. J. Drug Metab. Pharmacokinet. 16:325-328. [DOI] [PubMed] [Google Scholar]
- 5.Bethell, D. B., P. Teja-Isavadharm, X. T. Cao, T. T. Pham, T. T. Ta, T. N. Tran, T. T. Nguyen, T. P. Pham, D. E. Kyle, N. P. Day, and N. J. White. 1997. Pharmacokinetics of oral artesunate in children with moderately severe Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 91:195-198. [DOI] [PubMed] [Google Scholar]
- 6.Bilia, A. R., D. Lazari, L. Messori, V. Taglioli, C. Temperini, and F. F. Vincieri. 2002. Simple and rapid physico-chemical methods to examine action of antimalarial drugs with hemin: its application to Artemisia annua constituents. Life Sci. 70:769-778. [DOI] [PubMed] [Google Scholar]
- 7.Bland, J. M., and D. G. Altman. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307-310. [PubMed] [Google Scholar]
- 8.Chan, K. L., K. H. Yuen, S. Jinadasa, K. K. Peh, and W. T. Toh. 1997. A high-performance liquid chromatography analysis of plasma artemisinin using a glassy carbon electrode for reductive electrochemical detection. Planta Med. 63:66-69. [DOI] [PubMed] [Google Scholar]
- 9.Chimanuka, B., M. Gabriels, M. R. Detaevernier, and J. A. Plaizier-Vercammen. 2002. Preparation of beta-artemether liposomes, their HPLC-UV evaluation and relevance for clearing recrudescent parasitaemia in Plasmodium chabaudi malaria-infected mice. J. Pharm. Biomed. Anal. 28:13-22. [DOI] [PubMed] [Google Scholar]
- 10.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hein, T. T., and N. J. White. 1993. Qinghaosu. Lancet 341:603-608. [DOI] [PubMed] [Google Scholar]
- 12.Idowu, O. R., G. Edwards, S. A. Ward, M. L. Orme, and A. M. Breckenridge. 1989. Determination of arteether in blood plasma by high-performance liquid chromatography with ultraviolet detection after hydrolysis acid. J. Chromatogr. 493:125-136. [DOI] [PubMed] [Google Scholar]
- 13.Ilett, K. F., B. T. Ethell, J. L. Maggs, T. M. Davis, K. T. Batty, B. Burchell, T. Q. Binh, T. A. Thu le, N. C. Hung, M. Pirmohamed, B. K. Park, and G. Edwards. 2002. Glucuronidation of dihydroartemisinin in vivo and by human liver microsomes and expressed UDP-glucuronosyltransferases. Drug Metab. Dispos. 30:1005-1012. [DOI] [PubMed] [Google Scholar]
- 14.Ittarat, W., S. Looareesuwan, P. Pootrakul, P. Sumpunsirikul, P. Vattanavibool, and S. R. Meshnick. 1998. Effects of α-thalassemia on pharmacokinetics of the antimalarial agent artesunate. Antimicrob. Agents Chemother. 42:2332-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karbwang, J., K. Na-Bangchang, P. Molunto, V. Banmairuroi, and K. Congpuong. 1997. Determination of artemether and its major metabolite, dihydroartemisinin, in plasma using high-performance liquid chromatography with electrochemical detection. J. Chromatogr. B Biomed. Sci. Appl. 690:259-265. [DOI] [PubMed] [Google Scholar]
- 16.Kotecka, B. M., and K. H. Rieckmann. 1993. Chloroquine bioassay using malaria microcultures. Am. J. Trop. Med. Hyg. 49:460-464. [DOI] [PubMed] [Google Scholar]
- 17.Li, Q. G., J. O. Peggins, L. L. Fleckenstein, K. Masonic, M. H. Heiffer, and T. G. Brewer. 1998. The pharmacokinetics and bioavailability of dihydroartemisinin, arteether, artemether, artesunic acid and artelinic acid in rats. J. Pharm. Pharmacol. 50:173-182. [DOI] [PubMed] [Google Scholar]
- 18.Li, X., and K. Rieckmann. 1992. A bioassay for derivatives of qinghaosu (artemisinin). Trop. Med. Parasitol. 43:195-196. [PubMed] [Google Scholar]
- 19.Maggs, J. L., S. Madden, L. P. Bishop, P. M. O'Neill, and B. K. Park. 1997. The rat biliary metabolites of dihydroartemisinin, an antimalarial endoperoxide. Drug Metab. Dispos. 25:1200-1204. [PubMed] [Google Scholar]
