Abstract
CIB1 is a 22-kDa regulatory protein previously implicated in cell survival and proliferation. However, the mechanism by which CIB1 regulates these processes is poorly defined. Here we report that CIB1 depletion in SK-N-SH neuroblastoma and MDA-MB-468 breast cancer cells promotes non-apoptotic, caspase-independent cell death that is not initiated by increased outer mitochondrial membrane permeability or translocation of apoptosis-inducing factor to the nucleus. Instead, cell death requires nuclear GAPDH accumulation. Furthermore, CIB1 depletion disrupts two commonly dysregulated, oncogenic pathways– PI3K/AKT and Ras/MEK/ERK, resulting in a synergistic mechanism of cell death, which was mimicked by simultaneous pharmacological inhibition of both pathways, but not either pathway alone. In defining each pathway’s contributions, we found that AKT inhibition alone maximally induced GAPDH nuclear accumulation, whereas MEK/ERK inhibition alone had no effect on GAPDH localization. Concurrent GAPDH nuclear accumulation and ERK inhibition were required however, to induce a significant DNA damage response, which was critical to subsequent cell death. Collectively, our results indicate that CIB1 is uniquely positioned to regulate PI3K/AKT and MEK/ERK signaling and that simultaneous disruption of these pathways synergistically induces a nuclear GAPDH-dependent cell death. The mechanistic insights into cell death induced by CIB1 interference suggest novel molecular targets for cancer therapy.
Keywords: cell death, CIB1, GAPDH, AKT, ERK
INTRODUCTION
Cancerous cells develop resistance to apoptotic stimuli and drug therapy through a number of dysregulated cell survival pathways. Two frequently mutated and persistently activated oncogenic pathways are PI3K/AKT and Ras/RAF/MEK/ERK (1). Other intersecting aberrantly activated pathways include PAK1, which contributes to MEK1, ERK (2) and AKT activation (3), further promoting cell survival.
Concurrent activation and crosstalk between the PI3K/AKT and MEK/ERK pathways may contribute to drug resistance by converging on common downstream targets (1, 4). For instance, both AKT and ERK phosphorylate and inactivate the proapoptotic protein BAD (5, 6) and the translational repressor 4E-BP1 (7). It is therefore becoming increasingly evident that simultaneous targeting of multiple convergent signaling pathways is more effective in promoting tumor cell death than targeting a single pathway (8).
Here, we report that CIB1 depletion disrupts tumor cell proliferation and promotes non-apoptotic cell death by interference with AKT and ERK signaling. CIB1 is a 22-kDa EF-hand calcium-binding protein, homologous to calmodulin (9, 10). CIB1 interacts with and regulates proteins involved in tumor cell survival and proliferation, including p21-activated kinase 1 (PAK1) (11) and apoptosis signal-regulating kinase 1 (ASK1) (12). CIB1−/− mice show compromised tumor growth, most likely due to deficient tumor-induced angiogenesis (13) and primary cells derived from CIB1−/− mice exhibit delayed proliferation (14, 15), emphasizing the role of CIB1 in cell survival and proliferation. However, the precise molecular mechanism by which CIB1 promotes these processes is not well defined.
Interestingly, we find that the marked cell death occurring upon CIB1 depletion is independent of caspase activation and mitochondrial permeabilization. Instead, cell death is caused by a loss of AKT signaling, facilitating GAPDH nuclear accumulation, which synergizes with loss of ERK signaling. While AKT and ERK inhibition alone minimally induce cell death, concurrent pharmacologic inhibition recapitulates the extensive non-apoptotic cell death, induction of DNA damage and cell cycle disruption that we observed with CIB1 depletion. Importantly, overexpression of GAPDH mutants that prevent post-translational modification of GAPDH rescues CIB1-depleted cells from DNA damage and cell death. In conclusion, these results identify previously unrecognized links between CIB1, the ERK and AKT pathways, and GAPDH in non-apoptotic tumor cell death and provide strategic opportunities to impair tumor cell survival.
RESULTS
CIB1 depletion induces cell death and inhibits cell proliferation
CIB1 regulates PAK1 activation and downstream signaling to ERK both in vitro and in vivo (11, 15). Because ERK is frequently hyperactivated in many cancers, we hypothesized that CIB1 may regulate tumor cell survival and proliferation. To test this, we silenced CIB1 expression in two distinct tumor cell models, the SK-N-SH neuroblastoma (MYCN-non-amplified, wild-type p53 and Rb) and the triple-negative MDA-MB-468 (MDA-468) breast epithelial cancer cell line (also mutant p53, PTEN and Rb negative). Endogenous CIB1 expression levels were consistently reduced by at least 80–90% in both tumor cell lines (Figure 1a and supplemental S1a).
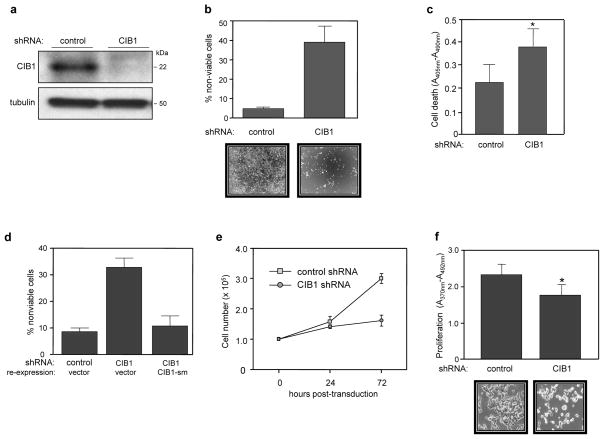
Figure 1. CIB1 depletion induces cell death and slows proliferation.
(a) Immunoblot showing efficient shRNA-induced CIB1 depletion from SK-N-SH cells. (b) Increased cell death in CIB1-depleted SK-N-SH cells as determined by trypan blue dye exclusion. Results are expressed as the mean percentage of dead cells (i.e., trypan blue positive cells) from both adherent and floating cell populations, data represent means +/− SEM, n=5. Representative phase contrast images show significant loss of adherent CIB1-depleted cells (bottom panels). (c) Analysis of control and CIB1-depleted SK-N-SH cells by immunochemical ELISA assays that detect histone-complexed DNA fragments as a marker of cell death (*p<0.05). (d) Ectopic expression of a shRNA-resistant CIB1 silent mutant (CIB1-sm) in CIB1-depleted SK-N-SH cells prevents cell death. Cell death was quantified as in (a), n=4. (e) Cell proliferation over 72 h, quantifed as total cell numbers at the indicated times post-transduction, n=2. (f) CIB1 depleted SK-N-SH cells show decreased cell proliferation as determined by BrdU proliferation assays (*p<0.05). Representative phase contrast images show significant loss of cell number from wells containing CIB1-depleted cells (bottom panels).
Microscopic analysis revealed that a marked proportion of CIB1-depleted cells became detached or rounded within 48–72 h post-lentiviral transduction suggesting increased cell death (Figure 1b, bottom panels). Trypan blue staining of adherent and floating cell populations indicated a marked ~6-fold increase in cell death in both CIB1-depleted SK-N-SH (Figure 1b) and MDA-MB-468 cells (supplemental figure S1c). Moreover, >95% of the detached CIB1-depleted cells were nonviable as detected by trypan blue uptake. Nucleosome formation was also significantly increased in CIB1-depleted SK-N-SH cells (Figure 1c), suggesting that CIB1 depletion may induce DNA fragmentation. Ectopic reexpression of a CIB1 silent mutant (CIB1-sm) resistant to shRNA knockdown prevented cell death induced by CIB1 shRNA in both SK-N-SH (Figure 1d) and MDA-468 cells (supplemental figure S1d), demonstrating that CIB1 depletion specifically induces cell death.
Cell counts of CIB1-depleted SK-N-SH and MDA-468 cells indicated a significant 1.9-and 1.6-fold decrease in proliferation rates, respectively (Figure 1e and supplemental figure S1e), which was also confirmed by bromodeoxyuridine (BrdU) ELISA analysis (Figure 1f). These results indicate that CIB1 depletion disrupted cell proliferation in two separate tumor cell types.
