Abstract
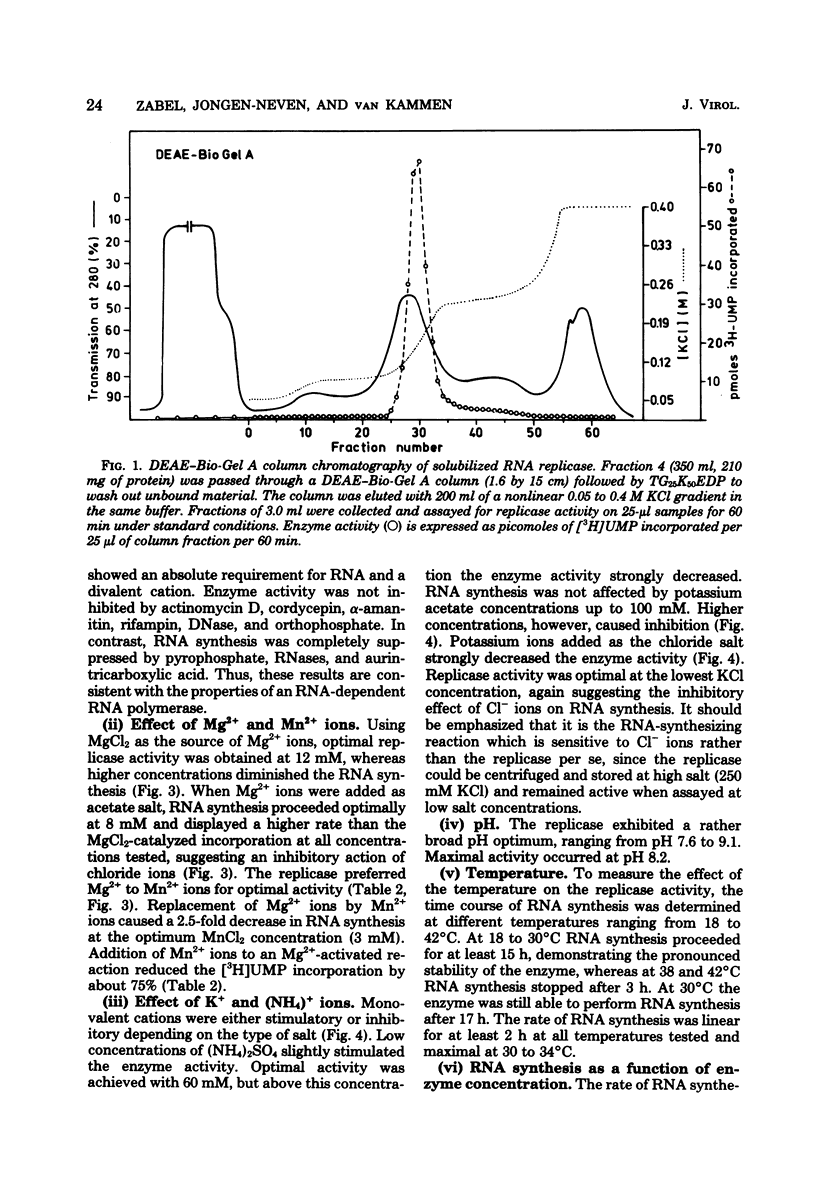
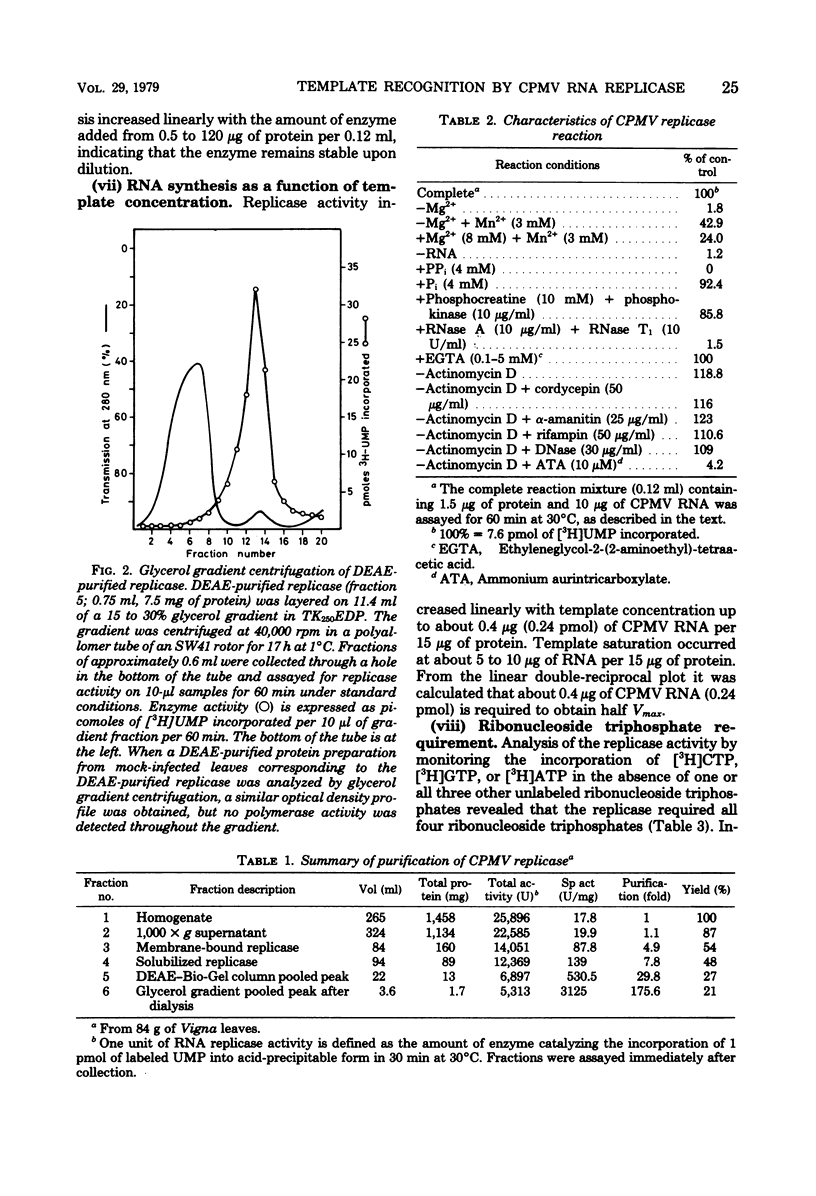
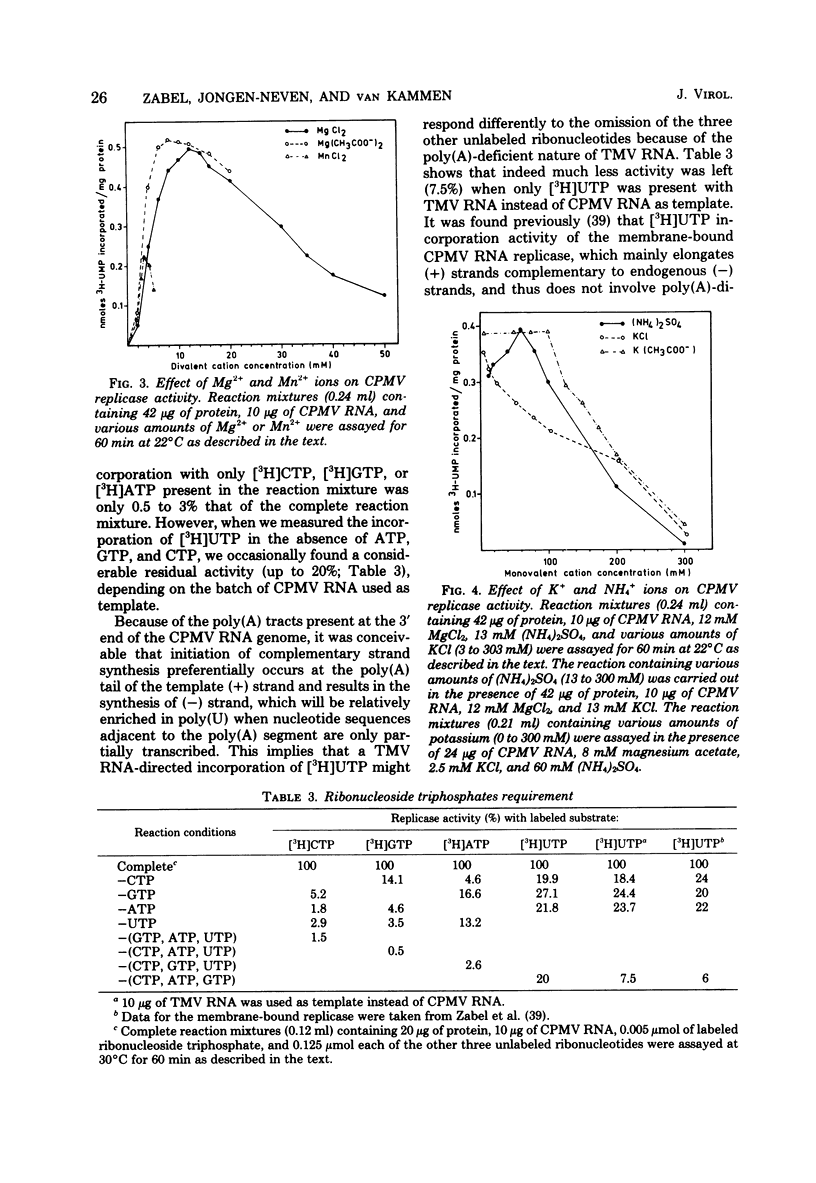
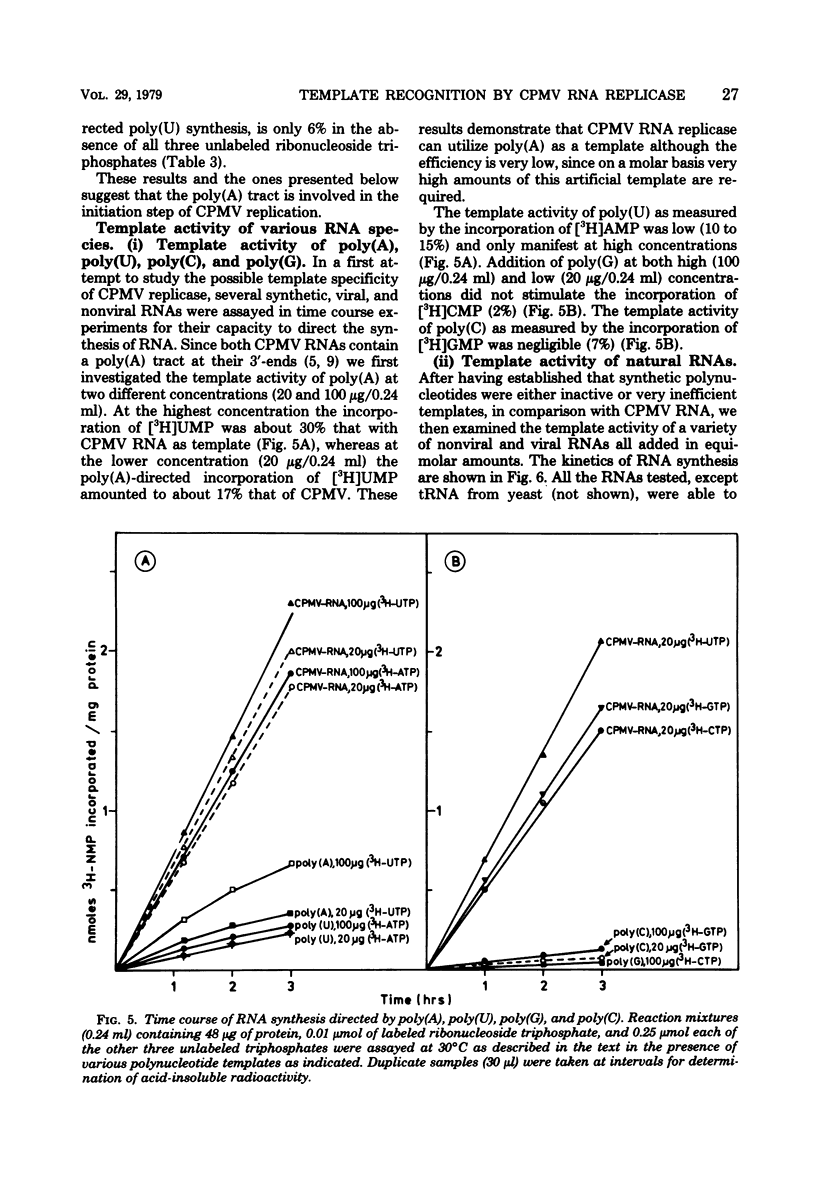
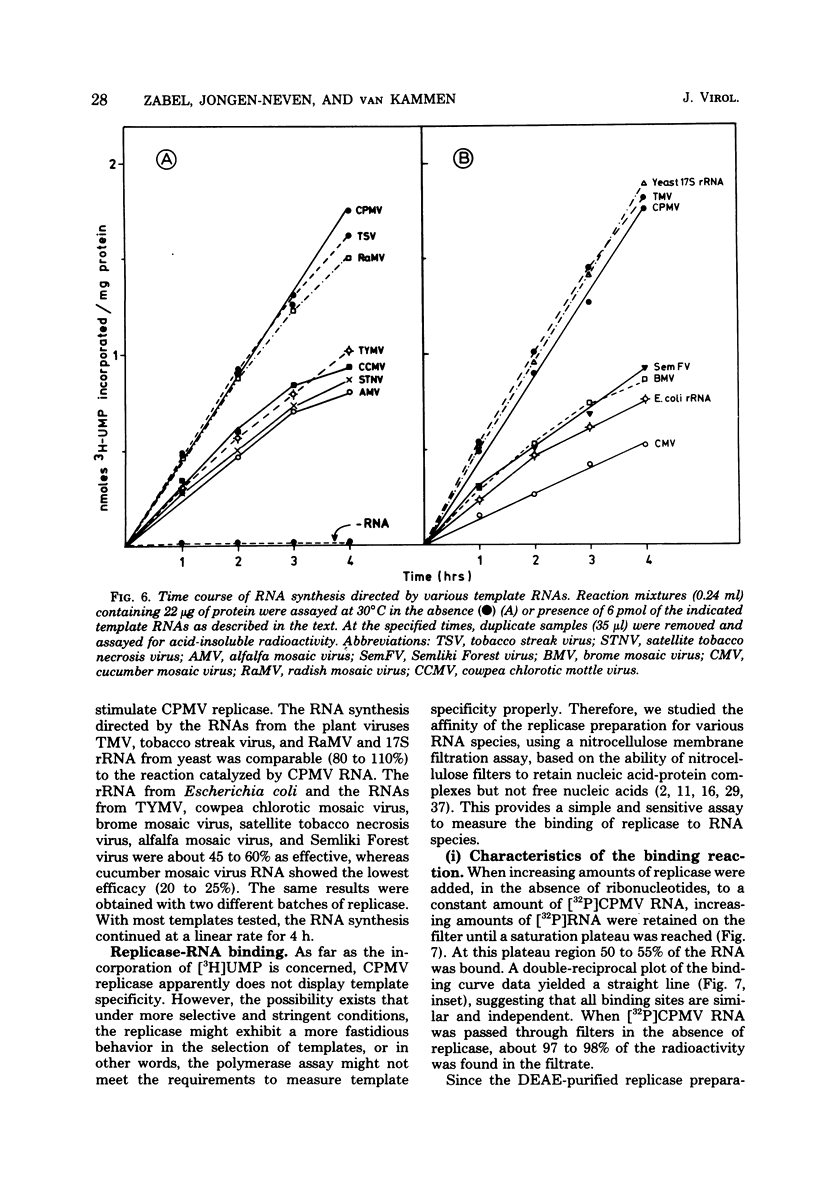
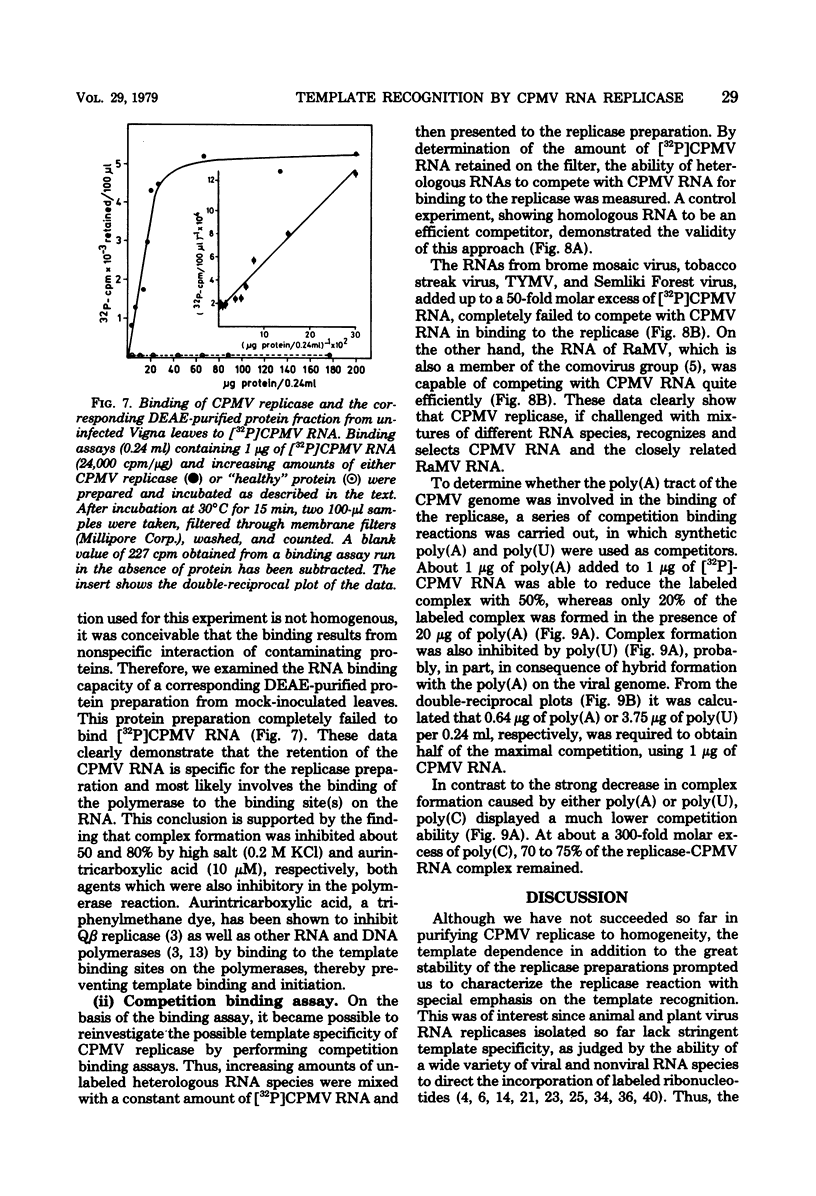
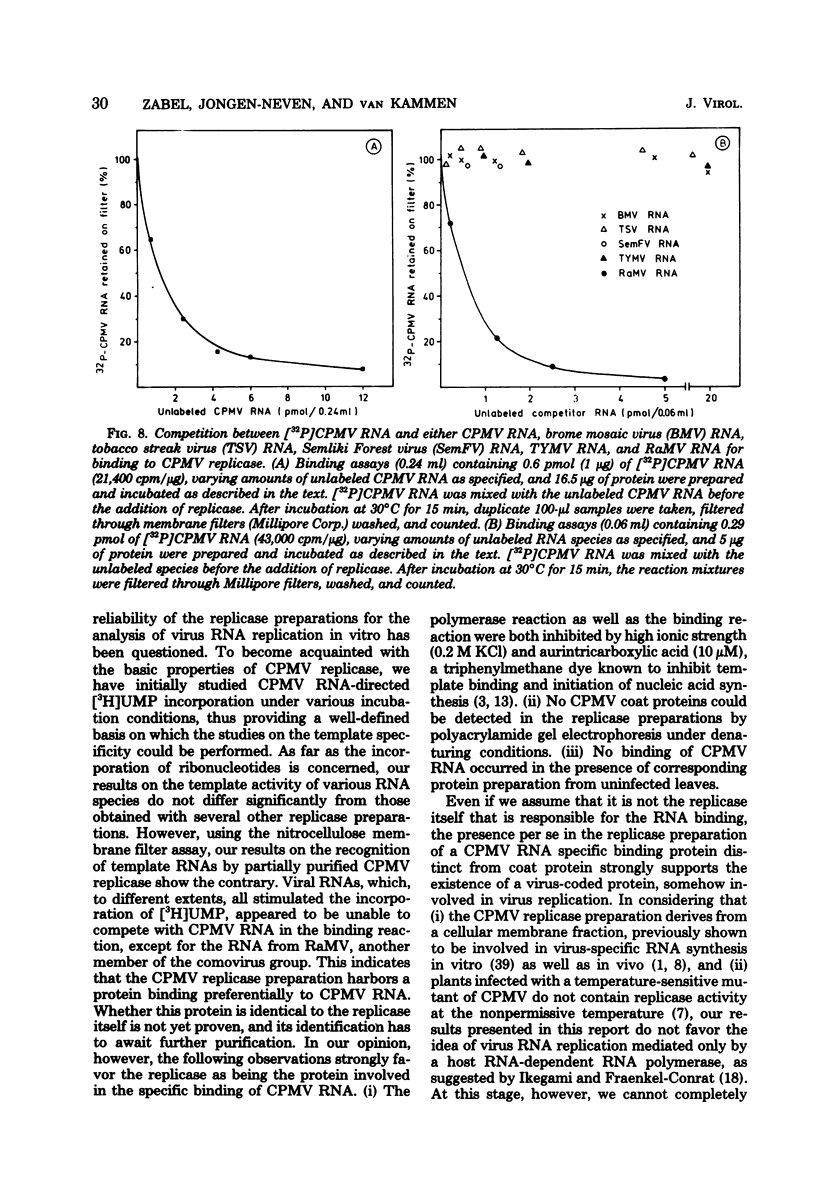
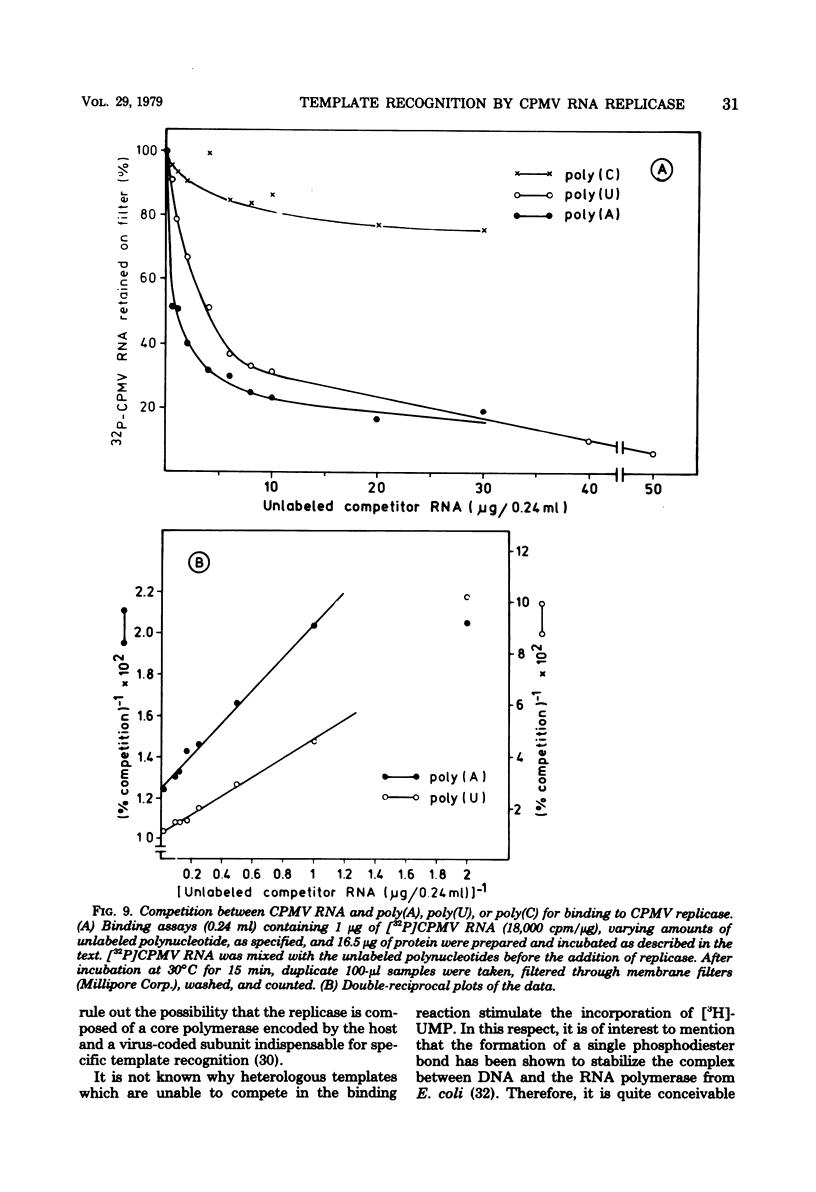
Cowpea mosaic virus (CPMV) RNA replicase has been purified about 200-fold from CPMV-infected Vigna unguiculata leaves. Optimal reaction conditions for replicase activity have been established that allow RNA synthesis to proceed for at least 15 h. Using a polymerase assay under conditions optimal for CPMV RNA-directed RNA synthesis, all natural RNA species tested appeared to be able to direct the incorporation of labeled ribonucleotides, whereas synthetic homoribopolymers were either inactive or only slightly active. Using a nitrocellulose membrane filter assay to measure complex formation between the replicase preparation and various RNA species, all natural RNA species tested, except that of the comovirus radish mosaic virus, appeared to be unable to compete with 32P-labeled CPMV RNA in binding to replicase. We propose that CPMV replicase is actually template specific but does not display this property in a polymerase