Abstract
The reaction mechanisms of (E)-β-farnesene synthase (EBFS) and isoprene synthase (ISPS), enzymes that catalyze a formal regioespecific 1,4-conjugate elimination of hydrogen-diphosphate from (E, E)-farnesyl and dimethylallyl diphosphate (FDP and DMADP) to generate the semiochemicals (E)-β-farnesene and isoprene, respectively, were probed with substrate analogs and kinetic measurements. The results support stepwise reaction mechanisms through analogous enzyme-bound allylic cationic intermediates. For EBFS, we demonstrate that the elimination reaction can proceed via the enzyme-bound intermediate trans-nerolidyl diphosphate, while for ISPS the intermediacy of 2-methylbut-3-enyl 2-diphosphate can be inferred from the product outcome when deuterated DMADPs are used as substrates. Possible implications derived from the mechanistic details of the EBFS catalyzed reaction for the evolution of sesquiterpene synthases are discussed.
INTRODUCTION
Class I terpene synthases rely on a shared protein fold to catalyze the metal dependent turnover of linear isoprenyl diphosphates to generate families of natural products characterized by their enormous diversity in structure, stereochemistry, biological function and application. Most mono-, sesqui- and diterpene synthases catalyze complex cyclization cascades of reactive carbocations with high regio- and stereochemical precision.1 On the other hand, the hemiterpene isoprene synthase (ISPS), the monoterpene myrcene synthase (MS) and the sesquipterpene (E)-β-farnesene synthase (EBFS) generate linear hydrocarbons through the regiospecific 1,4-conjugated elimination of hydrogen diphosphate (HOPP, i.e., inorganic pyrophosphate plus a proton) from diphosphates 1, 2 and 3, respectively (Scheme 1). From a mechanistic viewpoint, these enzymes catalyze one of the simplest biochemical transformations of prenyl diphosphates.
Scheme 1.
Conversion of FDP (3) and DMADP (1) to EBF (6) and isoprene (4) along concerted (a)12f or stepwise (b and c)2a,12f reaction pathways.
The semiochemical (E)-β-farnesene (EBF, 6) is an acyclic sesquiterpene produced both by plants and animals.2 EBF has been described as a defensive allomone (bees), a trail pheromone (ants), a prey-finding kairomone (beetles), a feeding stimulant (fly), an oviposition stimulant (European corn borer) and a pollination stimulant (bumblebees).2 More importantly, since EBF is used by the majority of aphid species as an alarm pheromone,3 this sesquiterpene is a valuable chemical to control aphid pests in crops.2a,4 To date, cDNAs coding for EBFS have been isolated from several plants,4g,5 and some have been over-expressed in bacterial2a, 6 and plant hosts.2b,4f,g,7 The amino acid sequence of EBFSs,2a amino acid sequence alignments4h,5b,6a,c–e and molecular modeling suggest that EBFSs possess the characteristic class I terpene fold found in all sesquiterpene synthases. 1c EBFS from Mentha x piperita has the diagnostic Asp-rich DDXXD motif (residues 301-305) that coordinates essential Mg2+-ions, and the non-catalytic N-terminal domain found in most plant-derived sesquiterpene synthases. 8,9
Despite the prominent ecological role and economical potential of (E)-β-farnesene, a detailed mechanistic study of the enzymatic reaction catalyzed by EBFS has not been reported. The obvious formation of 6 via the transoid farnesyl cation 9 (Scheme 1, path b) or the possible recombination of 9 with inorganic pyrophosphate (PPi) to yield trans-nerolidyl diphosphate (NDP, 12) as an enzyme-bound intermediate en route to 6 (Scheme 1, path c) were briefly discussed by Crock and colleagues, although no compelling evidence for either proposal was provided.2a The maize sesquiterpene synthase TPS1 has been found to produce, in addition to 6,6b equal amounts of (E)-nerolidol and (3E, 6E)-farnesol, thus supporting the formation of the intermediate trans-farnesyl carbocation (9). Similarly, the co-production of myrcene (5) and linalool or mixtures of myrcene and ocimene by the myrcene synthases from P. frutensens and A. thaliana supports the formation of the geranyl cation intermediate 8 during the elimination reaction. 10,11
An interesting alternative mechanistic possibility for a 1,4-conjugate elimination has recently been considered for isoprene synthase (ISPS). This hemiterpene synthase converts dimethylallyl diphosphate (DMADP, 1) to isoprene and hydrogen diphosphate.12 In plants, isoprene emission protects plants from environmental stresses such as elevated temperatures and oxidative damage; the atmospheric emission of plant-derived isoprene is approximately 100 Tg per year.13 While the dimethyl allyl cation 7 was favored as an intermediate in catalysis,12f a plausible concerted syn-periplanar elimination mechanism was considered based on X-ray crystallographic data, in which the diphosphate-leaving group could serve as the catalytic base (Scheme 1, path a).12f
RESULTS AND DISCUSSION
Here, we examine the mechanistic details of the elimination reactions catalyzed by EBFS and ISPS with the FDP (3) analogs (2Z,6E)-2-fluorofarnesyl diphosphate (2F-3), (6E)-3-fluoromethylfarnesyl diphosphate (3CH2F-3) and (6E)-3-trifluoromethylfarnesyl diphosphate (3CF3-3)15–17 and with DMADP (1) analogs (Z)-[4,4,4-2H3]DMADP and (E)-[4,4,4-2H3]DMADP.14
Depending on the mode of proton elimination from the DMADP analogs, alternative deuterated isoprene products would result that could be distinguished easily by mass spectrometry; regiospecific proton/deuteron elimination should yield a single deuterated product, which could be consistent with a concerted reaction path, whereas non-regiospecific proton/deuteron elimination should yield two deuterated products, consistent with a common dimethylallyl cation intermediate that would exclude a concerted pathway (Scheme 2).
