Abstract
Organophosphates (OP) inhibit acetylcholinesterase (AChE, E.C.3.1.1.7), both in peripheral tissues and central nervous system (CNS), causing adverse and sometimes fatal effects due to the accumulation of neurotransmitter acetylcholine (ACh). The currently used therapy, focusing on the reactivation of inhibited AChE, is limited to peripheral tissues because commonly used quaternary pyridinium oxime reactivators do not cross the blood brain barrier (BBB) at therapeutically relevant levels. A directed library of thirty uncharged oximes that contain tertiary amine or imidazole protonable functional groups that should cross the BBB as unionized species was tested as tabun-hAChE conjugate reactivators along with three reference oximes: DAM (diacetylmonoxime), MINA (monoisonitrosoacetone), and 2-PAM. The oxime RS150D [N-((1-(3-(2-((hydroxyimino)methyl)-1H-imidazol-1-yl)propyl)-1H-1,2,3-triazol-4-yl)methyl)benzamide] was highlighted as the most promising reactivator of the tabun-hAChE conjugate. We also observed that oximes RS194B [N-(2-(azepan-1-yl)ethyl)-2-(hydroxyimino)acetamide] and RS41A [2-(hydroxyimino)-N-(2-(pyrrolidin-1-yl)ethyl)acetamide], which emerged as lead uncharged reactivators of phosphylated hAChE with other OPs (sarin, cyclosarin and VX), exhibited only moderate reactivation potency for tabun inhibited hAChE. This implies that geometry of oxime access to the phosphorus atom conjugated to the active serine is an important criterion for efficient reactivation, along with the chemical nature of the conjugated moiety: phosphorate, phosphonate, or phosphoramidate. Moreover, modification of the active center through mutagenesis enhances the rates of reactivation. The phosphoramidated-hAChE choline-binding site mutant Y337A showed three-times enhanced reactivation capacity with non-triazole imidazole containing aldoximes (RS113B, RS113A and RS115A) and acetamide derivative (RS194B) than with 2PAM.
Keywords: Oxime reactivation, organophosphate intoxication, CNS AChE reactivation, hydroxyiminoacetamides, butyrylcholinesterase, tabun
1. Introduction
Organophosphate (OP), nerve agents are small lipophilic organic molecules that readily diffuse through biological membranes and quickly reach CNS. OPs inhibit both peripheral and CNS acetylcholinesterase (AChE, E.C.3.1.1.7) causing adverse effects due to the accumulation of neurotransmitter acetylcholine (ACh), which can eventually lead to death. Currently employed antidotal therapy, focused on the reactivation of inhibited AChE, is limited to the peripheral circulation because commonly used quaternary pyridinium aldoxime reactivators cannot cross the blood brain barrier (BBB) at therapeutically relevant levels due to their positive charge. We recently designed a library of more than hundred uncharged oximes that contain a tertiary amine or imidazole protonable functional group where a significant fraction of species are non-ionized at physiological pH and could cross the BBB. N-substituted 2-hydroxyimino acetamidoalkyl amines, though devoid of a permanent cationic charge, can efficiently reactivate the OP conjugated hAChE in vitro [1]. Ionization equilibria around two ionizable groups, an oxime and an amine group results in coexistence of a cationic, zwitterionic, uncharged and anionic reactivator species around physiological pH values. While zwitterionic and cationic species have the best chance of productive interaction with OP-hAChE conjugates, the uncharged species can be expected to cross the BBB delivering reactivator into CNS.
Among 135 new oximes, R194B [N-(2-(azepan-1-yl)ethyl)-2-(hydroxyimino)acetamide] proved to be efficient reactivator upon VX, sarin, and cyclosarin exposure [1,2]. Paraoxon- and tabun-derived conjugates with larger substituents occupying the OP-hAChE acyl pocket had different structural requirements and proved to be more refractory to reactivation [1]. Herein, we tested 29 uncharged oximes following tabun exposure along with three reference oximes: DAM (diacetylmonoxime), MINA (monoisonitrosoacetone), and 2-PAM. In addition, comparative rates of reaction were investigated on phosphylated hBChE and two hAChE mutants.