assay, since labile complexes between heterologous templates and replicase become stabilized by the formation of phosphodiester bonds. From homoribopolymer competition binding experiments we conclude that the polyadenylic acid on the CPMV genome might be a part of the replicase binding site.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assink A. M., Swaans H., Van Kammen A. The localization of virus-specific double-stranded RNA of cowpea mosaic virus in subcellular fractions of infected Vigna leaves. Virology. 1973 Jun;53(2):384–391. doi: 10.1016/0042-6822(73)90218-3. [DOI] [PubMed] [Google Scholar]
- August J. T., Banerjee A. K., Eoyang L., Franze de Fernandez M. T., Hori K., Kuo C. H., Rensing U., Shapiro L. Synthesis of bacteriophage Q-beta RNA. Cold Spring Harb Symp Quant Biol. 1968;33:73–81. doi: 10.1101/sqb.1968.033.01.013. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A. The inhibition of nucleic acid-binding proteins by aurintricarboxylic acid. Biochem Biophys Res Commun. 1973 Dec 10;55(3):680–688. doi: 10.1016/0006-291x(73)91198-4. [DOI] [PubMed] [Google Scholar]
- Brishammar S., Juntti N. Partial purification and characterization of soluble TMV replicase. Virology. 1974 May;59(1):245–253. doi: 10.1016/0042-6822(74)90219-0. [DOI] [PubMed] [Google Scholar]