Scheme 2.
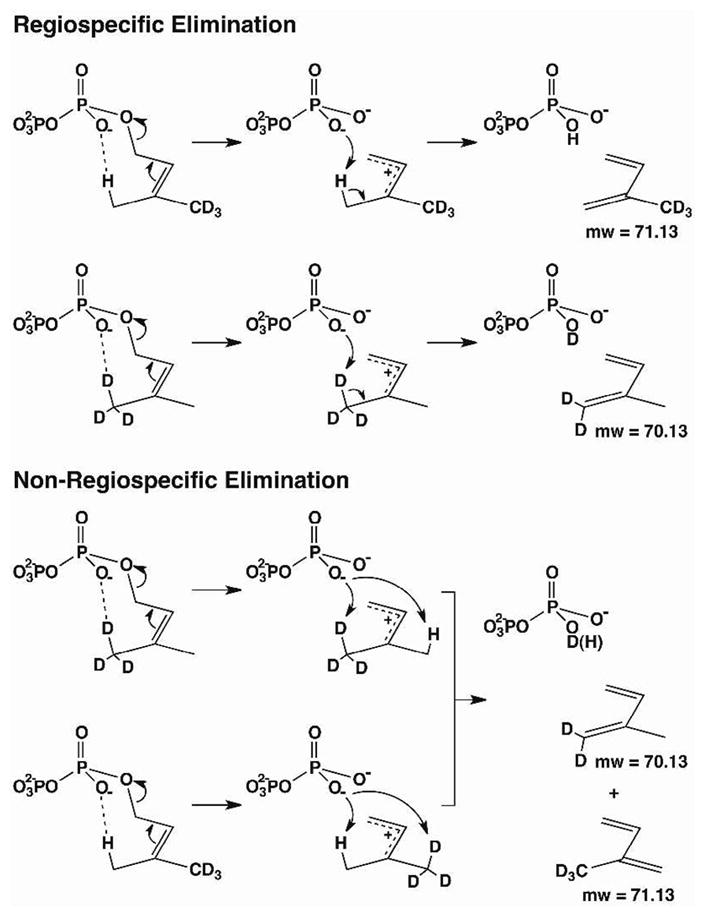
Possible product profiles for the conversion of DMADP (1) to isoprene (4).
For pathways b and c (Scheme 1), the strong electron-withdrawing effect of the vinylic (2F-3) and allylic (3CH2F-3 and 3CF3-3) fluorine substituent(s) is expected to diminish the rate of the formation of trans-farnesyl cation (9). Hence these substrate analogs should act as competitive inhibitors of EBFS. While diphosphates 2F-315,16 and 3CH2F-316c have been used previously, the kinetic evaluation of 3CF3-3 is without precedent in sesquiterpene chemistry. 8a We have also probed the intermediacy of trans-nerolidyl diphosphate (12) in the catalytic cycle of EBFS with (2Z, 6E)-FDP (cis-3) and (3RS)-trans-NDP (12), which were prepared as indicated previously.16b,18,19
Isoprene Synthase
Recombinant ISPS from grey poplar hybrid Populus x canescens with an N-terminal hexahistidine tag to facilitate purification was produced and purified as described.12f Major peaks for isoprene appear in mass spectra at m/z = 68, 67 and 53, which are believed to correspond to the molecular ion and its dehydrogenated and demethylated forms (Figure S3, Supporting Information).