CIB1 depletion results in caspase-independent cell death
To determine the mechanism of cell death, we first investigated the status of caspase activation. In the absence of the apoptotic agent staurosporine (STS), no evidence of caspase-7 or -9 cleavage or cleavage of the caspase-3 and -6 substrates, poly-ADP ribose polymerase (PARP) and lamin A/C, respectively, was observed in either control or CIB1-depleted SK-N-SH cells (Figure 2a), demonstrating that key caspases were not activated simply by the absence of endogenous CIB1. In contrast, STS induced a dose-dependent increase in the activation of these caspases in both control and CIB1-depleted SK-N-SH cells that was not further enhanced by CIB1 depletion (Figure 2a). Identical results were obtained with increasing concentrations of another apoptotic agent, the topoisomerase II inhibitor, etoposide (data not shown). Taken together, these results indicate that, while the apoptotic pathway is intact in these cells, CIB1 depletion does not induce cell death by triggering or enhancing caspase-dependent apoptotic cell death.
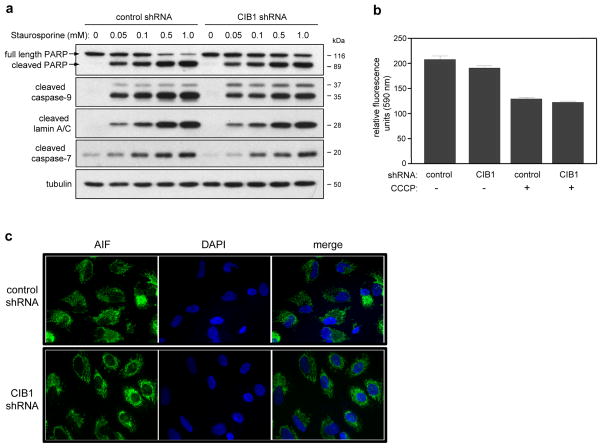
Figure 2. Effect of CIB1 depletion on caspase activation and mitochondrial function.
(a) Control and CIB1 depleted SK-N-SH cells were incubated in the absence (0) and presence of increasing concentrations (0.05–1.0 mM) of staurosporine (STS); whole cell lysates were immunoblotted using the indicated antibodies, n=3. (b) CIB1-depletion does not affect mitochondrial membrane potential (Δψm). The mitochondrial dye JC-10 was used to quantify Δψm in adherent control and CIB1-depleted cells. In non-compromised mitochondria, JC-10 accumulates as J-aggregates which fluoresce at 590 nm (data expressed in relative fluorescence units). As positive control, cells were treated with 4 μM of the mitochondrial depolarizing agent carbonyl cyanide 3-chlorophenylhydrazone (CCCP), resulting in the release of JC-10 monomers, as indicated by decreased fluorescence at 590 nm. Data represent means +/− SEM, n=2. (c) Control and CIB1-depleted SK-N-SH cells were immunostained with an anti-AIF antibody (green fluorescence) and nuclei were visualized by counterstaining with DAPI (blue fluorescence). Images are representative of at least 3 separate experiments. Scale bar = 20 μm
CIB1 depletion does not affect mitochondrial function
DNA fragmentation, mitochondrial outer membrane permeability and cell death can occur independently of caspase activation (16). Therefore, mitochondrial membrane potential (Δψm) was analyzed using the membrane permeable fluorescent cationic dye, JC-10, which accumulates in non-compromised mitochondria and is released into the cytosol as monomers upon mitochondrial disruption. Because no overall difference in JC-10 fluorescence was observed between adherent CIB1-depleted and control shRNA cells (Figure 2b), these results suggest that mitochondrial dysfunction does not precede the onset of CIB1-depletion-induced cell death. As a positive control, the mitochondrial depolarization agent, carbonyl cyanide 3-chlorophenylhydrazone (CCCP), did decrease the fluorescent emission signals in both control and CIB1-depleted SK-N-SH (Figure 2b) and MDA-468 cells (supplementary figure S2a), consistent with a collapse of mitochondrial membrane potential and increased mitochondrial permeabilization.
Caspase-independent cell death can also be initiated by translocation of proteins such as apoptosis-inducing factor (AIF) from the mitochondrial intermembrane to the nucleus (17). Here we found that AIF localized mainly to mitochondrial structures in both control and CIB1-depleted SK-N-SH cells (Figure 2c), further confirming that CIB1 depletion does not initiate cell death by affecting mitochondrial membrane integrity or release of apoptotic proteins.
Because CIB1 depletion can enhance ASK1/JNK-mediated activation and apoptosis in response to oxidative stress in 293T and HeLa cells (12), we examined JNK activation as a mechanism of cell death. The JNK inhibitor SP00125, however, had no effect on CIB1-depleted SK-N-SH or MDA-468 cell death (supplementary figures S2b and S2c, respectively), suggesting that JNK signaling does not contribute significantly to cell death in CIB1-depleted SK-N-SH and MDA-MB-468 cells.
In certain cellular contexts, the degradative process of autophagy can serve as an alternative cell death mechanism. However, no difference in levels of the well-established autophagic marker LC3-II was observed between control and CIB1-depleted cells (data not shown), suggesting that autophagy was not induced.
CIB1-depletion induces GAPDH nuclear accumulation and GAPDH-dependent cell death
Because nuclear translocation and accumulation of the glycolytic enzyme GAPDH contributes to neuronal and non-neuronal cell death (18), we examined GAPDH localization in CIB1-depleted cells. Subcellular analysis of cytoplasmic and nuclear fractions revealed a significant accumulation of GAPDH in the nuclear fraction of both CIB1-depleted SK-N-SH cells (Figure 3a) and MDA-468 cells (supplementary figure S3a), indicating that CIB1 depletion induces GAPDH nuclear accumulation.
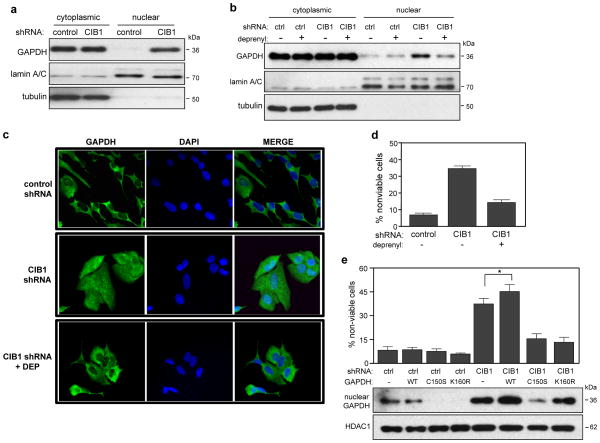
Figure 3. Cell death induced by CIB1 depletion requires GAPDH nuclear accumulation and GAPDH S-nitrosylation and acetylation.
(a) Western blot analysis of cytoplasmic and nuclear fractions shows increased nuclear GAPDH in CIB1-depleted SK-N-SH cells. αTubulin and lamin A/C antibodies were used to confirm cytoplasmic and nuclear fractions, respectively. (b) Deprenyl blocks nuclear accumulation of GAPDH. Control (ctrl) and CIB1-depleted cells were treated with or without 25 nM deprenyl (DEP) and analyzed as in (a), n=4. (c) Immunohistochemical analysis of GAPDH localization. Control and CIB1-depleted cells were treated with and without deprenyl as in (b) and immunostained with anti-GAPDH (green) and nuclei were visualized with DAPI (blue). Bar = 20 μm. (d) Deprenyl protects CIB1-depleted cells from cell death. Cells were treated with or without DEP as in (b). Cell death was determined as in Figure 1b. Data represent means ± SEM, n=8. (e) Cell death in control and CIB1-depleted cells expressing empty vector, HA-GAPDH-WT, -C150S or -K160R was quantified as in Figure 1b. Data represent means +/− SEM, n=4 (t-test; * p<0.05 versus CIB1 shRNA). Effect of GAPDH-WT, -C150, or -K160R expression on GAPDH nuclear accumulation. Nuclear lysates were immunoblotted with anti-GAPDH or -histone deacetylase-1 (HDAC1) antibodies, lower panels.
We next asked if increased nuclear accumulation of GAPDH per se contributes to the cell death observed in CIB1-depleted cells. The monoamine oxidase-B (MAO-B) inhibitor, R(−)-Deprenyl (deprenyl) binds directly to GAPDH, inhibits its nuclear translocation, and has been used by others to link GAPDH nuclear translocation to cell death (19). Deprenyl treatment markedly reduced GAPDH nuclear accumulation in both CIB1-depleted SK-N-SH (Figure 3b) and MDA-468 cells (supplementary figure S3b). Immunocytochemical staining also confirmed the predominantly cytoplasmic GAPDH localization in control cells and increased nuclear GAPDH localization in CIB1-depleted cells that was blocked by deprenyl (Figure 3c).