- Clark G. L., Peden K. W., Symons R. H. Cucumber mosaic virus-induced RNA polymerase: partial purification and properties of the template-free enzyme. Virology. 1974 Dec;62(2):434–443. doi: 10.1016/0042-6822(74)90405-x. [DOI] [PubMed] [Google Scholar]
- Flanegan J. B., Petterson R. F., Ambros V., Hewlett N. J., Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5'-terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci U S A. 1977 Mar;74(3):961–965. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman E. J., Jones O. W. Binding of RNA polymerase to T7 DNA: evidence for minimal number of polymerase molecules required to cause retention of polymerase-T7 DNA complex on membrane filters. Biochem Biophys Res Commun. 1967 Oct 11;29(1):45–52. doi: 10.1016/0006-291x(67)90538-4. [DOI] [PubMed] [Google Scholar]
- Frisby D., Eaton M., Fellner P. Absence of 5' terminal capping in encephalomyocarditis virus RNA. Nucleic Acids Res. 1976 Oct;3(10):2771–2787. doi: 10.1093/nar/3.10.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens J. F., Manly K. F. Inhibition of RNA-directed DNA polymerase by aurintricarboxylic acid. Nucleic Acids Res. 1976 Feb;3(2):405–418. doi: 10.1093/nar/3.