If the ionization and elimination steps are concerted in the ISPS reaction, or if the allylic carbocation-PPi ion pair initially formed by ionization of DMADP is tightly bound, then preferential elimination of a proton from the (Z)-methyl group would be expected based on the conformation of dimethylallyl-S-thiolodiphosphate found in the ISPS active site.12f Consequently, proton elimination from (E)-[4,4,4-2H3]DMADP would exclusively yield [4,4,4-2H3]isoprene, which would generate ions with m/z = 71, 70, 53; proton elimination from (Z)-[4,4,4-2H3]DMADP would exclusively yield [1,1-2H2]isoprene, which would generate ions with m/z = 70, 69, 55 (Scheme 2). However, both (Z)-[4,4,4-2H3]DMADP and (E)-[4,4,4-2H3]DMADP give rise to isoprene yielding ions with m/z = 71, 70, 53 and 70, 69, 55 (Supporting Info). Therefore, both the (Z)-methyl and (E)-methyl groups of DMADP (1) can undergo elimination to generate isoprene, i.e., there is no regiospecificity in the proton elimination step. It follows that the ISPS reaction must proceed through an allylic carbocation intermediate, since DMADP cannot achieve a conformation that would support the concerted departure of PPi with proton abstraction from the (E)-methyl group. If PPi is indeed the general base that receives the proton, as implied from the lack of alternative residues that can perform this function, 12f then there must be sufficient flexibility in the ISPS active site to allow the allylic cation to shift, so that both (E)-methyl and (Z)-methyl groups of 7 are equally accessible to bound PPi, which could serve as the general base. Alternatively, if 10 is an intermediate in the ISPS reaction, then a concerted or stepwise elimination reaction would similarly yield both deuterated isomers of isoprene (Figure 1).
Figure 1.
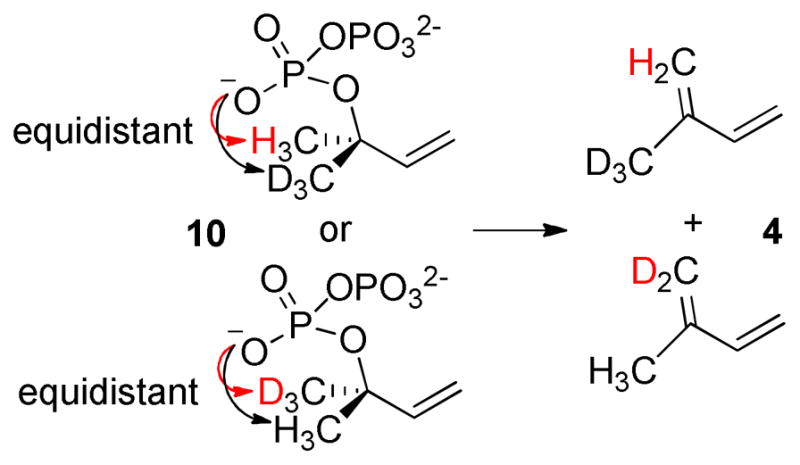
Proposed formation of MW 71 and 70 isoprene (4) products (Scheme 2) from (Z)- and (E)-[4,4,4-2H3]DMADPs via 10. This reaction could be concerted via a 6-membered ring transition state involving inorganic pyrophosphate, or it could proceed in a stepwise fashion through the re-formation of allylic carbocation intermediate 7.
Farnesene synthase
Recombinant (E)-β-farnesene synthase from Mentha x piperita2a was overproduced in E. coli to yield the expected monomeric protein.2 The steady-state kinetic parameters were measured with tritiated 3 (kcat = 0.028 ± 0.002 s−1; KM = 1.8 ± 0.2 μM, Table 1) and were in reasonable agreement with previous reports (KM = 0.6 μM, kcat not determined,2a or KM = 1 μM and kcat = 0.01 s−1).6e However, the product distribution observed here, 95% EBF (6), 1.5% (Z)-β-farnesene (ZBF, 13), 1.3% (Z)-α-farnesene (ZAF, 14), 0.2% (E)-α-farnesene (EAF, 15) and approximately 2% of unidentified material (Figure 2), differs from that previously reported from a partially purified recombinant EBFS (85% 6; 8% 13 and 5% δ-cadinene).2a The identities of EBF (6), ZAF (14) and EAF (15) were established by GC-MS comparisons with an authentic mixture of standards generated from farnesyl acetate with a Pd(0)-catalyst.20
Table 1.
Steady-state kinetic parameters and inhibition constants.a
| KM (μM) | kcat × 10−3 (s−1) | Ki (μM) | |
|---|---|---|---|
| 3 | 1.8 ± 0.2 | 28 ± 0.2 | - |
| 2F-3 | 1.6 ± 0.2 | 0.2 ± 0.1 | 1.3 ± 0.1 |
| 3CH2F-3 | - | - | 2.3 ± 0.2 |
| 3CF3-3 | - | - | 1.6 ± 0.2 |
| (±)-12 | 25.0 ± 4.2 | 23 ± 0.1 | - |
Assays were carried out according to the standard, linear range, microassay procedure (see Supporting Info and Refs 16b and 20). Reported values are the average of 3 (Michaelis-Menten) or 2 (inhibition) measurements; all values were within 5% of the average. Errors are standard deviations for one sigma.
Figure 2.