If nuclear GAPDH accumulation is the primary cause of death in CIB1-depleted cells, deprenyl treatment, by preventing nuclear GAPDH accumulation, should prevent cell death. Deprenyl treatment did reduce cell death in both CIB1-depleted SK-N-SH (Figure 3d) and MDA-468 cells (supplementary figure S3b), providing strong evidence that GAPDH nuclear accumulation promotes cell death.
S-nitrosylation of cysteine 150 (Cys150) within the GAPDH active site is known to facilitate GAPDH nuclear translocation and subsequent cell death (20). Since GAPDH S-nitrosylation and nuclear translocation can be blocked by deprenyl or a GAPDH mutant resistant to S-nitrosylation at Cys150 (GAPDH-C150S) (20, 21), we predicted that GAPDH-C150S should prevent CIB1-depletion-induced cell death. Indeed, overexpression of GAPDH-C150S but not WT-GAPDH in both CIB1-depleted SK-N-SH (Figure 3e) and MDA-468 cells (supplementary figure S3c) blocked CIB1-depletion induced cell death and GAPDH nuclear accumulation. This result implies a role for GAPDH S-nitrosylation and additionally supports a role for GAPDH nuclear accumulation in cell death.
Under stress conditions, nuclear GAPDH also becomes acetylated at lysine 160 (K160) by the acetyltransferase p300/CBP (22). Acetylated GAPDH in turn, binds to p300/CBP to stimulate p300/CBP acetylation of nuclear proteins, triggering their degradation (20, 22). The GAPDH acetylation-resistant mutant, GAPDH-K160R, functions in a dominant-negative manner by blocking endogenous GAPDH acetylation and cell death (22). To test whether GAPDH acetylation is involved in the cell death mechanism in CIB1-depleted cells, GAPDH-K160R was overexpressed and cell viability assessed as above. Notably, a significant decrease in cell death was observed in CIB1-depleted SK-N-SH (Figure 3e) and MDA-468 cells (supplementary figure S3c) that expressed this mutant, strongly implicating a role for GAPDH acetylation in mediating cell death in CIB1-depleted cells. Consistent with previous reports that the K160R mutation does not affect GAPDH nuclear translocation (22), we still observed significant nuclear GAPDH in CIB1-depleted cells (Figures 3e and S3c).
S-nitrosylation of Cys150 reversibly inactivates GAPDH, but under extreme cellular stress, Cys150 can undergo further oxidative modification that results in irreversible inactivation of its catalytic activity (23). Since S-nitrosylation of GAPDH plays a role in the observed cell death, we asked whether GAPDH activity was globally suppressed by CIB1 depletion. No difference in global GAPDH activity, however, was observed in either CIB1-depleted SK-N-SH or MDA-468 cells (supplementary figures S4a and S4b, respectively) or in the same cells overexpressing GAPDH-C150S (supplementary figures S4a and S4b). As a positive control, treatment of SK-N-SH cells with a GAPDH inhibitor, iodoacetate (IOA), dose-dependently inhibited GAPDH activity (supplementary figure S4c), which correlated with increased cell death 24 h-post treatment (supplementary figure S4d). This effect is consistent with shRNA depletion of GAPDH, which also induced a significant decrease in overall GAPDH activity (supplemental figure S4b) and increased cell death in both cell lines (data not shown). Taken together, these results indicate that CIB1 depletion induces GAPDH S-nitrosylation but has minimal effect on global GAPDH activity. Therefore, cell death in CIB1-depleted cells is unlikely due to increased metabolic stress.
To more firmly confirm nuclear GAPDH accumulation and rule out caspase activation as a mediator of cell death, CIB1-depleted cells were treated with the pan-caspase inhibitor zVAD-FMK. Unlike deprenyl, zVAD-FMK did not prevent cell death induced by CIB1-depletion in either SK-N-SH (Figure 4b, upper graph) or MDA-468 cells (supplementary figure S3b, upper graph) and had no effect on GAPDH nuclear accumulation in either CIB1-depleted cell type (Figure 4b and supplementary figure S3b, bottom panels). These results indicate that nuclear GAPDH accumulation, but not caspase activation, promotes cell death in CIB1-depleted cells.
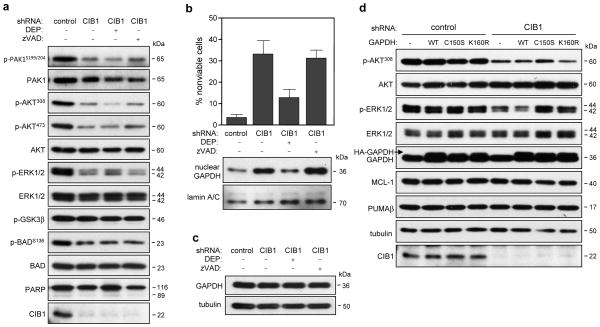
Figure 4. Effect of CIB1 depletion on AKT, ERK and PAK1 signaling pathways in the absence and presence of deprenyl or zVAD-FMK.
(a) Immunoblots of whole cell lysates prepared from control and CIB1-depleted SK-N-SH cells, n ≥ 3. (b) Deprenyl (DEP), but not caspase inhibition with zVAD-FMK (zVAD) blocks nuclear GAPDH accumulation and cell death. (c) GAPDH expression is unchanged in the absence and presence of either DEP or zVAD. (d) Whole cell lysates from SK-N-SH cells expressing emtpy vector, HA-GAPDH-WT, -CI50S or -K160R were immunoblotted with the indicated antibodies.
CIB1 depletion disrupts ERK1/2 and AKT signaling
Since we previously found that endothelial cells lacking CIB1 exhibited decreased cell proliferation and ERK activation (15), we asked whether ERK signaling was compromised in CIB1-depleted SK-N-SH and MDA-468 cells. Consistent with our previous findings, reduced ERK activation was observed in both CIB1-depleted SK-N-SH (Figure 4a) and MDA-468 cells (supplementary figure S5c). CIB1 can activate PAK1 both in vitro and in vivo (11, 15) and PAK1 is a well-established activator of the MEK/ERK pathway. Like ERK activation, PAK1 activation (as determined by PAKS199/204 phosphorylation) was markedly reduced in both CIB1-depleted tumor cell lines (Figure 4a and supplementary figure S5c). Because PAK1 was also recently reported to regulate AKT (3), we assessed the status of AKT activation in CIB1-depleted cells. A marked reduction of AKT activity was observed in both cell types, as detected by decreased AKT308 and AKT473 phosphorylation (Figure 4a and supplementary figure S5c). These results indicate that CIB1 expression regulates both AKT and ERK activation in multiple tumor cell types.
A common downstream target of PAK1, ERK, and AKT is the proapoptotic protein BAD, which when phosphorylated, promotes cell survival (24). Decreased BADS136 phosphorylation was also observed in CIB1-depleted SK-N-SH (Figure 4a) and MDA-468 cells (supplementary figure S5c), consistent with decreased PAK1 and/or AKT activation in both cell types. While AKT phosphorylation and inactivation of GSK3α/β can contribute to cell survival and cell cycle regulation (25), no difference in phosphorylation levels of either GSK3 isoform (data not shown) was observed upon CIB1 depletion in either tumor cell line (Figure 4a, supplementary figure S5c and data not shown).
We then assessed whether PAK1, AKT, ERK, or BAD activation in CIB1-depleted cells occurred upstream or downstream of nuclear GAPDH translocation to the nucleus, by blocking nuclear accumulation with deprenyl. Interestingly, deprenyl had no effect on the activation status of these kinases (Figure 4a and supplementary figure S5c). Deprenyl treatment also had no effect on overall GAPDH expression levels in either SK-N-SH (Figure 4c) or MDA-468 cells (supplementary figure S5b). Similar to deprenyl treatment, overexpression of GAPDH-C150S and -K160R mutants had no effect on AKT and ERK activation in either SK-N-SH (Figure 4d) or MDA-468 cells (supplementary figure S6a). Taken together, these results suggest that the effects of blocking GAPDH nuclear accumulation and signaling occur downstream of AKT, PAK1 and ERK.