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadidi A., Fraenkel-Conrat H. Characterization and specificity of soluble RNA polymerase of brome mosaic virus. Virology. 1973 Apr;52(2):363–372. doi: 10.1016/0042-6822(73)90331-0. [DOI] [PubMed] [Google Scholar]
- Hibi T., Rezelman G., Van Kammen A. Infection of cowpea mesophyll protoplasts with cowpea mosaic virus. Virology. 1975 Apr;64(2):308–318. doi: 10.1016/0042-6822(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Hizi A., Leis J. P., Joklik W. K. The RNA-dependent DNA polymerase of avian sarcoma virus B77. Binding of viral and nonviral ribonucleic acids to the alpha, beta2, and alphabeta forms of the enzyme. J Biol Chem. 1977 Oct 10;252(19):6878–6884. [PubMed] [Google Scholar]
- Hruby D. E., Roberts W. K. Encephalomyocarditis virus RNA. III. Presence of a genome-associated protein. J Virol. 1978 Jan;25(1):413–415. doi: 10.1128/jvi.25.1.413-415.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami M., Fraenkel-Conrat H. RNA-dependent RNA polymerase of tobacco plants. Proc Natl Acad Sci U S A. 1978 May;75(5):2122–2124. doi: 10.1073/pnas.75.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klootwijk J., Klein I., Zabel P., van Kammen A. Cowpea mosaic virus RNAs have neither m7GpppN ... nor mono-, di- or triphosphates at their 5' ends. Cell. 