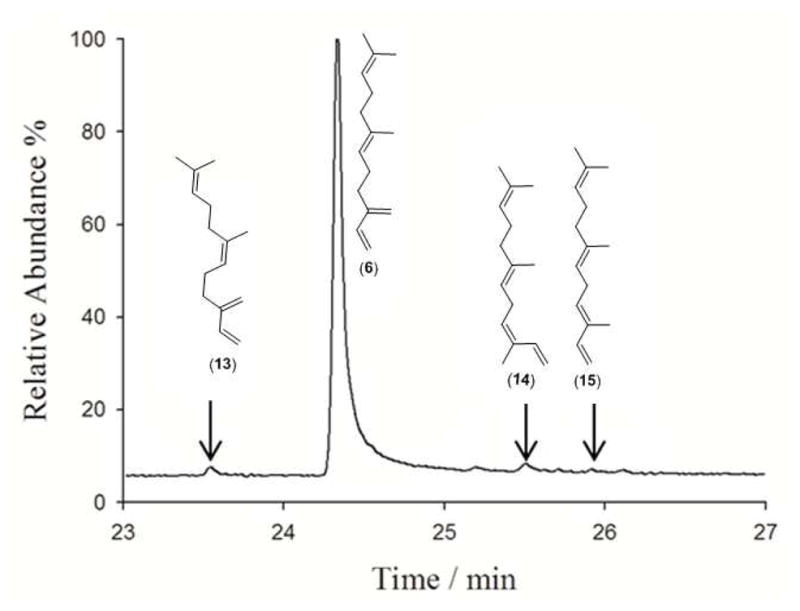
Product profile for the EBFS catalyzed conversion of FDP (3) to (E)-β-farnesene (6), (Z)-β-farnesene (13), (Z)-α-farnesene (14) and (E)-α-farnesene (15).
(2Z, 6E)-2-Fluorofarnesyl (2F-3) proved to be a potent competitive inhibitor of EBFS (Ki of 1.3 ± 0.1 μM), thus suggesting a reaction along either path b or c (Scheme 1). The strong inhibition of EBFS by 2F-3 is comparable with that observed previously for several monoterpene cyclases with 2-fluorogeranyl (2F-2) and 2-fluorolinalyl diphosphate (2F-11). In these cases, fluorinated products were formed albeit at reduced rates.21 Similarly, prolonged incubations (16–18 h) of EBFS (10 μM) with saturating concentrations of 2F-3 (500 μM) generated a single fluorinated hydrocarbon (m/z 222), which was identified by GC-MS as (E)-β-2F-farnesene (2F-6).22 While this observation could in principle be reconciled with a reaction along pathways b or c, it could be interpreted to suggest a concerted process (path a) similar to the one previously discussed for ISPS catalysis.12 To distinguish between the concerted and the stepwise mechanisms, (1RS)-2-fluoro[1-3H1]FDP (2F-[1-3H1]-3) was synthesized23 and assayed with EBFS under standard Michaelis-Menten conditions. While the replacement of trans-FDP (3) by this ‘trans’ fluorinated analog had a negligible effect on the Michaelis constant (KM = 1.6 ± 0.2 μM), the strong electron-withdrawing effect of fluorine reduced the turnover number 140-fold (kcat = 2.0 ± 0.5 × 10−4 s−1, Table 1), thereby confirming the most likely electrophilic nature of the elimination reaction catalyzed by EBFS.
Further support for the stepwise mechanism was obtained from the observation that 15-fluorofarnesyl diphosphate (3CH2F-3) and 15-trifluorofarnesyl diphosphate (3CF3-3) acted as potent competitive inhibitors of EBFS with Ki values of 2.3 ± 0.2 μM and 1.6 ± 0.2 μM, respectively. As expected for reactions proceeding through positively charged intermediates,24 the substitution of hydrogen atoms in the allylic substrate by the strongly electron-withdrawing fluorine atom abolished the formation of fluorinated α- or β-farnesenes as judged by GC-MS, even after incubations of up to 72 h. The kinetic behavior of 3CH2F-3 and 3CF3-3 during EBFS catalysis parallels that previously observed in a study of yeast farnesyl-transferase, in which 3CF3-3 was shown to act as the stronger inhibitor of the farnesylation reaction and the weaker substrate of the transferase enzyme.25
As inferred for the reaction catalyzed by ISPS (Figure 1), the possible involvement of the tertiary allylic diphosphate trans-NDP (12, Scheme 1) as an enzyme-bound intermediate in catalysis by EBFS was examined using (2Z, 6E)-FDP18 (cis-3) and (3RS)-trans-NDP (12).19 GC-MS analysis reveled that EBFS converted cis-3 (and 12) almost exclusively and with high efficiency to (E)-β-farnesene (93%) suggesting that the reactions for both FDP isomers proceed via a common intermediate arising from the plausible collapse of either cis-farnesyl or trans-farnesyl cation to NDP (12). Indeed, (3RS)-(1Z)-trans-[1-3H1]NDP, prepared by stereoselective γ-cis-vinylic metallation26 of racemic transnerolidol, 27 displayed a turnover number (kcat = 0.023 ± 0.001 s−1)28 similar to that measured for FDP (kcat = 0.028 ± 0.002 s−1) in good agreement with a reaction along pathway c (Scheme 1). It is noteworthy that racemic trans-NDP was used in the kinetic analysis and hence the Michaelis constant measured for racemic trans-[1-3H1]NDP (KM = 25.0 ± 4.2 μM, Table 1) is not easily compared with that measured for 3. The steady-state kinetic parameters for trans-FDP (3) and (3RS)-trans-NDP (12) resemble the well-established kinetic behavior observed for the monoterpene substrates 2 and 11 (Scheme 1).1a The higher kcat values observed for the tertiary (3S)- or (3R)-linalyl diphosphate (11) isomers suggest that they are indeed biosynthetic intermediates in reactions catalyzed by several monoterpene synthases.1a,21 Similarly, for trichodiene synthase and δ-cadinene synthase, the formation of trans-NDP (12) from FDP 3 was inferred from comparisons of their kcat values, although in these cases, the turnover number for the presumed intermediate (12) was slightly lower that that measured for 3.29 Thus, in catalysis by EBFS, the almost identical kcat values for trans-NDP (12) and trans-FDP (1) strongly support a stepwise elimination reaction via path c and intermediate 12 (Scheme 1).