Activation of the GAPDH/p300/CBP signaling cascade induces p53-upregulated mediator of apoptosis (PUMA), and PUMA upregulation can be blocked by GAPDH-K160R (22). Since we found that GAPDH acetylation is important in mediating cell death in CIB1-depleted cells, we asked if a p300/CBP/PUMA signaling cascade was activated as a result of CIB1 depletion. No changes in PUMA expression, however, were found in either CIB1-depleted SK-N-SH (Figure 4d) or MDA-468 cells (supplementary figure S5a). We also observed no change in the expression of the anti-apoptotic BCL-2 family member, MCL-1, which is degraded in response to apoptotic stimuli (26, 27) in either CIB1-depleted cell line (Figure 4d and supplementary figure S5a). Collectively, these results are consistent with a non-apoptotic mechanism of cell death in CIB1-depleted cells.
AKT inhibition induces nuclear GAPDH accumulation
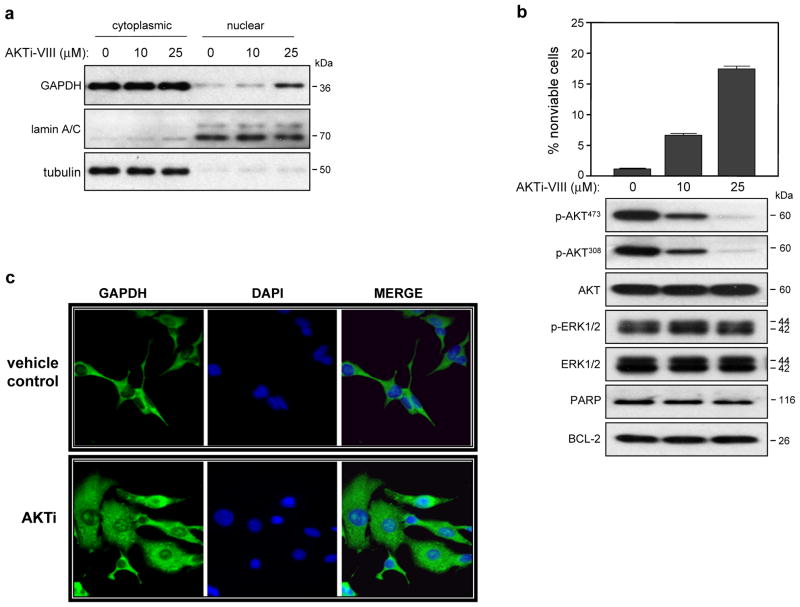
Inhibition of PI3K/AKT signaling can block the nuclear export of GAPDH (28), suggesting that PI3K/AKT negatively regulates nuclear GAPDH localization. We therefore asked if inhibition of AKT would mimic CIB1 depletion and induce GAPDH nuclear accumulation. SK-N-SH cells treated with increasing concentrations of AKT inhibitor VIII (AKTi), exhibited increased nuclear GAPDH accumulation (Figure 5a) that correlated with decreasing levels of AKT phosphorylation. However, total AKT expression or activation of other MAP kinases such as p38 (data not shown) or ERK were unaffected (Figure 5b). Importantly, AKTi induced a modest, dose-dependent increase in cell death that correlated with decreased AKT phosphorylation (Figure 5b). Like CIB1 depletion, AKT inhibition failed to induce caspase activation, as detected by the absence of PARP cleavage products or changes in BCL-2 expression (Figure 5b). Immunocytochemical anaylsis of SK-N-SH cells treated with AKTi also showed a significant increase in nuclear GAPDH staining (Figure 5c), further supporting our finding that CIB1 depletion induces GAPDH nuclear accumulation due to loss of AKT activity.
Figure 5. AKT inhibition induces cell death and GAPDH nuclear accumulation.
Untransduced SK-N-SH cells were treated in the absence and presence of either 10 or 25 μM AKT inhibitor VIII (AKTi-VIII) for 48 h. (a) Analysis of cytoplasmic and nuclear fractions was performed as in Figure 3a, n=4. (b) AKT inhibition induces SK-N-SH cell death. Cell death was determined as in Figure 1b. Data represent means ± SEM, n=4. Immunoblot analysis of whole cell lysates, n=3. (c) SK-N-SH cells were treated as in (a) and GAPDH cellular localization was visualized as in Figure 3c.
Concurrent inhibition of AKT and ERK mimic cell death induced by CIB1 depletion
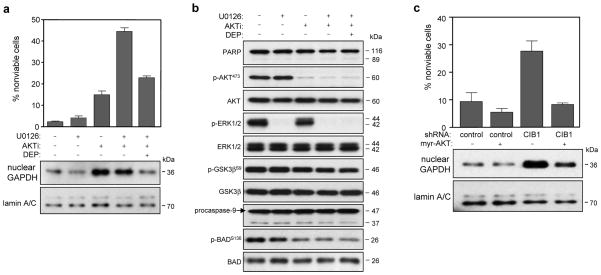
While AKT inhibition alone induced a modest increase in SK-N-SH cell death and significant GAPDH nuclear accumulation, it did not induce the same extent of cell death observed in CIB1-depleted cells. This suggests the contribution of additional signaling pathways. Since ERK activation was also reduced in CIB1-depleted cells (Figure 4a and supplementary figure S4e), we asked whether the combined pharmacological inhibition of AKT and ERK would more closely mimic cell death induced by CIB1 depletion. Indeed, treatment of cells with both AKTi and the MEK inhibitor U0126 induced a significant ~12-fold increase in cell death (Figure 6a) whereas MEK/ERK inhibition alone had a minimal effect (Figure 6a). Western blot analysis confirmed that AKTi and U0126 effectively inhibited AKT and ERK phosphorylation, respectively (Figure 6b). Inhibition of both AKT and ERK also failed to induce caspase activation as evidenced by the lack of PARP and caspase-9 cleavage products (Figure 6b), indicating a caspase-independent mechanism of death in these cell types. Similar to our findings with CIB1-depleted cells, deprenyl treatment significantly reduced cell death induced by the combined inhibition of ERK and AKT (Figure 6a) further supporting a role for nuclear GAPDH in promoting cell death upon AKT and ERK inhibition. Interestingly, inhibition of both pathways did not further increase nuclear GAPDH accumulation relative to inhibition of either enzyme alone (Figure 6a, lower panels). Moreover, deprenyl blocked GAPDH nuclear accumulation in cells incubated with AKTi and U0126 (Figure 6a, lower panels). To further implicate AKT activity in regulating GAPDH translocation, we expressed a constitutively active AKT mutant (myr-AKT) in control and CIB1-depleted cells. Restoration of AKT activity in CIB1-depleted cells prevented cell death in both SK-N-SH (Figure 6c) and MDA-468 cells (supplementary figure S6b), and importantly, reduced GAPDH nuclear accumulation in both cell lines (Figure 6c and supplementary figure S6b, bottom panels). These results imply that AKT inhibition is sufficient to induce nuclear GAPDH accumulation, but that concurrent inhibition of the MEK/ERK pathway is necessary to mimic CIB1 depletion-induced, GAPDH-dependent tumor cell death.
Figure 6. Effects of combined AKT and ERK inhibition on SK-N-SH cell death and GAPDH nuclear accumulation.
Untransduced SK-N-SH cells were treated in the absence and presence of either 10 μM U0126 (MEK inhibitor) and/or 20 μM AKTi. Cells exposed to both inhibitors were also treated with 25 nM deprenyl. (a) Concurrent inhibition of AKT and ERK significantly increases cell death but not GAPDH nuclear accumulation. Cell death was quantified as in Figure 1b (upper graph). Data represent means ± SEM, n=3. Analysis of cytoplasmic and nuclear fractions was performed as in Figure 3a (bottom panels). (b) Immunoblotting of whole cell lysates with the indicated antibodies, prepared from cells treated with or without AKTi and/or U0126. (c) Overexpression of a constitutively active AKT mutant (myr-AKT) blocks cell death in CIB1-depleted cells. Cell viability was quantified as Figure 1b. Data represent +/- SEM, n=3. Expression of myr-AKT blocks GAPDH nuclear accumulation (lower panels). Nuclear lysates were immunobloted with anti-GAPDH or -lamin A/C antibodies.