1977 May;11(1):73–82. doi: 10.1016/0092-8674(77)90318-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Roy C., Stussi-Garaud C., Hirth L. RNA-dependent RNA polymerases in uninfected and in alfalfa mosaic virus-infected tobacco plants. Virology. 1977 Oct 1;82(1):48–62. doi: 10.1016/0042-6822(77)90031-9. [DOI] [PubMed] [Google Scholar]
- Lee Y. F., Nomoto A., Detjen B. M., Wimmer E. A protein covalently linked to poliovirus genome RNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):59–63. doi: 10.1073/pnas.74.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J. T., Symons R. H. Specificity of the cucumber mosaic virus-induced RNA polymerase for RNA and polynucleotide templates. Virology. 1971 Jun;44(3):517–526. doi: 10.1016/0042-6822(71)90365-5. [DOI] [PubMed] [Google Scholar]
- Mouches C., Bove C., Bove J. M. Turnip yellow mosaic virus-RNA replicase: partial purification of the enzyme from the solubilized enzyme-template complex. Virology. 1974 Apr;58(2):409–423. doi: 10.1016/0042-6822(74)90076-2. [DOI] [PubMed] [Google Scholar]
- Mouchès C., Bové C., Barreau C., Bové J. M. TYMV RNA replicase: formation of a complex between the purified enzyme and TYMV RNA. Ann Microbiol (Paris) 1976 Jan;127A(1):75–90. [PubMed] [Google Scholar]