2. Material and Methods
2.1. Chemicals
Design, synthesis, and initial functional characterization of novel oxime reactivators were reported recently [1,2]. 2PAM (2-Pyridinealdoxime methiodide), MINA (monoisonitrosoacetone), and DAM (2,3-butanedione monoxime), acetylthiocholine iodide (ATCh) and 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) were purchased from Sigma-Aldrich. Tabun [ethyl N,N-dimethylphosphoroamidocyanidate] was purchased from the NC Laboratory, Spiez, Switzerland.
2.2. Enzyme
Highly purified monomeric hAChE was prepared as described earlier [1,3]. Purified oligomeric hBChE isolated from human plasma was kindly donated by Drs. Douglas Cerasoli and David Lenz, USAMRICD, Edgewood, MD.
2.3. Oxime reactivation assays
Phosphoroamidated enzyme conjugates were prepared by incubating (0.1 to 1.0 μM enzyme with 5 μM tabun for 30-45 minutes until inhibition exceeded 95%. Such inhibited enzyme was passed through a Sephadex G-50 spin column (Roche Diagnostic GmbH, Mannheim, Germany) to remove excess of unconjugated tabun. After filtration enzyme was incubated with oxime (0.05 – 10.0 mM) in 0.1 mM sodium phosphate buffer pH 7.4 containing 0.01% BSA at 37 °C. At specified time intervals, 10 μl aliquots were diluted 100-fold in phosphate buffer containing DTNB. Upon addition of ATCh residual enzyme activity was measured by the Ellman method [4]. Equivalent samples of uninhibited enzyme were passed through a parallel column, diluted to the same extent as the inhibition mixture, and control activity was measured in the presence of oxime at concentrations used for reactivation. Activities of control and reactivation mixture were corrected for oxime-induced hydrolysis of ATCh. Kinetic parameters of reactivation, constants k+2 (maximal first order reactivation rate constant), KOX (the apparent phosphylated enzyme-oxime dissociation constant) and kr (second-order rate constant of reactivation), were calculated from experimental data obtained in at least three separate experiments [5].
