Abstract
Two experiments tested the effects of food deprivation on discounting in pigeons. An adjusting-amount procedure was used to estimate the subjective value of food at delays ranging from 1 to 24 s. Experiment 1 compared pigeons’ discounting of delayed food reinforcers at 75-80% and 90-95% of free-feeding weight. Experiment 2 compared discounting under 1-hr and 23-hr food deprivation. In both experiments at both deprivation levels, discounting was well described by the hyperboloid discounting function. No systematic effect of level of deprivation on degree of discounting was observed in either experiment. This finding is consistent with the view that pigeons’ choices are controlled by the relative, rather than the absolute, value of reinforcers.
The choices made by humans and other animals often follow simple rules when the alternatives differ on a single dimension: larger rewards are preferred to smaller rewards; certain rewards are preferred to uncertain rewards; and immediate rewards are preferred to delayed rewards. When alternatives differ on two or more dimensions, however, predicting an organism’s choices becomes more difficult (Green & Myerson, 2004; Keeney & Raiffa, 1993). For example, consider the case when the choice is between a smaller, sooner reward and a larger, later reward. Delay discounting refers to the increase in the tendency to choose the smaller, sooner reward as the delay to the larger, later reward increases, and is assumed to reflect a decrease in the subjective value of the delayed reward. Both human and nonhuman discounting data are well described by a hyperboloid discounting function of the form:
| (1) |
where V is the present, subjective value of a delayed reward of amount A, D is the delay to its receipt, k is a parameter that reflects the discount rate, with larger values representing steeper discounting, and s is a parameter that represents the nonlinear scaling of the delay to the reward and its amount (Green, Fry, & Myerson, 1994).
Although Equation 1 describes the discounting results for every species and reinforcer studied to date (Green & Myerson, 2010), several differences between the discounting behavior of human and nonhuman animals have been consistently observed. With nonhumans, for example, the s parameter rarely differs significantly from 1.0 (Green, Myerson, Holt, Slevin, & Estle, 2004), so that Equation 1 reduces to a simple hyperbola (Mazur, 1987), whereas with humans, the value of s is often significantly less than 1.0 (for a review, see Green & Myerson, 2004).
Another difference concerns the occurrence of magnitude effects, which are a reliable finding with human subjects. For example, Green, Myerson, and McFadden (1997) studied humans’ discounting of four different amounts of delayed hypothetical rewards ($100, $2,000, $25,000, and $100,000) and found that the degree of discounting decreased as the amount of the delayed reward increased. A similar magnitude effect has been obtained with real delayed monetary rewards (Johnson & Bickel, 2002) and also with real delayed liquid rewards (Jimura, Myerson, Hilgard, Braver, & Green, 2009). In contrast, using an adjusting-amount procedure similar to that used in human studies to assess subjective value, researchers have found that the degree to which nonhuman animals discount delayed rewards is usually not affected by the amount of the reward (Calvert, Green, & Myerson, 2010; Green et al., 2004; Richards, Mitchell, de Wit, & Seiden, 1997). Grace, Sargisson, and White (2012) have reported a magnitude effect in pigeons using relative rate of key pecking as a measure (cf Grace, 1999), but this measure is quite different from that used to establish magnitude effects in humans.
The degree to which humans discount delayed monetary rewards also is affected by their income level but in a manner opposite to what might be expected based on the magnitude effect: Individuals with lower incomes discount delayed rewards more steeply than individuals with higher incomes, as first predicted by Fisher (1907), and subsequently observed in both economic data (e.g., Hausman, 1979; Lawrance, 1991) and laboratory studies (e.g., Green, Myerson, Lichtman, Rosen, & Fry, 1996). This finding is somewhat surprising given the magnitude effect, because one might have expected that individuals with lower incomes, who are presumably more deprived, would place a greater value on monetary rewards and therefore discount them less steeply. However, the reverse appears to be true: Poorer individuals discount more steeply. Similarly, depriving drug-dependent individuals of their drug of choice (nicotine or opiates), thereby increasing the value of the drug reward, increases the degree of discounting (e.g., Field, Santarcangelo, Sumnall, Goudie, & Cole, 2006; Giordano, Bickel, Loewenstein, Jacobs, Marsch, & Badger, 2002). Further research is needed to resolve the paradoxical relations among deprivation, value, and discounting.