- Nomoto A., Detjen B., Pozzatti R., Wimmer E. The location of the polio genome protein in viral RNAs and its implication for RNA synthesis. Nature. 1977 Jul 21;268(5617):208–213. doi: 10.1038/268208a0. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F., Flanegan J. B., Rose J. K., Baltimore D. 5'-Terminal nucleotide sequences of polio virus polyribosomal RNA and virion RNA are identical. Nature. 1977 Jul 21;268(5617):270–272. doi: 10.1038/268270a0. [DOI] [PubMed] [Google Scholar]
- Reijnders L., Aalbers A. M., van Kammen A., Thuring R. W. Molecular weights of plant viral RNAs determined by gel electrophoresis under denaturing conditions. Virology. 1974 Aug;60(2):515–521. doi: 10.1016/0042-6822(74)90345-6. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Romaine C. P., Zaitlin M. RNA-dependent RNA polymerases in uninfected and tobacco mosaic virus-infected tabacco leaves: viral induced stimulation of a host polymerase activity. Virology. 1978 May 1;86(1):241–253. doi: 10.1016/0042-6822(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Sangar D. V., Rowlands D. J., Harris T. J., Brown F. Protein covalently linked to foot-and-mouth disease virus RNA. Nature. 1977 Aug 18;268(5621):648–650. doi: 10.1038/268648a0. [DOI] [PubMed] [Google Scholar]