CONCLUSION
The data presented here exclude concerted processes and strongly support electrophilic reaction mechanisms for the EBFS and ISPS catalyzed conversions of FDP (3) and DMADP (1) to EBF (6) and isoprene (4), respectively. Furthermore, the kinetic values (Table 1) and the observed deuterium patterns (non-regiospecific elimination, Figure 2) obtained for EBFS and ISPS are consistent with electrophilic reaction pathways via the enzyme bound tertiary diphosphates 12 and 10. By implication, it seems reasonable to speculate that the synthesis of the monoterpene myrcene (5) from geranyl diphosphate (2) will also proceed along a stepwise mechanism presumably via the intermediate linalyl diphosphate (11). Indeed, tertiary diphosphate intermediates could comprise a general feature of all 1,4-conjugate elimination reactions catalyzed by terpene synthases.
The presence of NDP (12), the effective substrate of sesquiterpene cyclases that follow a 1,6 cyclization mechanism, as an intermediate of the reaction catalyzed by EBFS from Mentha x piperita is intriguing, since this plant produces EBF (6) as the only reported acyclic sesquiterpene; however, EBF constitutes only approximately 2% of the total sesquiterpene fraction in the essential oil of pepermint. 2a,30 Furthermore, since the sesquiterpene fraction is rich in cyclic olefins such as 39% β-caryophyllene, 33% γ-cadinene, 2% δ-cadinene, 1.5% germacrene D, 1.3% copaene and 1.3 % α-humulene, which mechanistically may be derived from enzyme-bound trans-NDP (12), it is tempting to suggest that the common precursor2a,6e of sesquiterpene cyclases and EBFS in the secretory glands of Mentha x piperita31 may have been an eliminase without the ability to form C-C bonds. This proposal is in good agreement with the results from a mutational study of two sesquiterpene synthases from Mentha x piperita with homology to EBFS, MxpSS1 (a cyclase utilizing 12 and with 96% amino acid identity to EBFS) and MxpSS2 (an enzyme with 99.6% sequence identity to EBFS, but no activity towards FDP).6e The sesquiterpene cyclases epi-isozizaene synthase (EIZS) from Streptomyces coelicolor and aristolochene synthase from Penicillium roqueforti could be converted into eliminases through single amino acid substitutions that produced EBF in excess of 70%.32 Interestingly, the catalytic efficiency (kcat/KM) of F96A-EIZS is only approximately 14-fold lower than that of peppermint EBFS making the mutant an enzyme with a catalytic performance approaching that of wild type EBFS. Hence, a single point mutation is sufficient to convert a cyclase into an eliminase, or vice versa. While this evolutionary scenario is highly plausible, it is nevertheless not possible to completely exclude that the modern EBFS derives from a peppermint sesquiterpene cyclase that has lost its cyclase activity. 2a The discovery of additional sesquiterpenes cyclases from peppermint, sequence alignments, reciprocal mutagenesis and a phylogenetic reconstruction should allow us to distinguish between these two proposals.
Supplementary Material
Acknowledgments
This work was supported by the United Kingdom’s Biotechnology and Biological Sciences Research Council (BBSRC) through grant BB/G003572/1, the UK’s Engineering and Physical Sciences Research Council (EPSRC) through grant EP/D06958/1 and by Cardiff University. It was also supported by grant GM56838 to D.W.C. from the National Institutes of Health. We thank Dr C. Dale Poulter (University of Utah, USA) for the generous gift of (Z)-[4,4,4-2H3]DMADP and (E)-[4,4,4-2H3]DMADP and Dr. Linda Field (Rothamsted Research, Harpenden, UK) for a cDNA coding for (E)-β-farnesene synthase.