Of course, one cannot experimentally manipulate people’s income levels and thereby affect their level of deprivation. One can manipulate people’s hypothetical incomes (Weatherly, 2012), but another approach to this issue would be to use an animal model and manipulate level of deprivation directly. Past research with animal models investigating the relation between deprivation and delay to different rewards has yielded inconsistent results. Some studies have reported that choice of a larger or more highly valued reinforcer was greater at higher deprivation levels than at lower deprivation levels (e.g., Bradshaw & Szabadi, 1992; Christensen-Szalanski, Goldberg, Anderson, & Mitchell, 1980; Hastjarjo & Silberberg, 1992), a pattern reminiscent of a magnitude effect. However, other studies have reported that choice of a larger reinforcer was greater at lower deprivation levels (e.g., Eisenberger, Masterson, & Lowman, 1982; Snyderman, 1983), a result similar to Green et al. (1996), or that deprivation had no systematic effect on choice (e.g., Logue & Peña-Correal, 1985).
More recently, the effect of deprivation on discounting has been examined by mapping out the delay discounting function in rats. Richards et al. (1997) examined the effect of water deprivation and found no systematic effect. Ostaszewski, Karzel, and Szafrańska (2003) examined the effect of restricting access to food on delay discounting in two experiments. In the first experiment, there were no significant differences in rate of discounting between deprivation conditions; in the second experiment, older rats discounted more steeply under strong deprivation but younger rats did not. The present study, unlike these previous efforts, addresses this issue by manipulating deprivation level in pigeons in two separate ways so as to unconfound two important aspects of deprivation: In Experiment 1, body weight was manipulated while time since last feeding was held constant, and in Experiment 2, time since the last feeding was varied while body weight was held constant.
The question of whether deprivation affects discounting has taken on additional significance in the light of recent studies examining the effect of reinforcer value on discounting rate. Calvert et al. (2010) examined rats’ discounting of highly preferred and less preferred reinforcers, and no systematic differences in discounting rates were observed, regardless of whether liquid reinforcers or flavored food pellets were used for the comparison. Similarly, Freeman, Nonnemacher, Green, Myerson, and Woolverton (2012) found that monkeys discounted delayed 10% and 20% sucrose solutions at equivalent rates, despite the fact that they preferred the sweeter solution. The present study takes a different approach to the issue of the discounting of reinforcers of higher and lower value by using deprivation as a way to manipulate reinforcer value.
EXPERIMENT 1
Method
Subjects
Five female White Carneau pigeons (Columba livia), all of whom had previous experience with discounting procedures, were used as subjects. Four completed all three phases of an ABA design; the fifth pigeon died after completing the first two phases. In the different phases of the experiment, the pigeons were maintained at two different levels of food deprivation: high deprivation (75-80% of free-feeding weight) and low deprivation (90-95% of free-feeding weight). Deprivation level was maintained by providing post-session feeding when necessary. The pigeons were housed in individual home cages where they had continuous access to water and grit and were maintained on a 12:12 hr light:dark cycle.
Apparatus
Two experimental chambers (Med Associates, Inc.), each measuring 29-cm long by 25-cm wide by 28.5-cm high, were located within sound- and light-attenuating enclosures equipped with ventilation fans. Three response keys, spaced 8 cm apart, center to center, were mounted on the front panel of the chamber. The right- and left-most keys, located 23.5 cm above the grid floor and 3.5 cm from the side walls of the chamber, could be transilluminated with green and red light, respectively. The center key, located 19 cm above the floor, could be transilluminated with yellow light, and a triple-cue light, equipped with green, yellow, and red bulbs, was located 7.5 cm above the center key. Two food magazines, mounted directly below the right and left keys and 4 cm above the grid floor, were each equipped with an infrared head entry detector and a 7-W white light that was illuminated during reinforcement. Two pellet dispensers (Med Associates, Inc.), mounted behind the front panel, delivered 14-mg precision food pellets (TestDiet®) at the rate of one pellet every 0.6 s. A 7-W houselight was mounted centrally on the ceiling of the chamber. Med-PC™ software (Med-Associates, Inc.) was used to control experimental events and record responses.
Procedure
The first phase of the ABA design was low deprivation for three pigeons (P65, P75, and P77) and high deprivation for two pigeons (P61 and P64). Within each phase, each pigeon experienced the six conditions (delays of 1, 2, 4, 8, 16, and 24 s to the larger reinforcer) in a different order.