3. Results and Discussion
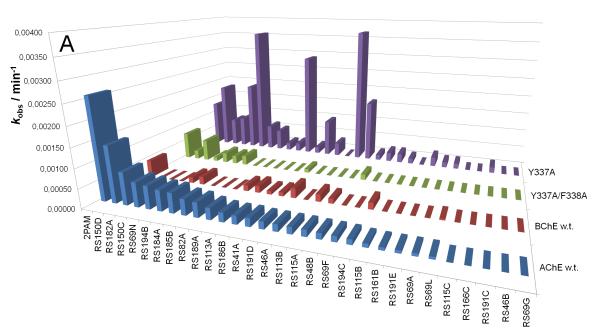
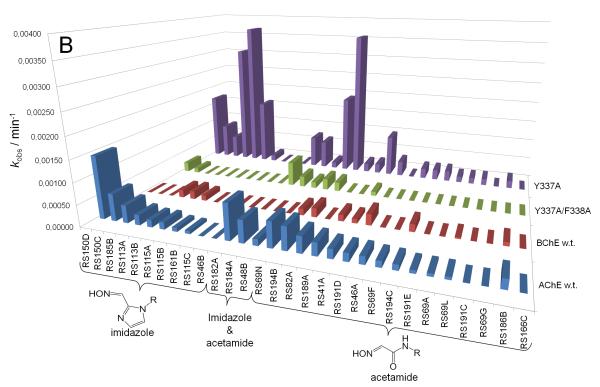
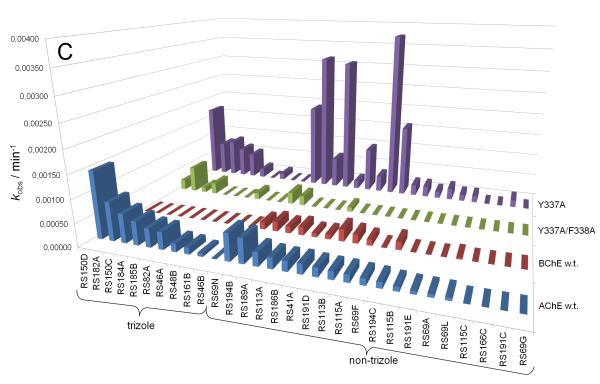
Our recent characterization of a library of 135 uncharged, structurally diverse oximes revealed hydroxyimino acetamido alkyl amines as efficient reactivators of OP-hAChE conjugates [1,2]. Although varied oxime structures seemed to affect similarly reactivation of methyl phosphonyl OP-hAChE conjugates (derived from sarin, cyclosarin and VX), tabun-derived conjugates proved to be resistant to reactivation. Indeed, out of 29 new RS oximes (Fig. 1), three best reactivating oximes were predominately imidazole-containing aldoximes (for hAChE) and hydroxyimino acetamido amine derivatives (for hBChE) (Fig. 2). These structural features of the reactivator differ from previous studies with the methylphosphonate OPs where the opposite structure/activity relationship was observed [1]. Only RS191D, RS194B and RS194C reactivated tabun-hBChE conjugate appreciably with a maximal degree of reactivation of 30% showing that larger moieties like azepane (RS194B and RS194C) or tetrahydroisoquinoline (RS191D) are preferable structural motives for binding to hBChE. The phosphoramidated-hAChE choline-binding site mutant Y337A showed three-times enhanced reactivation capacity with non-triazole imidazole containing aldoximes (RS113B, RS113A and RS115A) and acetamide derivative (RS194B) than with 2PAM. Bis-oxime RS182A reactivated the double mutant Y337A/F338A conjugate up to 50% with a rate similar to 2PAM.
Fig. 1.
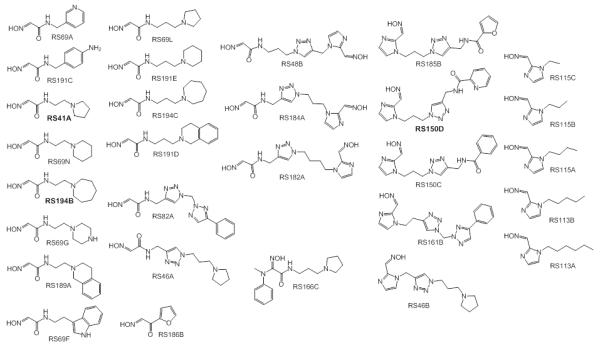
Uncharged oximes that contain tertiary amine or imidazole protonable functional groups.
Fig. 2.
Reactivation rates (kobs) of tabun-inhibited hAChE, hBChE and two hAChE mutants by 1 mM oximes recorded within 22 h. Oximes are ordered by their reactivity to hAChE (A) and segregated by their general structure as imidazole, acetamide, and other oximes (B) or triazole ring in oxime structure (C).