Footnotes
ASSOCIATED CONTENT
Detailed experimental procedures, gas chromatograms, mass spectra and/or NMR spectra of key compounds, as well as inhibition kinetics studies are described in supplementary information. This information is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Croteau R. Chem Rev. 1987;87:929–954. [Google Scholar]; (b) Cane DE. Chem Rev. 1990;90:1089–1093. [Google Scholar]; (c) Christianson DW. Chem Rev. 2006;106:3412–3442. doi: 10.1021/cr050286w. [DOI] [PubMed] [Google Scholar]; (d) Tantillo DE. Nat Prod Rep. 2011;28:1035–1053. doi: 10.1039/c1np00006c. [DOI] [PubMed] [Google Scholar]; (e) Miller DJ, Allemann RK. Nat Prod Rep. 2012;29:60–71. doi: 10.1039/c1np00060h. [DOI] [PubMed] [Google Scholar]; (f) Gao Y, Honzatko RB, Peters RJ. Nat Prod Rep. 2012;29:1153–1175. doi: 10.1039/c2np20059g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crock J, Wildung M, Croteau R. Proc Natl Acad Sci U S A. 1997;94:12833–12838. doi: 10.1073/pnas.94.24.12833.and references cited herein Beale MH, Birkett MA, Bruce TJA, Chamberlain K, Field LM, Huttly AK, Martin JL, Parker R, Phillips AL, Pickett JA, Prosser IA, Shewry PR, Smart LE, Wadhams LJ, Woodcock CM, Zhang Y. Proc Natl Acad Sci U S A. 2006;103:10509–10513. doi: 10.1073/pnas.0603998103.
- 3.(a) Bowers WS, Nault LR, Webb RE, Dutky SR. Science. 1972;177:1121–1122. doi: 10.1126/science.177.4054.1121. [DOI] [PubMed] [Google Scholar]; (b) Edwards LJ, Siddall JB, Dunham LL, Uden P, Kislow CJ. Nature. 1973;241:126–127. [Google Scholar]; (c) Nault LR, Montgomery ME. Science. 1976;192:1349–1351. doi: 10.1126/science.1273595. [DOI] [PubMed] [Google Scholar]; (d) Pickett JA, Griffith DC. J Chem Ecol. 1980;6:349–360. [Google Scholar]; (e) Francis F, Vandermoten S, Verheggen F, Longay G, Haubruge E. J App Entol. 2005;129:6–11. [Google Scholar]; (f) Vandermoten S, Mescher MC, Francis F, Haubruge E, Verheggen FJ. Insect Biochem Mol Biol. 2012;42:155–163. doi: 10.1016/j.ibmb.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 4.(a) Griffiths DC, Pickett JA. Entomol Exp Appl. 1980;27:199–201. [Google Scholar]; (b) Dawson GW, Griffiths DC, Pickett JA, Plumb RT, Woodcock CM, Zhang ZN. Pest Sci. 1988;22:17–30. [Google Scholar]; (c) Gibson RW, Pickett JA. Nature. 1983;302:608–609. [Google Scholar]; (d) Mostafavi R, Henning JA, Gardea-Torresday J, Ray IM. J Chem Ecol. 1996;22:1629–1638. doi: 10.1007/BF02272403. [DOI] [PubMed] [Google Scholar]; (e) Birkett MA, Pickett JA. Phytochemistry. 2003;62:651–656. doi: 10.1016/s0031-9422(02)00568-x. [DOI] [PubMed] [Google Scholar]; (f) Bruce TJA, Birkett MA, Blande J, Hooper AM, Martin JL, Khambay B, Prosser I, Smart LE, Wadhams LJ. Pest Manag Sci. 2005;61:1115–1121. doi: 10.1002/ps.1102. [DOI] [PubMed] [Google Scholar]; (g) Yu XD, Jones HD, Ma Y, Wang G, Xu Z, Zhang B, Zhang Y, Ren G, Pickett JA, Xia LQ. Funct Integr Genomics. 2012;12:207–213. doi: 10.1007/s10142-011-0244-1. [DOI] [PubMed] [Google Scholar]; (h) Yu XD, Pickett JA, Ma YZ, Bruce T, Napier J, Jones HD, Xia LQ. J Integr Plant Biol. 2012;54:282–299. doi: 10.1111/j.1744-7909.2012.01107.x. [DOI] [PubMed] [Google Scholar]; (i) Cui LL, Dong J, Francis F, Liu YJ, Heuskin S, Lognay G, Chen JL, Bragard C, Tooker JF, Liu Y. Crop Protection. 2012;35:91–96. [Google Scholar]