Each experimental session consisted of both free-choice and forced-choice trials. On free-choice trials, the pigeons chose between a smaller, immediate reinforcer, which was associated with the left (red) choice key, and a larger, but delayed reinforcer, which was associated with the right (green) choice key. Only one choice key was transilluminated on forced-choice trials. Each session began with four forced-choice trials, two of each kind, presented in a random order, which were followed by 20 free-choice trials. If a pigeon chose the same key on four consecutive free-choice trials, a single forced-choice trial involving the other alternative would follow. Sessions were conducted daily and ended after the subjects had completed the 20 free-choice trials or 75 minutes had passed, whichever occurred first.
The beginning of a free-choice trial was signaled by the illumination of the center yellow response key and the houselight. A single response on the yellow key turned off the center key light, and illuminated the red and green choice keys and the corresponding cue lights; on forced-choice trials, only one key and its corresponding cue light were illuminated. A single response on the green choice key darkened both choice keys, the red cue light, and the houselight, and initiated the delay to reinforcement during which the green cue light remained illuminated. After the delay interval had elapsed, the cue light was extinguished, the right magazine light was illuminated, and 32 food pellets were delivered. A single response on the red choice key darkened both choice keys, the green cue light, and the houselight. The red cue light remained illuminated for 0.5 s, after which the cue light was extinguished, the left magazine light was illuminated, and an adjusting number of pellets was delivered. The magazine remained illuminated throughout pellet delivery and until 3 s had elapsed without the pigeon breaking the infrared beam, after which the magazine light was turned off and the pigeon remained in blackout for the remainder of the trial. All trials had a fixed duration of 70 seconds regardless of which alternative was chosen.
An adjusting-amount procedure was used to estimate the subjective value of the delayed reinforcer in each condition. If a pigeon preferred the smaller, immediate reinforcer on more than 60% of the trials for three consecutive days, the amount of immediate reinforcer was decreased beginning with the next session; similarly, if the pigeon preferred the smaller, immediate reinforcer on less than 40% of the trials for three consecutive days, the amount of immediate reinforcer was increased. Indifference was defined as a period of three consecutive sessions in which the pigeon chose the smaller, immediate reinforcer on 40 to 60% of the trials (i.e., between 8 and 12 out of 20 free-choice trials in each session). The subjective value of a delayed reinforcer was estimated as the number of pellets available immediately when the pigeon was indifferent between the smaller, immediate amount and the 32-pellet, delayed amount.
At the beginning of each delay condition, pigeons chose between 32 delayed pellets and 16 immediate pellets. If the pigeon preferred the delayed reinforcer, then the immediate amount was increased to 24 pellets (i.e., half of the difference between the smaller and larger rewards); if the pigeon preferred the immediate reinforcer, its amount was decreased to 8 pellets. Each subsequent adjustment was half the size of the preceding adjustment until the size of the adjustment was one pellet, after which the immediate amount was increased or decreased by one pellet until indifference was observed. The mean number of sessions was 26.8 (SD = 6.3) for the low deprivation phase and 29.7 (SD = 12.5) for the high deprivation phase. For eight conditions, replications were conducted, and for these conditions, the average was used in all data analyses.
Results and Discussion
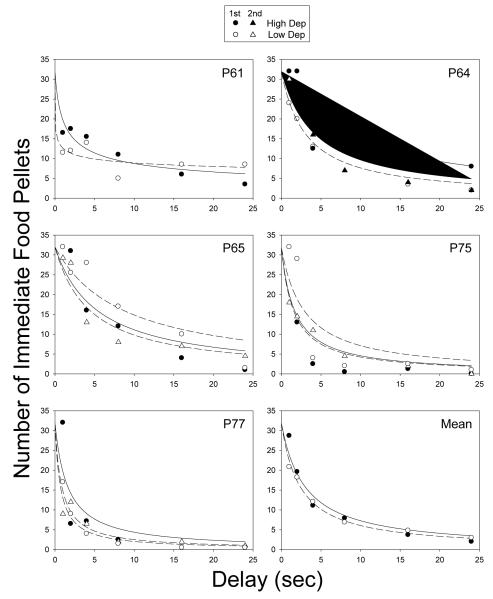
Figure 1 shows the number of immediate food pellets at indifference (i.e., the subjective value of the 32-pellet reinforcer) plotted as a function of delay. As may be seen, the subjective value of the delayed 32-pellet reinforcer tended to decrease systematically as the delay to its receipt increased. For P61, the s parameter was significantly less than 1.0 for both the high and low deprivation phases, t(4) = 3.77 (p = .02) and 7.81 (p = .001), respectively, and the s parameter was nearly significantly less than 1.0 for P77 in the first low deprivation phase, t(4) = 2.56 (p = .06). In all other cases, the s parameter was not significantly less than 1.0 for either deprivation phase, all ts < 1.50 (all ps > .20). Excluding P61, the fits of the simple hyperbola (Eq. 1 with s = 1.0) to the data from each deprivation phase were generally good, with a median R2 of .85. Estimates of the k parameter and R2s for each pigeon in each deprivation phase are provided in Table 1. There was no significant difference between the logarithms of the k values for the high and low deprivation phases; t(4) = 1.73, ns.