CIB1 depletion affects cell cycle progression and induces DNA damage
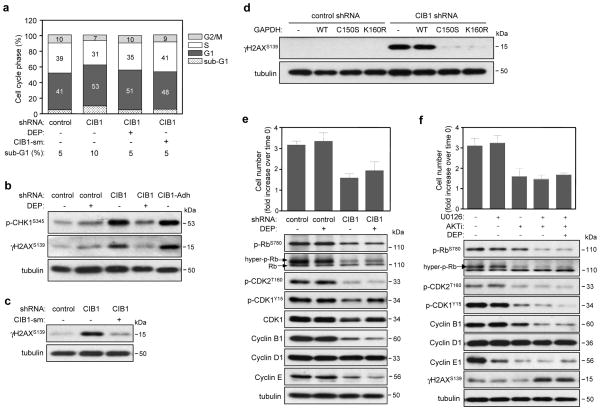
To better understand the cell proliferation defect in CIB1-depleted cells, flow cytometry was used to assess cell cycle profiles of asynchronously growing cells. CIB1-depleted SK-N-SH cells showed a moderate accumulation of cells in the G1 phase relative to control cells (53% vs. 41%, respectively) (Figure 7a), whereas CIB1-depleted MDA-468 cells showed a more significant G1 accumulation (supplementary figure S7a). This increased G1 accumulation was also associated with a reduced percentage of cells in S and G2/M in both cell types (Figure 7a and supplementary figure S7a), suggesting that CIB1 depletion induces G1 cell cycle arrest. Since cell cycle arrest can be triggered by DNA damage, it was noteworthy that both CIB1-depleted SK-N-SH (Figure 7a) and MDA-468 cells (supplementary figure S7a) showed a ~ 2-fold increase of cells in the sub-G1 fraction (<2N DNA ploidy) that is often associated with DNA fragmentation. We also assessed markers commonly associated with induction of DNA damage. A significant increase in phosphorylation of both the histone variant H2AX at Ser139 (γH2AX), a sensitive marker of early DNA damage (29) and checkpoint kinase CHK1 (30) was observed in CIB1-depleted SK-N-SH and MDA-468 cells (Figure 7b and supplementary figure S7b). This DNA damage response appears to precede cell death since these markers were observed in the adherent, viable CIB1-depleted cell population (Figure 7b), suggesting that DNA damage may be a cause of cell death rather than a secondary consequence. This effect is specific for CIB1 depletion since ectopic re-expression of CIB1 in CIB1-depleted SK-N-SH and MDA-468 cells blocked induction of γH2Ax (Figure 7c and supplementary figure S7c, respectively). While deprenyl did not significantly rescue cell proliferation in either CIB1-depleted SK-N-SH (Figure 7c, upper panel) or MDA-468 cells (data not shown), it did reduce the sub-G1 population (Figure 7a and supplementary figure S7a) and γH2AX induction (Figure 7b and supplementary figure S7b), suggesting a role for abnormal nuclear GAPDH accumulation in DNA damage. Since blocking GAPDH nuclear accumulation with deprenyl correlated with decreased H2AX phosphorylation, we predicted that expression of either GAPDH-C150S or GAPDH-K150R would reduce the extent of DNA damage in CIB1-depleted cells. As expected, overexpression of either GAPDH mutant effectively blocked induction of H2AX phosphorylation in CIB1-depleted SK-N-SH (Figure 7d) and MDA-468 cells (supplementary figure S7d), suggesting that both GAPDH nuclear accumulation and acetylation are required to induce DNA damage.
Figure 7. Increased DNA damage and disruption of cell cycle regulation by CIB1 depletion is recapitulated by concurrent AKT and ERK inhibition.
(a) Cell cycle analysis of propidium iodide-stained control and CIB1-depleted SK-N-SH cells treated with 25 nM deprenyl or expressing CIB1-sm (CIB1-silent mutation). (b) CIB1-depleted cells show induction of DNA damage markers. Analysis of whole cell lysates prepared from control and CIB1-depleted cells in the absence and presence of deprenyl (DEP). Analysis of adherent CIB1-depleted (CIB1-Adh) cells (excluding floating cell populations) shows induction of both γH2AX and p-CHK1. (c) Ectopic expression of CIB1-sm in CIB1-depleted cells blocks induction of γH2AX. (d) Ectopic expression of either HA-GAPDH-C150S or -K160R blocks induction of γH2AX. (e) Deprenyl does not significantly rescue the cell proliferation defect in CIB1-depleted cells. Proliferation was quantified as in Figure 1c. Data represent means ± SEM, n=7. Effect of DEP on the activation and expression of cell cycle proteins (blots, lower panels). (f) Effect of AKTi and/or U0126 on proliferation and cell cycle regulatory proteins. Proliferation of SK-N-SH cells treated with and without inhibitors expressed as the fold increase over the cell number at time of cell plating (time 0) (upper graph). Data represent means ± SEM, n=3. Effect of AKT and/or ERK inhibition on the activation and expression of cell cycle regulatory proteins. Whole cell lysates were analyzed as in (b) (lower panels).
CIB1 depletion disrupts key cell cycle signaling pathways
The accumulation of CIB1-depleted cells in G1 suggested that regulatory proteins associated with G1 could be disrupted or dysregulated. An important regulatory step required for G1/S transition is phosphorylation of Thr160 within the cyclin-dependent kinase 2 (CDK2) activation loop. A marked reduction in CDK2 phosphorylation was observed in both CIB1-depleted tumor cell lines (Figures 7e and supplementary figure S7e), suggesting a potential mechanism of G1 arrest. Active cyclin E/A CDK2 complexes also regulate the G1/S transition by phosphorylation and activation of retinoblastoma (Rb) (31). Consistent with a loss of CDK2 activation, CIB1-depleted SK-N-SH cells also showed a marked reduction in RbS780 phosphorylation (Figure 7e). Rb phosphorylation was not detected in MDA-468 cells, consistent with the absence of Rb in this cell type (data not shown). Expression levels of cyclin D1, which along with other cyclins, regulates CDK activity, were similar in either CIB1-depleted SK-N-SH (Figure 7e) or MDA-468 cells (supplementary figure S7e). Cyclin E, however, was downregulated in CIB1-depleted SK-N-SH cells (Figure 7e) and upregulated in CIB1-depleted MDA-468 cells (supplementary Figure S7e), suggesting cell type specific effects of CIB1 depletion on G1 regulation. Taken together, these results suggest that loss of CIB1 disrupts CDK2 and Rb phosphorylation, and cyclin E expression—key cell cycle regulatory proteins necessary for G1/S transition.
Activation of the CDK1/cyclin B1 complex is an important regulatory step in the G2/M transition. Moreover, cyclin B1 depletion can increase cell death and decrease tumor cell proliferation (32). CIB1-depleted tumor cell lines exhibited decreases in both cyclin B1 expression and inhibitory CDK1Y15 phosphorylation (Figure 7e and supplementary figure S7e), indicating abnormally activated CDK1. Interestingly, deprenyl rescued CDK1 phosphorylation in both CIB1-depleted cell types (Figure 7c and supplementary figure S7e), but had no effect on G1/S-related cyclins or CDK2. Deprenyl however, did not rescue cell proliferation in either CIB1-depleted SK-N-SH or MDA-468 (Figure 7e and supplemental figure S7e), suggesting that CIB1 depletion induces G1/S disruption independent of nuclear GAPDH accumulation.
Concurrent inhibition of ERK and AKT mimics the effects of CIB1 depletion on proliferation and cell cycle regulatory proteins
If disrupted cell proliferation in CIB1-depleted cells occurs through inhibition of AKT and ERK, pharmacological inhibition of these kinases should similarly disrupt proliferation and cell cycle regulatory proteins. Indeed, AKTi alone decreased cell proliferation 1.5-fold; concurrent ERK inhibition with U0126 did not cause a further reduction (Figure 7f, upper graph). Concurrent treatment with AKTi and U0126 markedly decreased Rb, CDK2 and CDK1 phosphorylation as well as cyclin B1 expression but had no effect on cyclin D1 levels (Figure 7f). These results mimic the activation and expression profile of these proteins in CIB1-depleted cells. In addition, AKT inhibition alone maximally inhibited CDK2 phosphorylation, indicating that ERK inhibition is not necessary for this effect. While U0126 effectively inhibited MEK/ERK activation, it had no effect on expression or activation of several cell cycle regulatory proteins (Figure 7f), consistent with the inability of U0126 alone to significantly inhibit cell survival and proliferation. Importantly, concurrent AKT and ERK inhibition did induce phosphorylation of the DNA damage marker γH2AX (Figure 7f). Therefore, pharmacological inhibition of both pathways synergizes to recapitulate the effects of CIB1 depletion on DNA damage, cell cycle disruption and cell death.