- So A. G., Downey K. M. Studies on the mechanism of ribonucleic acid synthesis. II. Stabilization of the deoxyribonucleic acid-ribonucleic acid polymerase complex by the formation of a single phosphodiester bond. Biochemistry. 1970 Nov 24;9(24):4788–4793. doi: 10.1021/bi00826a024. [DOI] [PubMed] [Google Scholar]
- Traub A., Duskin B., Rosenberg H., Kalmar E. Isolation and properties of the replicase of encephalomyocarditis virus. J Virol. 1976 May;18(2):375–382. doi: 10.1128/jvi.18.2.375-382.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. L., Murakishi H. H. In vitro replication of tobacco mosaic virus RNA in tobacco callus cultures: solubilization of membrane-bound replicase and partial purification. J Virol. 1977 Feb;21(2):484–492. doi: 10.1128/jvi.21.2.484-492.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M., Berg P. Recognition of tRNA by aminoacyl tRNA synthetases. J Mol Biol. 1967 Sep 28;28(3):479–490. doi: 10.1016/s0022-2836(67)80098-6. [DOI] [PubMed] [Google Scholar]
- Zabel P., Jongen-Neven I., Van Krammen A. In vitro replication of cowpea mosaic virus RNA. II. Solubilization of membrane-bound replicase and the partial purification of the solubilized enzyme. J Virol. 1976 Mar;17(3):679–685. doi: 10.1128/jvi.17.3.679-685.