Figure 1.
Number of immediate food pellets at indifference (i.e., the subjective value of the 32-pellet reinforcer) plotted as a function of delay for Experiment 1. Symbols represent the estimated indifference points, and the curves represent the best-fitting hyperboloid discounting function (Eq.1) with s = 1.0 (i.e., a simple hyperbola), for all pigeons except P61 for whom s was a free parameter (see text). The high-deprivation phases are represented by solid curves and filled symbols; the low-deprivation phases are represented by dashed curves and open symbols. For individual pigeons, circles represent the first determination and triangles represent the second determination; for the group mean, symbols represent the average of the first and second determinations. (In cases where symbols appear to be missing, it is because data values from different deprivation phases are nearly equivalent.)
Table 1.
Proportion of variance accounted for and parameters of Equation 1 for the high and low deprivation phases for each pigeon in Experiment 1, presented in the order in which the phases were studied. Rk2 indicates the proportion of variance accounted for by Equation 1 with s = 1.0, and Rk,s2 indicates the proportion of variance accounted for by Equation 1 with s as a free parameter for the one pigeon (P61) for which s was significantly different from 1.0.
| Pigeon | Deprivation Phase* |
Rk2 [Rk,s2] |
k [k; s] |
|---|---|---|---|
| 61 | High | .62 [.81] |
0.383 [2.25; 0.411] |
| Low | .00 [.34] |
0.734 [4.56; 0.152] |
|
| 64 | High | .75 | 0.123 |
| Low | .96 | 0.319 | |
| High | .88 | 0.232 | |
| 65 | Low | .86 | 0.116 |
| High | .85 | 0.188 | |
| Low | .88 | 0.227 | |
| 75 | Low | .71 | 0.350 |
| High | .72 | 0.600 | |
| Low | .96 | 0.653 | |
| 77 | Low | .94 | 1.173 |
| High | .68 | 0.631 | |
| Low | .65 | 1.468 |
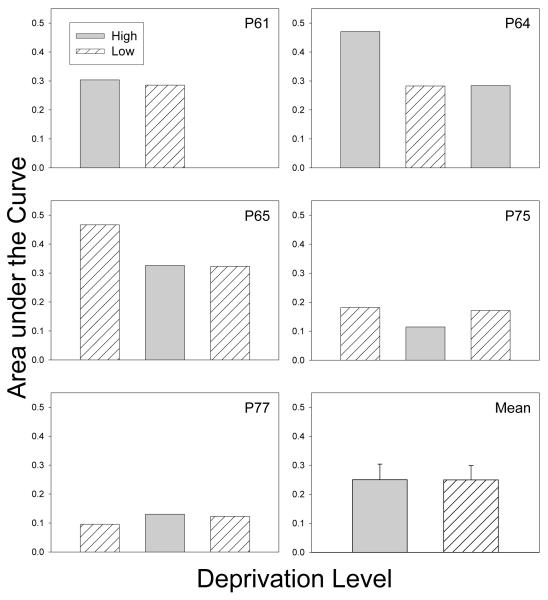
The degree of discounting in the low and high deprivation phases also was compared using the atheoretical Area-Under-the-Curve (AuC) measure. The AuC is calculated based on the obtained indifference points (rather than a fitted curve) and can vary between 0.0 and 1.0; the steeper the discounting, the closer the AuC will be to 0.0 (for details, see Myerson, Green, & Warusawitharana, 2001). Figure 2 shows the AuCs for the different deprivation phases. For each pigeon, data are presented in the order in which the phases were studied. The grand means for the low and high deprivation phases (lower right panel) were calculated based on one low-deprivation AuC and one high-deprivation AuC for each pigeon (after averaging across the two A conditions of the ABA design). There was no significant difference between the AuCs for the high and low deprivation phases; t(4) < 1.0. Thus, regardless of whether the degree of discounting is measured by the AuC or the individual estimates of the k parameter, there was no systematic difference in discounting under low and high deprivation.