DISCUSSION
Here we report that CIB1 is necessary for tumor cell survival and proliferation. Specifically, CIB1 knockdown significantly increases non-apoptotic tumor cell death and decreases cell proliferation by separate but related mechanisms. CIB1 depletion results in AKT inhibition, which is sufficient to induce nuclear GAPDH accumulation. Nuclear GAPDH accumulation itself is necessary, but concurrent ERK inhibition is also required to induce the extent of cell death observed in CIB1-depleted cells. CIB1 depletion also induces GAPDH-dependent DNA damage and cell death. This DNA damage response requires GAPDH nuclear accumulation and GAPDH acetylation. These results highlight the novel link between loss of CIB1 expression, dysregulation of AKT and ERK signaling, GAPDH nuclear accumulation, and non-apoptotic cell death.
CIB1 depletion in two distinct cancer cell lines significantly decreased cell viability independent of caspase activation or loss of mitochondrial function. Rather, we observed a marked increase in nuclear GAPDH accumulation. Increasing evidence indicates that nuclear accumulation or translocation of GAPDH can induce cell death in both neuronal and non-neuronal cells (18, 22). GAPDH nuclear accumulation in and of itself, however, does not establish nuclear GAPDH as a causative factor in promoting cell death. The MAO-B inhibitor, deprenyl, specifically interacts with and inhibits GAPDH nuclear accumulation, thereby preventing nuclear GAPDH-induced cell death (20, 33). Since deprenyl significantly reduced nuclear GAPDH accumulation and cell death in CIB-depleted cells, this finding provides a strong link between these events. Deprenyl also had no effect on GAPDH activity, survival or proliferation of control cells indicating that the deprenyl concentration used in our study is unlikely to have adverse effects on normal cellular glycolysis.
Posttranslational modification of GAPDH occurs as a result of oxidative stress and is thought to govern GAPDH nuclear localization (23). For example, S-nitrosylation of Cys150 (Cys152 in human GAPDH) within the GAPDH active site facilitates its association with the E3 ubiquitin ligase Siah-1 resulting in GAPDH nuclear translocation via the Siah-1 nuclear localization sequence. Deprenyl inhibits GAPDH S-nitrosylation and subsequent GAPDH binding to Siah-1, thereby preventing GAPDH translocation to the nucleus (20). Cell death and GAPDH translocation to the nucleus is also blocked by mutation of Cys150 in GAPDH (20, 34). Since we found that both deprenyl and GAPDH-C150S block GAPDH nuclear accumulation and cell death, our results suggest that GAPDH undergoes S-nitrosylation in CIB1-depleted cells. While S-nitrosylation at this catalytic site inhibits GAPDH activity in vitro (35, 36), we found no decline in global GAPDH activity in CIB1-depleted cells, suggesting that the cell death observed in CIB1-depleted cells is unlikely due to metabolic stress. Aggregation of GAPDH, in the absence of GAPDH nuclear accumulation is also reported to induce cell death under increased cellular stress conditions (34). Under low to moderate stress conditions however, the authors did find that cell death is accompanied by GAPDH nuclear translocation (34). Western blot analysis did not reveal the presence of GAPDH aggregates in CIB1-depleted cells (data not shown), suggesting that CIB1 depletion likely induces a low to moderate oxidative stress signaling cascade. Because we also observed a significant proportion of GAPDH remaining in the cytoplasmic compartment, it may be likely that this low to moderate oxidative stress modifies a subpopulation of GAPDH in CIB1-depleted cells. In agreement, Hara, et al., report that a small population of GAPDH is S-nitrosylated and translocated to the nucleus under their experimental conditions and is sufficient to induce cell death (20). The authors also find that cell death triggered by nuclear GAPDH-Siah1 is largely independent of glycolytic impairment (20).
Several mechanisms have been proposed to explain how nuclear GAPDH promotes cell death. One mechanism involves Siah-1-dependent degradation of nuclear proteins via the nuclear GAPDH/Siah-1 complex (20). Nuclear GAPDH can also bind and stimulate acetyltransferase p300/CBP, leading to the activation of p53 and induction of cell death (22). These events are known to be blocked by expression of a GAPDH mutant resistant to acetylation at lysine 160 (GAPDH-K160R) (22). Consistent with these findings, we observed that expression of GAPDH-K160R blocks cell death, indicating that GAPDH acetylation is required to induce cell death in CIB1-depleted cells. However, since cell death occurs in MDA-468 cells that express mutant p53, CIB1 depletion appears to induce cell death independent of p53 status per se. Because tumors bearing mutant p53 are more resistant to chemotherapeutic agents and strongly correlate with poor patient prognosis (37), our results indicate that CIB1 inhibition could provide an effective therapeutic approach for treating these tumors.
Tumor cells commonly exhibit hyperactivated PI3K/AKT and Ras/MEK/ERK signaling pathways. Since CIB1 depletion markedly reduced both ERK and AKT activation, it is important to consider the contribution of each pathway in GAPDH-dependent cell death. Inhibition of PI3K/AKT signaling is known to block nuclear export of GAPDH upon growth factor stimulation (28, 38), indicating a link between PI3K/AKT survival signaling and GAPDH localization. In agreement, we find that restoration of AKT activity in CIB1-depleted cells blocks GAPDH nuclear accumulation and cell death, demonstrating the importance of AKT in regulating GAPDH localization. Furthermore, our results showed that AKT but not ERK inhibition markedly and maximally increased nuclear GAPDH localization, which correlated with a moderate increase in cell death. Inhibition of both pathways, however, induced similar levels of cell death to that observed in CIB1-depleted cells. These results show that GAPDH nuclear accumulation is necessary, but not sufficient to induce the extent of cell death observed in CIB1-depleted cells. Other reports also indicate that in certain instances, nuclear GAPDH accumulation alone is not sufficient to induce cell death (39). Notably, forced nuclear targeting of GAPDH fused to a nuclear localization sequence (GAPDH-NLS) fails to induce cell death in SH-SY5Y neuroblastoma (40) and HEK293 cells (20). Yet, depletion of GAPDH or blocking GAPDH nuclear accumulation is protective against genotoxic or stress-induced stimuli (41, 42), indicating that GAPDH is necessary for cell death under these stress conditions.
We previously demonstrated that CIB1 directly activates PAK1, which affects downstream signaling to ERK (11, 15). CIB1-depleted SK-N-SH and MDA-MB-468 cells exhibit significantly reduced PAK1 activation that correlated with a reduction in ERK activity. Recently, PAK1 was found to directly regulate AKT in colorectal cancer (3) and Erb-B2 positive breast cancer cells (43), thereby possibly explaining how CIB1 regulates both ERK and AKT.
Another consequence of CIB1 depletion is increased DNA damage. CIB1-depleted adherent and floating cell populations showed a moderate increase in the sub-G1 content that is typically associated with cell shrinkage, large scale DNA fragmentation, and condensed nuclei. Adherent, viable CIB1-depleted cells however, showed no noticeable increase in these apoptotic nuclear characteristics. Instead, this adherent cell population exhibited a marked increase in γH2AX and CHK1 activation, suggesting more moderate DNA damage that precedes cell detachment and cell death. Concurrent inhibition of AKT and ERK mimicked CIB1 depletion by inducing γH2AX, indicating that loss of these signaling pathways is sufficient to induce DNA damage. GAPDH depletion is known to protect against DNA damage and subsequent γH2AX formation induced by the cytotoxic agent cytosine arabinoside (AraC) (41), thus implicating a role for nuclear GAPDH in the DNA damage response. In agreement, we found that inhibition of GAPDH nuclear accumulation in CIB1-depleted cells markedly reduced the induction of γH2AX and appearance of the sub-G1 population, further suggesting a link between GAPDH nuclear accumulation, DNA damage and subsequent cell death. This response also requires GAPDH acetylation since expression of an acetylation mutant blocked CIB1-depletion-induced cell death. Interestingly, GAPDH directly interacts with DNA as well as with several nuclear components involved in DNA repair/damage and transcription such as the NAD+-dependent deacetylase SIRT1 (21) and AP endonuclease1 (APE1) (44). Exactly how nuclear GAPDH expression induces DNA damage in CIB1-depleted cells or other scenarios where this occurs (41, 45) is unknown and warrants future investigation.