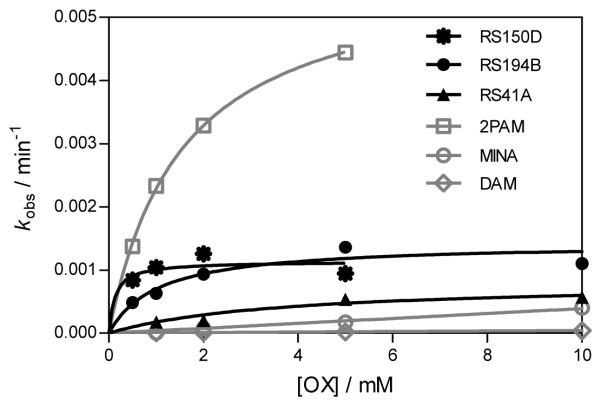
Reactivation rates for the tabun-hAChE conjugates by the best, overall reactivator, RS150D, and other leads, RS194B and RS41A, was additionally measured over a range of oxime concentrations, from 0.05 mM up to 10 mM, and compared to uncharged (DAM and MINA) and charged (2PAM) reference compounds (Fig. 3). While reactivation by MINA and in particular DAM was very slow, RS150D and RS194B displayed more rapid reactivation over the entire measured concentration range, but with no rate enhancement when compared to the quaternary cationic 2PAM. This suggests that the maximal reactivation rate constant for 2PAM is generally superior to other oximes, while its binding to covalent tabun-hAChE conjugates (reflected in the constant KOX) is comparable to that of RS194B (Table 1). Large KOX constants (i.e. low affinity) for uncharged aldoximes are consistent with AChE having preference for binding cationic ligands, even in the OP conjugated state [5]. One third of the oximes contained the triazole ring (as RS150D) showing that this heterocycle positioned some distance from the oxime group enhances tabun-hAChE conjugate reactivation (Fig. 2c). The triazole moiety was earlier observed to contribute synergistically to the total binding energy of high affinity AChE inhibitors [6]. All three RS oximes possess the oxime group positioned farther from the part of the molecule largely responsible for molecular recognition. This relationship is in accordance with our previous study conducted with VX and other OPs [1]. Optimal separation equivalent to a five- or six-atom linker is consistent with structures of all five best uncharged reactivators of tabun-hAChE conjugate. Maximum of reactivation reached 70% suggesting that reactions such as aging and reinhibition with phosphylated oxime could have competed with reactivation. However, owing primarily to its relatively moderate affinity, RS150D was singled out as the best hAChE reactivator among these uncharged aldoximes. Interestingly it is consistent with large percentage of oximate anion calculated for RS150D at pH 7.4 (58%) vs. RS194B (0.25%) or RS41A (0.21%) [1,2]. Although several classes of novel compounds have been suggested as promising centrally [7] or primarily peripherally [8,9] acting reactivators of tabun-hAChE conjugate, therapy following tabun exposure presents unique features for finding reactivators with enhanced reactivity and favorable pharmacokinetic properties.
Fig. 3.
Concentration dependence of oxime reactivation of tabun-inhibited hAChE.
Table 1.
Detailed kinetic analysis of tabun-hAChE conjugate reactivation by varying concentrations of oximes.a
| Oxime | k+2 / min−1 | KOX / mM | kr / M−1min−1 | React.max / % |
|---|---|---|---|---|
| RS194B | 0.0018 ± 0.0002 | 1.8 ± 0.5 | 1.00 ± 0.29 | 70 |
| RS150D | 0.0015 ± 0.0001 | 0.41 ± 0.12 | 3.7 ± 1.2 | 70 |
| RS41A | 0.00082 ± 0.0001 | 3.6 ± 1.5 | 0.22 ± 0.10 | 40 |
| 2PAM | 0.0058 ± 0.0006 | 1.5 ± 0.4 | 3.8 ± 1.1 | 70 |
| DAM* | - | - | 0.0042 ± 0.0003 | <10 |
| MINA* | - | - | 0.039 ± 0.008 | 25 |
k+2 - the maximal first order reactivation rate constant, KOX - the apparent phosphylated enzyme-oxime dissociation constant, kr - the second-order rate constant of reactivation, React.max - the maximal percentage of reactivation
4. Conclusion
Oxime RS150D was highlighted as the most promising uncharged reactivator of the tabun-hAChE conjugate, although still falls short of bispyridinium aldoximes [8,9]. We also observed that oximes RS194B and RS41A, which emerged as potential reactivators of hAChE inhibited with other methyl phosphonate OPs (sarin, cyclosarin and VX)[1], did not show such high reactivation potency for tabun inhibited hAChE. This is due to larger substituents on the phosphorus that occupy the tabun-hAChE acyl pocket in phosphorylated conjugates [5]. A limiting potency to reactivate tabun-enzyme conjugates proves that oxime access to phosphorus atom within the AChE active center gorge is an important criterion for efficient reactivation as is the organophosphorus structure itself. Although the enhancement achieved by mutation Y337A is too low for an effective catalytic scavenger [10] optimized for tabun, it confirms the importance of examining mutants for enhancing catalysis in plasma.