- 20.Melendez, V., J. O. Peggins, T. G. Brewer, and A. D. Theoharides. 1991. Determination of the antimalarial arteether and its deethylated metabolite dihydroartemisinin in plasma by high-performance liquid chromatography with reductive electrochemical detection. J. Pharm. Sci. 80:132-138. [DOI] [PubMed] [Google Scholar]
- 21.Muhia, D. K., C. G. Thomas, S. A. Ward, G. Edwards, E. K. Mberu, and W. M. Watkins. 1994. Ferriprotoporphyrin catalysed decomposition of artemether: analytical and pharmacological implications. Biochem. Pharmacol. 48:889-895. [DOI] [PubMed] [Google Scholar]
- 22.Murphy, S. A., E. Mberu, D. Muhia, M. English, J. Crawley, C. Waruiru, B. Lowe, C. R. Newton, P. Winstanley, K. Marsh, and W. M. Watkins. 1997. The disposition of intramuscular artemether in children with cerebral malaria; a preliminary study. Trans. R. Soc. Trop. Med. Hyg. 91:331-334. [DOI] [PubMed] [Google Scholar]
- 23.Navaratnam, V., S. M. Mansor, L. K. Chin, M. N. Mordi, M. Asokan, and N. K. Nair. 1995. Determination of artemether and dihydroartemisinin in blood plasma by high-performance liquid chromatography for application in clinical pharmacological studies. J. Chromatogr. B Biomed. Appl. 669:289-294. [DOI] [PubMed] [Google Scholar]
- 24.Newton, P., Y. Suputtamongkol, P. Teja-Isavadharm, S. Pukrittayakamee, V. Navaratnam, I. Bates, and N. J. White. 2000. Antimalarial bioavailability and disposition of artesunate in acute falciparum malaria. Antimicrob. Agents Chemother. 44:972-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newton, P., M. Vugt, P. Teja-Isavadharm, D. Siriyanonda, M. Rasameesoraj, P. Teerapong, R. Ruangveerayuth, T. Slight, F. Nosten, Y. Suputtamongkol, S. Looareesuwan, and N. J. White. 2002. Comparison of oral artesunate and dihydroartemisinin antimalarial bioavailabilities in acute falciparum malaria. Antimicrob. Agents Chemother. 46:1125-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramu, K., and J. K. Baker. 1997. Identification of the glucuronides of the hydroxylated metabolites of the antimalarial arteether in rat plasma and urine by thermospray high-performance liquid chromatography/mass spectrometry. J. Pharm. Sci. 86:915-920. [DOI] [PubMed] [Google Scholar]
- 27.Sabarinath, S., M. Rajanikanth, K. P. Madhusudanan, and R. C. Gupta. 2003. A sensitive and selective liquid chromatographic/electrospray ionization tandem mass spectrometric assay for the simultaneous quantification of alpha-, beta-arteether and its metabolite dihydroartemisinin in plasma, useful for pharmacokinetic studies. J. Mass Spectrom. 38:732-742. [DOI] [PubMed] [Google Scholar]
- 28.Sandrenan, N., A. Sioufi, J. Godbillon, C. Netter, M. Donker, and C. van Valkenburg. 1997. Determination of artemether and its metabolite, dihydroartemisinin, in plasma by high-performance liquid chromatography and electrochemical detection in the reductive mode. J. Chromatogr. B Biomed. Sci. Appl. 691:145-153. [DOI] [PubMed] [Google Scholar]
- 29.Scott, H. V., M. D. Edstein, J. R. Veenendall, and K. H. Rieckmann. 1988. A sensitive bioassay for serum cycloguanil using Plasmodium falciparum in vitro. Int. J. Parasitol. 18:605-609. [DOI] [PubMed] [Google Scholar]
- 30.Sipahimalani, A. T., D. P. Fulzele, and M. R. Heble. 1991. Rapid method for the detection and determination of artemisinin by gas chromatography. J. Chromatogr. 538:452-455. [Google Scholar]
- 31.Souppart, C., N. Gauducheau, N. Sandrenan, and F. Richard. 2002. Development and validation of a high-performance liquid chromatography-mass spectrometry assay for the determination of artemether and its metabolite dihydroartemisinin in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 774:195-203. [DOI] [PubMed] [Google Scholar]