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel P., Weenen-Swaans H., van Kammen A. In vitro replication of cowpea mosaic virus RNA: I. Isolation and properties of the membrane-bound replicase. J Virol. 1974 Nov;14(5):1049–1055. doi: 10.1128/jvi.14.5.1049-1055.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitlin M., Duda C. T., Petti M. A. Replication of tobacco mosaic virus. V. Properties of the bound and solubilized replicase. Virology. 1973 Jun;53(2):300–311. doi: 10.1016/0042-6822(73)90207-9. [DOI] [PubMed] [Google Scholar]
- de Jager C. P., Zabel P., van der Beek C. P., van Kammen A. Genetic and physiological characterization of a temperature-sensitive mutant of cowpea mosaic virus. Virology. 1977 Jan;76(1):164–172. doi: 10.1016/0042-6822(77)90293-8. [DOI] [PubMed] [Google Scholar]
- de Zoeten G. A., Assink A. M., van Kammen A. Association of cowpea mosaic virus-induced double-stranded RNA with a cytopathological structure in infected cells. Virology. 1974 Jun;59(2):341–355. doi: 10.1016/0042-6822(74)90449-8. [DOI] [PubMed] [Google Scholar]
- el-Manna M. M., Bruening G. Polyadenylate sequences in the ribonucleic acids of cowpea mosaic virus. Virology. 1973 Nov;56(1):198–206. doi: 10.1016/0042-6822(73)90299-7. [DOI] [PubMed] [Google Scholar]