Figure 2.
Area under the Curve for the low and high deprivation phases for each pigeon and for the group mean in Experiment 1. For the group means (bottom right panel), bars represent the standard errors.
The finding that pigeons’ discounting of delayed food is apparently unaffected by their level of deprivation is somewhat surprising. At the least, it suggests that high and low body weight may not be good analogs of low and high income levels in humans, because humans with lower incomes discount more steeply than those with higher incomes (Green et al., 1996). It is possible, however, that the present results are peculiar to the specific way in which deprivation was operationalized in Experiment 1. Accordingly, in Experiment 2 we held body weight constant and manipulated deprivation by varying the time from last feeding to the beginning of the experimental session.
EXPERIMENT 2
Method
Subjects and Apparatus
Six experimentally naïve male White Carneau pigeons (Columba livia) were used as subjects. The pigeons were maintained at 80-85% of their free-feeding weight. As in Experiment 1, the pigeons were housed in individual home cages on a 12:12 hr light:dark cycle with continuous access to water and grit. The experimental chambers were the same as those used in Experiment 1 but without infrared photo detectors.
Procedure
The procedure was similar to that used in Experiment 1, with the most significant difference being that in both low (1 hr) and high (23 hr) deprivation phases of the experiment, pigeons were at 80-85% of their free-feeding weight at the start of the session. To accomplish this, pigeons in the low deprivation phases were weighed and given supplemental food if necessary right after each experimental session, and weighed again and fed 15 grams of pigeon checkers 1 hr prior to the session, whereas pigeons in the high deprivation phases were weighed, but not fed, prior to the session and fed right after the session when necessary. In both phases, pigeons were not run on days on which they did not meet the 80-85% weight criterion. Each pigeon was exposed to five delay conditions (1, 3, 6, 12, and 24 s) at each level of deprivation in a unique order. Another notable difference in procedure was that deprivation level alternated between 23 hr and 1 hr every time the delay condition changed (e.g., for P81, the first four conditions, out of the ten total conditions, were 24-s delay at low deprivation, followed by 1-s delay at high deprivation, then 12-s delay at low deprivation, and 6-s delay at high deprivation).
As in Experiment 1, sessions consisted of four forced-choice trials followed by free-choice trials. Daily sessions ended after 20 free-choice trials or after 60 min had elapsed, whichever occurred first. Unlike in Experiment 1, the food magazine light remained illuminated for 5 s after the last pellet was delivered. Also as in Experiment 1, an adjusting-amount procedure was used to determine the subjective value of delayed reinforcers, and indifference was defined as a period of three consecutive sessions in which preference was between 40 to 60%. However, the procedure differed from that in the preceding experiment in that the smallest adjustment in immediate amount was 2 pellets, rather than l.
The procedure used to determine the subjective value of the delayed reinforcers also was modified from that in the preceding experiment. In the present experiment, a delay condition was terminated after a single determination of subjective value only if the pigeon was indifferent between 16 pellets immediately and 32 pellets later (in which case the subjective value of the delayed 32-pellet reinforcer was taken to be 16 pellets) or if the pigeon’s choices resulted in the immediate amount being adjusted to either of the two extreme values (2 or 30 pellets). If the immediate amount was adjusted to one of the extreme values, and the pigeon was then indifferent, that immediate amount was taken to be the subjective value of the delayed reinforcer. If, however, the pigeon maintained its preference for either the immediate or the delayed reinforcer, then the subjective value of the delayed reinforcer was taken to be either 1 or 31 pellets, respectively.