Highlights.
library of 30 uncharged oximes was tested as reactivators tabun-inhibited ChE
imidazole-containing oximes preferred tabun-hAChE conjugate
hydroxyimino acetamido amine derivatives preferred tabun-hBChE conjugate
3-times faster mutant Y337A reactivation with nontriazole oximes than with 2PAM
Acknowledgement
This research was supported by the CounterACT Program, National Institutes of Health, Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke (NINDS), Grant Number U01 NS058046.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest. None.
References
- [1].Sit RK, Radić Z, Gerardi V, Zhang L, Garcia E, Katalinić M, Amitai G, Kovarik Z, Fokin VV, Sharpless KB, Taylor P. New structural scaffolds for centrally acting oxime reactivators of phosphylated cholinesterases. J. Biol. Chem. 2011;286:19422–19430. doi: 10.1074/jbc.M111.230656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Radić Z, Sit RK, Kovarik Z, Berend S, Garcia E, Zhang L, Amitai G, Green C, Radić B, Fokin VV, Sharpless KB, Taylor P. Refinement of structural leads for centrally acting oxime reactivators of phosphylated cholinesterases. J. Biol. Chem. 2012;287:11798–11809. doi: 10.1074/jbc.M111.333732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cochran R, Kalisiak J, Küçükkilinç T, Radic Z, Garcia E, Zhang L, Ho KY, Amitai G, Kovarik Z, Fokin VV, Sharpless KB, Taylor P. Oxime-assisted acetylcholinesterase catalytic scavengers of organophosphates that resist aging. J. Biol. Chem. 2011;286:29718–29724. doi: 10.1074/jbc.M111.264739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ellman GL, Courtney KD, Andres V, Jr., Featherstone RM. New and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- [5].Kovarik Z, Radić Z, Berman HA, Simeon-Rudolf V, Reiner E, Taylor P. Mutant cholinesterases possessing enhanced capacity for reactivation of their phosphonylated conjugates. Biochemistry. 2004;43:3222–3229. doi: 10.1021/bi036191a. [DOI] [PubMed] [Google Scholar]
- [6].Manetsch R, Krasiński A, Radić Z, Raushel J, Taylor P, Sharpless KB, Kolb HC. In situ click chemistry: enzyme inhibitors made to their own specifications. J. Am. Chem. Soc. 2004;126:12809–12818. doi: 10.1021/ja046382g. [DOI] [PubMed] [Google Scholar]
- [7].Mercey G, Verdelet T, Saint-André G, Gillon E, Wagner A, Baati R, Jean L, Nachon F, Renard PY. First efficient uncharged reactivators for the dephosphylation of poisoned human acetylcholinesterase. Chem Commun (Camb) 2011;47:5295–5297. doi: 10.1039/c1cc10787a. [DOI] [PubMed] [Google Scholar]
- [8].Kovarik Z, Lucić Vrdoljak A, Berend S, Čalić M, Kuča K, Musilek K, Radić B. Evaluation of oxime K203 as antidote in tabun poisoning. Arh. Hig. Rada Toksikol. 2009;60:19–26. doi: 10.2478/10004-1254-60-2009-1890. [DOI] [PubMed] [Google Scholar]
- [9].Worek F, von der Wellen J, Musilek K, Kuca K, Thiermann H. Reactivation kinetics of a homologous series of bispyridinium bis-oximes with nerve agent-inhibited human acetylcholinesterase. Arch. Toxicol. 2012;86:1379–1386. doi: 10.1007/s00204-012-0842-2. [DOI] [PubMed] [Google Scholar]
- [10].Kovarik Z, Radić Z, Berman HA, Taylor P. Mutation of acetylcholinesterase to enhance oxime-assisted catalytic turnover of methylphosphonates. Toxicology. 2007;233:79–84. doi: 10.1016/j.tox.2006.08.032. [DOI] [PubMed] [Google Scholar]