ERK signaling can positively regulate DNA repair mechanisms to attenuate DNA damage (46, 47). This role of ERK may account for the absence of DNA damage and modest cell death in cells where only AKT is inhibited, despite a marked increase in nuclear GAPDH, and may explain why concurrent inhibition of ERK was required to induce DNA damage and significant cell death. DNA damage also normally induces activation of upstream kinases WEE/MYT1 that phosphorylate and inactivate CDK1 to prevent entry into mitosis thereby allowing sufficient time for DNA repair. Aberrant or unchecked CDK1 activation in the face of DNA damage results in premature entry into mitosis and has been associated with increased cell death (48). An interesting finding from our study is the marked decrease in CDK1 phosphorylation (i.e., increased CDK1 activation) that was blocked by preventing GAPDH nuclear accumulation in CIB1-depleted cells treated with deprenyl. These results therefore suggest that nuclear GAPDH also prevents sufficient G2/M delay by disrupting CDK1 phosphorylation and inactivation. How nuclear GAPDH prevents this delay is currently unknown. One possibility may be through the interaction of nuclear GAPDH with SET, a negative regulator of CDK1/cyclin B1 activity, thereby resulting in indirect CDK1 activation (49).
Here we find increased accumulation of cells in the G1 phase of the cell cycle suggesting that CIB1 depletion also results in G1 arrest. Two important regulatory steps for G1/S transition are CDK2 and retinoblastoma (Rb) activation by active cyclin E/A-CDK2 complexes (31). While the PI3K/AKT and MEK/ERK pathways have both been implicated in regulating CDK2 (50), we found that AKT but not ERK inhibition was sufficient to inhibit CDK2 activation and block cell proliferation. Our results therefore suggest that decreased AKT activation in CIB1-depleted cells likely contributes to decreased CDK2 activation and subsequent G1 arrest. In agreement, inhibition of PI3K/AKT signaling is also known to promote G1 arrest in several cancer cell lines (51, 52). In addition, our finding that blocking GAPDH nuclear accumulation with either deprenyl or GAPDH-C150S did not restore cell proliferation, suggests that cell cycle arrest occurs independently of GAPDH nuclear accumulation.
In summary, we have identified CIB1 as a novel upstream regulator of two major oncogenic pathways that promote cell survival and proliferation. Our results suggest a model whereby CIB1, via AKT and ERK signaling prevents abnormal nuclear GAPDH accumulation, DNA damage and cell death (supplementary figure S8). Additional studies will be needed to define the precise mechanisms by which nuclear GAPDH induces DNA damage and cell death in CIB1-depleted cells. Nevertheless, our findings indicate that CIB1 may represent a unique molecular target for inducing selective tumor cell death via regulation of these pathways.
EXPERIMENTAL PROCEDURES
Materials
Plasmids, reagents, antibodies, cell culture, lentiviral transduction, Western blotting, mitochondrial membrane potential assays, GAPDH activity assays and statistical analysis are described in detail in the Supplemental Information.
Subcellular Fractionation
Subcellular fractionation was performed as described (53) with minor modifications. Detailed methods are described in Supplemental information.
Immunocytochemistry
GAPDH and AIF immunostaining was performed as previously described (11).
Cell cycle analysis
Cells were harvested (1–2 × 106), resuspended in PBS and fixed overnight in cold 70% ethanol. Cells were washed once in PBS and resuspended in propidium iodide staining solution (20 μg/mL propidium iodide (Sigma, St. Louis, MO, USA), 0.1% Triton X-100, and 0.2 mg/mL DNAse-free RNAse (Sigma) in PBS) for 30 min at room temperature. Data was collected from ~20,000 cells using a Cyan ADP flow cytometer (Beckman-Coulter/Dako, Brea, CA, USA) and percentages of cells in G1, S, and G2/M were determined using FlowJo analysis software (Tree Star, Inc., Ashland, OR, USA)
Supplementary Material
Acknowledgments
We thank members of the Parise, Cook and A. Sancar laboratories, Drs. Yue Xiong, and Matthew Torres for critical review and suggestions. We also thank Dr. Akira Sawa for generously providing GAPDH plasmids. Special thanks to Dr. Howard Fried and Andrew McFadden for lentiviral plasmid construction. This work was funded by the postdoctoral fellowship grant HL07149T32 (C.M.) and 1R01HL092544 (L.V.P.) from the NIH.
References
- 1.McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–79. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Slack-Davis JK, Eblen ST, Zecevic M, Boerner SA, Tarcsafalvi A, Diaz HB, et al. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J Cell Biol. 2003;162(2):281–91. doi: 10.1083/jcb.200212141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huynh N, Liu KH, Baldwin GS, He H. P21-activated kinase 1 stimulates colon cancer cell growth and migration/invasion via ERK- and AKT-dependent pathways. Biochim Biophys Acta. 2010;1803(9):1106–13. doi: 10.1016/j.bbamcr.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Grant S. Cotargeting survival signaling pathways in cancer. The Journal of clinical investigation. 2008;118(9):3003–6. doi: 10.1172/JCI36898. Epub 2008/08/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 6.Fang X, Yu S, Eder A, Mao M, Bast RC, Jr, Boyd D, et al. Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene. 1999;18(48):6635–40. doi: 10.1038/sj.onc.1203076. [DOI] [PubMed] [Google Scholar]
- 7.She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, Sasazuki T, et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell. 2010;18(1):39–51. doi: 10.1016/j.ccr.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dent P, Curiel DT, Fisher PB, Grant S. Synergistic combinations of signaling pathway inhibitors: mechanisms for improved cancer therapy. Drug Resist Updat. 2009;12(3):65–73. doi: 10.1016/j.drup.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gentry HR, Singer AU, Betts L, Yang C, Ferrara JD, Sondek J, et al. Structural and biochemical characterization of CIB1 delineates a new family of EF-hand-containing proteins. J Biol Chem. 2005;280(9):8407–15. doi: 10.1074/jbc.M411515200. [DOI] [PubMed] [Google Scholar]
- 10.Naik UP, Patel PM, Parise LV. Identification of a novel calcium-binding protein that interacts with the integrin alphaIIb cytoplasmic domain. J Biol Chem. 1997;272(8):4651–4. doi: 10.1074/jbc.272.8.4651. [DOI] [PubMed] [Google Scholar]
- 11.Leisner TM, Liu M, Jaffer ZM, Chernoff J, Parise LV. Essential role of CIB1 in regulating PAK1 activation and cell migration. J Cell Biol. 2005;170(3):465–76. doi: 10.1083/jcb.200502090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon KW, Cho JH, Lee JK, Kang YH, Chae JS, Kim YM, et al. CIB1 functions as a Ca(2+)-sensitive modulator of stress-induced signaling by targeting ASK1. Proc Natl Acad Sci USA. 2009;106(41):17389–94. doi: 10.1073/pnas.0812259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zayed MA, Yuan W, Chalothorn D, Faber JE, Parise LV. Tumor growth and angiogenesis is impaired in CIB1 knockout mice. J Angiogenes Res. 2010;2(1):17. doi: 10.1186/2040-2384-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan W, Leisner TM, McFadden AW, Clark S, Hiller S, Maeda N, et al. CIB1 is essential for mouse spermatogenesis. Mol Cell Biol. 2006;26(22):8507–14. doi: 10.1128/MCB.01488-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zayed MA, Yuan W, Leisner TM, Chalothorn D, McFadden AW, Schaller MD, et al. CIB1 regulates endothelial cells and ischemia-induced pathological and adaptive angiogenesis. Circ Res. 2007;101(11):1185–93. doi: 10.1161/CIRCRESAHA.107.157586. [DOI] [PubMed] [Google Scholar]