- 5.(a) Salin F, Pauly G, Charon J, Gleizes M. J Plant Physiol. 1995;146:203–209. [Google Scholar]; (c) Kopke D, Beyaert I, Gershenzon J, Hilker M, Schmidt A. Phytochemistry. 2010;71:909–917. doi: 10.1016/j.phytochem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 6.(a) Maruyama T, Ito M, Honda G. Biol Pharm Bull. 2001;24:1171–1175. doi: 10.1248/bpb.24.1171. [DOI] [PubMed] [Google Scholar]; (b) Schnee C, Kollner TG, Gershenzon J, Degenhardt J. Plant Physiol. 2002;130:2049–2060. doi: 10.1104/pp.008326. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Huber DPW, Philippe RN, Godard KA, Sturrock RN, Bohlmann J. Phytochemistry. 2005;66:1427–1439. doi: 10.1016/j.phytochem.2005.04.030. [DOI] [PubMed] [Google Scholar]; (d) Picaud S, Brodelius M, Brodelius PE. Phytochemistry. 2005;66:961–967. doi: 10.1016/j.phytochem.2005.03.027. [DOI] [PubMed] [Google Scholar]; (e) Prosser IM, Adams RJ, Beale MH, Hawkins ND, Philips AL, Pickett JA, Field LM. Phytochemistry. 2006;67:1564–1571. doi: 10.1016/j.phytochem.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Schnee C, Kollner TG, Held M, Turlings TCJ, Gershenzon J, Degenhardt J. Proc Natl Acad Sci U S A. 2006;103:1129–1134. doi: 10.1073/pnas.0508027103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Starks CM, Back K, Chappell J, Noel JP. Science. 1997;277:1815–1820. doi: 10.1126/science.277.5333.1815. [DOI] [PubMed] [Google Scholar]; (b) Gennadios HA, Gonzalez V, Di Costanzo L, Li A, Yu F, Miller DJ, Allemann RK, Christianson DW. Biochemistry. 2009;48:6175–6183. doi: 10.1021/bi900483b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAndrew RP, Peralta-Yahya PP, DeGiovanni A, Pereira JH, Hadi MZ, Keasling JD, Adams PD. Structure. 2011;19:1876–1884. doi: 10.1016/j.str.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Hosoi M, Ito M, Yagura T, Adams RP, Honda G. Biol Pharm Bull. 2004;27:1979–1985. doi: 10.1248/bpb.27.1979. [DOI] [PubMed] [Google Scholar]
- 11.Bohlmann J, Martin D, Oldham NJ, Gershenzon J. Arch Biochem Biophys. 2000;375:261–269. doi: 10.1006/abbi.1999.1669. [DOI] [PubMed] [Google Scholar]
- 12.(a) Silver GM, Fall R. Plant Physiol. 1991;97:1588–1591. doi: 10.1104/pp.97.4.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Silver GM, Fall R. J Biol Chem. 1995;270:13010–13016. doi: 10.1074/jbc.270.22.13010. [DOI] [PubMed] [Google Scholar]; (c) Sharkey TD, Singsass EL. Nature. 1995;374:769. [Google Scholar]; (d) Sharkey TD, Yeh S. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:407–436. doi: 10.1146/annurev.arplant.52.1.407. [DOI] [PubMed] [Google Scholar]; (e) Miller B, Oschinski C, Zimmer W. Planta. 2001;213:483–487. doi: 10.1007/s004250100557. [DOI] [PubMed] [Google Scholar]; (f) Koksal M, Zimmer I, Schnitzler JP, Christianson DW. J Mol Biol. 2010;402:363–373. doi: 10.1016/j.jmb.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Singsass EL, Lerdau M, Winter K, Sharkey TD. Plant Physiol. 1997;115:1413–1420. doi: 10.1104/pp.115.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Behnke K, Ehlting B, Teuber M, Bauerfeind M, Louis S, Hansch R, Polle A, Bohlmann J, Schnitzler JP. Plant J. 2007;51:485–499. doi: 10.1111/j.1365-313X.2007.03157.x. [DOI] [PubMed] [Google Scholar]; (c) Loreto F, Velikova V. Plant Physiol. 2001;127:1781–1787. [PMC free article] [PubMed] [Google Scholar]; (d) Vickers CE, Possell M, Cojocariu CI, Velikova VB, Laothawornkitkul J, Ryan A, Mullineaux PM, Hewitt CN. Plant Cell Environ. 2009;32:520–531. doi: 10.1111/j.1365-3040.2009.01946.x. [DOI] [PubMed] [Google Scholar]; (e) Guenther A, Hewitt CN, Erickson D, Fall R, Geron C, Graedel T, Harley P, Klinger L, Lerdau M, Mckay WA, Pierce T, Scholes B, Steinbrecher R, Tallamraju R, Taylor J, Zimmerman P. J Geophys Res. 1995;100(D5):8873–8892. [Google Scholar]