- 32.Suputtamongkol, Y., P. N. Newton, B. Angus, P. Teja-Isavadharm, D. Keeratithakul, M. Rasameesoraj, S. Pukrittayakamee, and N. J. White. 2001. A comparison of oral artesunate and artemether antimalarial bioactivities in acute falciparum malaria. Br. J. Clin. Pharmacol. 52:655-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor, R. B., M. I. Awad, R. Reid, and R. R. Moody. 2000. Determination of sodium artesunate in plasma using ion-pairing high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 744:415-421. [DOI] [PubMed] [Google Scholar]
- 34.Teja-Isavadharm, P., F. Nosten, D. E. Kyle, C. Luxemburger, F. ter Kuile, J. O. Peggins, T. G. Brewer, and N. J. White. 1996. Comparative bioavailability of oral, rectal and intramuscular artemether in healthy subjects—use of simultaneous measurement by high performance liquid chromatography and bioassay. Br. J. Clin. Pharmacol. 42:599-604. [DOI] [PubMed] [Google Scholar]
- 35.Teja-Isavadharm, P., G. Watt, C. Eamsila, K. Jongsakul, Q. Li, D. Keeratithakul, N. Sirisopana, L. Loesuttiviboon, T. G. Brewer, and D. E. Kyle. 2001. Comparative pharmacokinetic-effect kinetics of oral sodium artesunate in healthy volunteers and patients with uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 65:717-721. [DOI] [PubMed] [Google Scholar]
- 36.Theoharides, A. D., M. H. Smyth, R. W. Ashmore, J. M. Halverson, Z. M. Zhou, W. E. Ridder, and A. J. Lin. 1988. Determination of dihydroqinghaosu in blood by pyrolysis gas chromatography/mass spectrometry. Anal. Chem. 60:115-130. [DOI] [PubMed] [Google Scholar]
- 37.Thomas, C. G., S. A. Ward, and G. Edwards. 1992. Selective determination, in plasma, of artemether and its major metabolite, dihydroartemisinin, by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. 583:131-136. [DOI] [PubMed] [Google Scholar]
- 38.Titulaer, H. A. C., J. Zuidema, P. A. Kager, J. C. F. M. Wetstyn, C. B. Lugt, and F. W. H. M. Merkus. 1990. The pharmacokinetics of artemisinin after oral, intramuscular and rectal administration to human volunteers. J. Pharm. Pharmacol. 42:810-813. [DOI] [PubMed] [Google Scholar]
- 39.Traore, B., E. Lazaro, and F. Gay. 1997. A bioassay for evaluating antimalarial activity and for measuring concentration in plasma. Trop. Med. Int. Health 2:929-933. [DOI] [PubMed] [Google Scholar]
- 40.van Agtmael, M. A., J. J Butter, E. J. Portier, and C. J. van Boxtel. 1998. Validation of an improved reversed-phase high-performance liquid chromatography assay with reductive electrochemical detection for the determination of artemisinin derivatives in man. Ther. Drug Monit. 20:109-116. [DOI] [PubMed] [Google Scholar]
- 41.Webster, H. K., E. F. Boudreau, K. Pavanand, K. Yongvanitchit, and L. W. Pang. 1985. Antimalarial drug susceptiblity testing of Plasmodium falciparum in Thailand using a microdilution radioisotope method. Am. J. Trop. Med. Hyg. 34:228-235. [DOI] [PubMed] [Google Scholar]
- 42.White, N. J. 1993. Clinical pharmacokinetics and pharmacodynamics of artemisinin and derivatives. Trans. R. Soc. Trop. Med. Hyg. 88(Suppl. 1):S41-S43. [DOI] [PubMed] [Google Scholar]
- 43.Yang, S. D., J. M. Ma, J. H. Sun, and Z. Y. Song. 1985. Determination of artesunate and dihydroqinghaosu in human plasma by high performance liquid chromatography with a reductive mode electrochemical detection. Yao Xue Xue Bao 20:457-462. [PubMed] [Google Scholar]
- 44.Yeo, A. E., and K. H. Rieckman. 1992. A bioassay for mefloquine. J. Parasitol. 78:1096-1097. [PubMed] [Google Scholar]
- 45.Zhou, Z. M., J. C. Anders, H. Chung, and A. D. Theoharides. 1987. Analysis of artesunic acid and dihydroqinghaosu in blood by high-performance liquid chromatography with reductive electrochemical detection. J. Chromatogr. 414:77-90. [DOI] [PubMed] [Google Scholar]