- 16.Broker LE, Kruyt FA, Giaccone G. Cell death independent of caspases: a review. Clin Cancer Res. 2005;11(9):3155–62. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- 17.Lartigue L, Kushnareva Y, Seong Y, Lin H, Faustin B, Newmeyer DD. Caspase-independent mitochondrial cell death results from loss of respiration, not cytotoxic protein release. Mol Biol Cell. 2009;20(23):4871–84. doi: 10.1091/mbc.E09-07-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawa A, Khan AA, Hester LD, Snyder SH. Glyceraldehyde-3-phosphate dehydrogenase: nuclear translocation participates in neuronal and nonneuronal cell death. Proc Natl Acad Sci USA. 1997;94(21):11669–74. doi: 10.1073/pnas.94.21.11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hara MR, Thomas B, Cascio MB, Bae BI, Hester LD, Dawson VL, et al. Neuroprotection by pharmacologic blockade of the GAPDH death cascade. Proc Natl Acad Sci USA. 2006;103(10):3887–9. doi: 10.1073/pnas.0511321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hara MR, Snyder SH. Nitric oxide-GAPDH-Siah: a novel cell death cascade. Cell Mol Neurobiol. 2006;26(4–6):527–38. doi: 10.1007/s10571-006-9011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kornberg MD, Sen N, Hara MR, Juluri KR, Nguyen JV, Snowman AM, et al. GAPDH mediates nitrosylation of nuclear proteins. Nat Cell Biol. 2010;12(11):1094–100. doi: 10.1038/ncb2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sen N, Hara MR, Kornberg MD, Cascio MB, Bae BI, Shahani N, et al. Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nat Cell Biol. 2008;10(7):866–73. doi: 10.1038/ncb1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tristan C, Shahani N, Sedlak TW, Sawa A. The diverse functions of GAPDH: views from different subcellular compartments. Cell Signal. 2011;23(2):317–23. doi: 10.1016/j.cellsig.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sastry KS, Karpova Y, Kulik G. Epidermal growth factor protects prostate cancer cells from apoptosis by inducing BAD phosphorylation via redundant signaling pathways. J Biol Chem. 2006;281(37):27367–77. doi: 10.1074/jbc.M511485200. [DOI] [PubMed] [Google Scholar]
- 25.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359(Pt 1):1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills JR, Malina A, Pelletier J. Inhibiting mitochondrial-dependent proteolysis of Mcl-1 promotes resistance to DNA damage. Cell Cycle. 2012;11(1):88–98. doi: 10.4161/cc.11.1.18408. Epub 2011/12/22. [DOI] [PubMed] [Google Scholar]
- 27.Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, et al. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes & development. 2003;17(12):1475–86. doi: 10.1101/gad.1093903. Epub 2003/06/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon HJ, Rhim JH, Jang IS, Kim GE, Park SC, Yeo EJ. Activation of AMP-activated protein kinase stimulates the nuclear localization of glyceraldehyde 3-phosphate dehydrogenase in human diploid fibroblasts. Exp Mol Med. 2010 doi: 10.3858/emm.2010.42.4.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273(10):5858–68. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 30.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 31.Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2(2):103–12. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 32.Yuan J, Kramer A, Matthess Y, Yan R, Spankuch B, Gatje R, et al. Stable gene silencing of cyclin B1 in tumor cells increases susceptibility to taxol and leads to growth arrest in vivo. Oncogene. 2006;25(12):1753–62. doi: 10.1038/sj.onc.1209202. [DOI] [PubMed] [Google Scholar]
- 33.Kragten E, Lalande I, Zimmermann K, Roggo S, Schindler P, Muller D, et al. Glyceraldehyde-3-phosphate dehydrogenase, the putative target of the antiapoptotic compounds CGP 3466 and R-(−)-deprenyl. J Biol Chem. 1998;273(10):5821–8. doi: 10.1074/jbc.273.10.5821. [DOI] [PubMed] [Google Scholar]
- 34.Nakajima H, Amano W, Kubo T, Fukuhara A, Ihara H, Azuma YT, et al. Glyceraldehyde-3-phosphate dehydrogenase aggregate formation participates in oxidative stress-induced cell death. The Journal of biological chemistry. 2009;284(49):34331–41. doi: 10.1074/jbc.M109.027698. Epub 2009/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohr S, Hallak H, de Boitte A, Lapetina EG, Brune B. Nitric oxide-induced S-glutathionylation and inactivation of glyceraldehyde-3-phosphate dehydrogenase. The Journal of biological chemistry. 1999;274(14):427–30. doi: 10.1074/jbc.274.14.9427. Epub 1999/03/27. [DOI] [PubMed] [Google Scholar]
- 36.Broniowska KA, Hogg N. Differential mechanisms of inhibition of glyceraldehyde-3-phosphate dehydrogenase by S-nitrosothiols and NO in cellular and cell-free conditions. American journal of physiology Heart and circulatory physiology. 2010;299(4):H1212–9. doi: 10.1152/ajpheart.00472.2010. Epub 2010/08/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y. Induction of genetic instability by gain-of-function p53 cancer mutants. Oncogene. 2008;27(25):3501–7. doi: 10.1038/sj.onc.1211023. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz HD. Reversible nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase upon serum depletion. Eur J Cell Biol. 2001;80(6):419–27. doi: 10.1078/0171-9335-00174. [DOI] [PubMed] [Google Scholar]
- 39.Colell A, Green DR, Ricci JE. Novel roles for GAPDH in cell death and carcinogenesis. Cell Death Differ. 2009;16(12):1573–81. doi: 10.1038/cdd.2009.137. [DOI] [PubMed] [Google Scholar]
- 40.Kodama R, Kondo T, Yokote H, Jing X, Sawada T, Hironishi M, et al. Nuclear localization of glyceraldehyde-3-phosphate dehydrogenase is not involved in the initiation of apoptosis induced by 1-Methyl-4-phenyl-pyridium iodide (MPP+) Genes Cells. 2005;10(12):1211–9. doi: 10.1111/j.1365-2443.2005.00911.x. [DOI] [PubMed] [Google Scholar]
- 41.Phadke MS, Krynetskaia NF, Mishra AK, Krynetskiy E. Glyceraldehyde 3-phosphate dehydrogenase depletion induces cell cycle arrest and resistance to antimetabolites in human carcinoma cell lines. J Pharmacol Exp Ther. 2009;331(1):77–86. doi: 10.1124/jpet.109.155671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xing C, LaPorte JR, Barbay JK, Myers AG. Identification of GAPDH as a protein target of the saframycin antiproliferative agents. Proc Natl Acad Sci USA. 2004;101(16):5862–6. doi: 10.1073/pnas.0307476101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arias-Romero LE, Villamar-Cruz O, Pacheco A, Kosoff R, Huang M, Muthuswamy SK, et al. A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells. Oncogene. 2010 doi: 10.1038/onc.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azam S, Jouvet N, Jilani A, Vongsamphanh R, Yang X, Yang S, et al. Human glyceraldehyde-3-phosphate dehydrogenase plays a direct role in reactivating oxidized forms of the DNA repair enzyme APE1. J Biol Chem. 2008;283(45):30632–41. doi: 10.1074/jbc.M801401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dastoor Z, Dreyer JL. Potential role of nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase in apoptosis and oxidative stress. J Cell Sci. 2001;114(Pt 9):1643–53. doi: 10.1242/jcs.114.9.1643. [DOI] [PubMed] [Google Scholar]
- 46.Golding SE, Rosenberg E, Neill S, Dent P, Povirk LF, Valerie K. Extracellular signal-related kinase positively regulates ataxia telangiectasia mutated, homologous recombination repair, and the DNA damage response. Cancer Res. 2007;67(3):1046–53. doi: 10.1158/0008-5472.CAN-06-2371. [DOI] [PubMed] [Google Scholar]
- 47.Dai Y, Chen S, Pei XY, Almenara JA, Kramer LB, Venditti CA, et al. Interruption of the Ras/MEK/ERK signaling cascade enhances Chk1 inhibitor-induced DNA damage in vitro and in vivo in human multiple myeloma cells. Blood. 2008;112(6):2439–49. doi: 10.1182/blood-2008-05-159392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23(16):2825–37. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- 49.Carujo S, Estanyol JM, Ejarque A, Agell N, Bachs O, Pujol MJ. Glyceraldehyde 3-phosphate dehydrogenase is a SET-binding protein and regulates cyclin B-cdk1 activity. Oncogene. 2006;25(29):4033–42. doi: 10.1038/sj.onc.1209433. [DOI] [PubMed] [Google Scholar]
- 50.Chambard JC, Lefloch R, Pouyssegur J, Lenormand P. ERK implication in cell cycle regulation. Biochim Biophys Acta. 2007;1773(8):1299–310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 51.Mikami I, Zhang F, Hirata T, Okamoto J, Koizumi K, Shimizu K, et al. Inhibition of activated phosphatidylinositol 3-kinase/AKT pathway in malignant pleural mesothelioma leads to G1 cell cycle arrest. Oncol Rep. 2010;24(6):1677–81. doi: 10.3892/or_00001033. [DOI] [PubMed] [Google Scholar]
- 52.She QB, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, et al. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS One. 2008;3(8):e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Milosevic J, Bulau P, Mortz E, Eickelberg O. Subcellular fractionation of TGF-beta1-stimulated lung epithelial cells: a novel proteomic approach for identifying signaling intermediates. Proteomics. 2009;9(5):1230–40. doi: 10.1002/pmic.200700604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.