- 14.Thulasiram HV, Phan RM, Rivera SB, Poulter CD. J Org Chem. 2006;71:1739–1741. doi: 10.1021/jo052384n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Shishova EY, Yu F, Miller DJ, Faraldos JA, Zhao Y, Coates RM, Allemann RK, Cane DE, Christianson DW. J Biol Chem. 2008;283:15431–15439. doi: 10.1074/jbc.M800659200. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Noel JP, Dellas N, Faraldos JA, Zhao M, Hess BA, Jr, Smentek L, Coates RM, O’Maille PE. ACS Chem Biol. 2010;5:377–392. doi: 10.1021/cb900295g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Vedula LS, Zhao YX, Coates RM, Koyama T, Cane DE, Christianson DW. Arch Biochem Biophys. 2007;466:260–266. doi: 10.1016/j.abb.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Faraldos JA, Miller DJ, Gonzalez V, Yoosuf-Aly Z, Cascon O, Li A, Allemann RK. J Am Chem Soc. 2012;134:5900–5908. doi: 10.1021/ja211820p. [DOI] [PubMed] [Google Scholar]; (c) Cascon O, Touchet S, Miller DJ, Gonzalez V, Faraldos JA, Allemann RK. Chem Commun. 2012;48:9702–9704. doi: 10.1039/c2cc35542f. [DOI] [PubMed] [Google Scholar]
- 17.Dolence JM, Poulter CD. Tetrahedron. 1996;52:119–130. [Google Scholar]
- 18.Faraldos JA, O’Maille PE, Dellas N, Noel JP, Coates RM. J Am Chem Soc. 2010;132:4281–4289. doi: 10.1021/ja909886q. [DOI] [PubMed] [Google Scholar]
- 19.(a) Cane DE, Ha HJ. J Am Chem Soc. 1988;110:6865–6870. [Google Scholar]; (b) Cuvigny T, Julia M, Rolando C. J Chem Soc, Chem Commun. 1984:8. [Google Scholar]; (c) Satterwhite DM, Wheeler CJ, Croteau R. J Biol Chem. 1985;260:13901–13908. [PubMed] [Google Scholar]
- 20.Faraldos JA, Gonzalez V, Senske MFRK. Org Biomol Chem. 2011;9:6920–6923. doi: 10.1039/c1ob06184d. [DOI] [PubMed] [Google Scholar]
- 21.(b) Croteau R. Arch Biochem Biophys. 1986;251:777–782. doi: 10.1016/0003-9861(86)90390-5. [DOI] [PubMed] [Google Scholar]; (c) Karp F, Zhao Y, Santhamma B, Assink B, Coates RM, Croteau RB. Arch Biochem Biophys. 2007;468:140–246. doi: 10.1016/j.abb.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hyatt DC, Youn B, Zhao Y, Santhamma B, Coates RM, Croteau R, Kang CH. Proc Natl Acad Sci USA. 2007;104:5360–5365. doi: 10.1073/pnas.0700915104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.An authentic sample of (2F-6) was generated by solvolysis of (2E, 6E)-2F-farnesyl methanesulfonate. Poulter CD, Argyle JC, Mash EA. J Biol Chem. 1978;253:7227–7233.Poulter CD, King CHR. J Am Chem Soc. 1982;104:1422–1424.
- 23.(1RS)-2F-[1-3H1]FDP was prepared exactly as described for (1RS)-trans-[1-3H1]FDP. Cane DE, Iyengar R, Shiao MS. J Am Chem Soc. 1981;103:914–931.Davisson VJ, Zabriskie TM, Poulter CD. Bioorg Chem. 1986;14:46–54.
- 24.Gebler JC, Woodside AB, Poulter CD. J Am Chem Soc. 1992;114:7354–7360. [Google Scholar]
- 25.Dolence JM, Poulter CD. Proc Natl Acad Sci USA. 1995;92:5008–5011. doi: 10.1073/pnas.92.11.5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuvigny T, Julia M, Rolando C. J Chem Soc Chem Commun. 1984:8. [Google Scholar]
- 27.Ha HJ, Cane DE. J Am Chem Soc. 1988;110:6865–6870. [Google Scholar]
- 28.A control reaction without EBFS was assayed at each concentration of diphosphate 12 to correct for the extensive nonenzymatic hydrolysis of (3RS)-trans-NDP.
- 29.(a) Cane DE, Yang G, Xue Q, Shim JH. Biochemistry. 1995;34:2471–2479. doi: 10.1021/bi00008a010. [DOI] [PubMed] [Google Scholar]; (b) Benedict CR, Lu JL, Pettigrew DW, Liu J, Stipanovic RD, Williams HJ. Plant Physiol. 2001;125:1754–1765. doi: 10.1104/pp.125.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croteau R, Karp F. Arch Biochem Biophys. 1979;198:512–522. doi: 10.1016/0003-9861(79)90526-5. [DOI] [PubMed] [Google Scholar]
- 31.Gershenzon J, McCaskill D, Rajaonarivony JIM, Mihaliak C, Karp F, Croteau R. Anal Biochem. 1992;200:130–138. doi: 10.1016/0003-2697(92)90288-i. [DOI] [PubMed] [Google Scholar]
- 32.(a) Aaron JA, Lin X, Cane DE, Christianson DW. Biochemistry. 2010;49:1787–1797. doi: 10.1021/bi902088z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Deligeorgopoulou A, Allemann RK. Biochemistry. 2003;42:7741–7747. doi: 10.1021/bi034410m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