If none of the preceding occurred, then the immediate amount was increased or decreased, depending on the pigeon’s preference, until its preference reversed. In this case, the pigeon’s indifference point was assumed to be midway between the amount that resulted in the reversal and the preceding amount, and a second determination of indifference was made. Again, the amount of immediate reinforcer was increased or decreased based on the pigeon’s choices until either a second preference reversal occurred or the immediate amount reached an extreme value. If a reversal occurred, then the second indifference point, like the first, was assumed to be midway between the amount that resulted in the reversal and the preceding amount. If a reversal did not occur, and therefore the immediate amount was ultimately adjusted to an extreme value, then the indifference point was assumed to be either the extreme value, if the pigeon was indifferent at that point, or if the pigeon still showed a preference, midway between the immediate amount and either 0 or 32 pellets, whichever was appropriate. Finally, the average of the first and second indifference points was used as the point of subjective equality between the immediate and delayed reinforcers. The mean number of sessions was 23.8 (SD = 9.2) for the low (1 hr) deprivation phases and 24.3 (SD = 7.2) for the high (23 hr) deprivation phases.
Results and Discussion
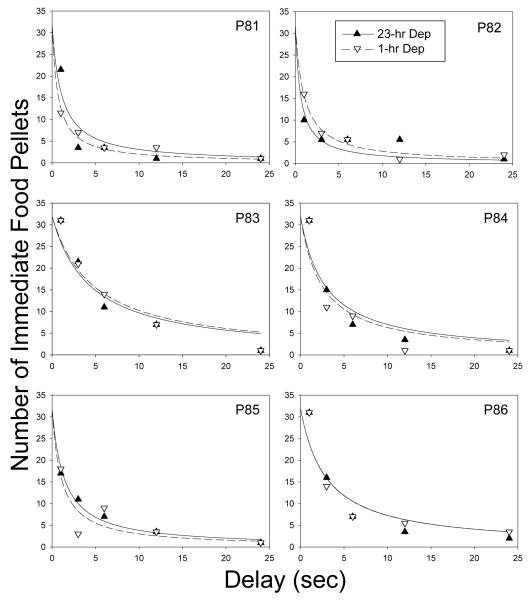
Figure 3 shows the number of immediate food pellets at indifference (i.e., the subjective value of the 32-pellet reinforcer) plotted as a function of delay for each of the six pigeons. Similar to what was observed in Experiment 1, the subjective value of the larger reinforcer decreased systematically for each pigeon as the delay to its receipt increased at both low and high deprivation. In 11 out of 12 cases (6 pigeons in 2 deprivation phases), discounting was well described by a simple hyperbola, with a median R2 of .87. The one exception was P82 in the 23-hr deprivation phase, for whom the s parameter in Equation 1 was significantly less than 1.0, t(3) = 4.33, p < .05. In only one other case, P81 in the 1-hr deprivation phase, was s significantly less than 1.0, t(3) = 3.35, p < .05. Estimates of the k parameter and R2s for each pigeon in the two deprivation phases (and s, where appropriate) are provided in Table 2. There was no significant difference between the logarithms of the k values for the 23-hr and 1-hr deprivation phases; t(5) < 1.0, ns.
Figure 3.
Number of immediate food pellets at indifference (i.e., the subjective value of the 32-pellet reinforcer) plotted as a function of delay for all pigeons in Experiment 2. The curves represent the best-fitting hyperboloid discounting function (Eq.1) with s = 1.0 (i.e., a simple hyperbola), for all pigeons.
Table 2.
Proportion of variance accounted for and parameters of Equation 1 for the 1-hr and 23- hr deprivation phases for each pigeon in Experiment 2. Rk2 indicates the proportion of variance accounted for by Equation 1 with s = 1.0, and Rk,s2 indicates the proportion of variance accounted for by Equation 1 with s as a free parameter in cases where s was significantly different from 1.0 (Pigeon 81 in the 1-hr deprivation phase and Pigeon 82 in the 23-hr deprivation phase).
| Pigeon | Deprivation condition |
Rk2 [Rk,s2] |
k [k; s] |
|---|---|---|---|
| 81 | 1-hr | .90 [.97] |
1.51 [3.77; 0.644] |
| 23-hr | .82 | 0.939 | |
| 82 | 1-hr | .97 | 1.04 |
| 23-hr | .34 [.81] |
1.70 [16.9; 0.407] |
|
| 83 | 1-hr | .92 | 0.212 |
| 23-hr | .90 | 0.231 | |
| 84 | 1-hr | .83 | 0.407 |
| 23-hr | .87 | 0.356 | |
| 85 | 1-hr | .74 | 0.958 |
| 23-hr | .97 | 0.733 | |
| 86 | 1-hr | .87 | 0.341 |
| 23-hr | .87 | 0.339 |
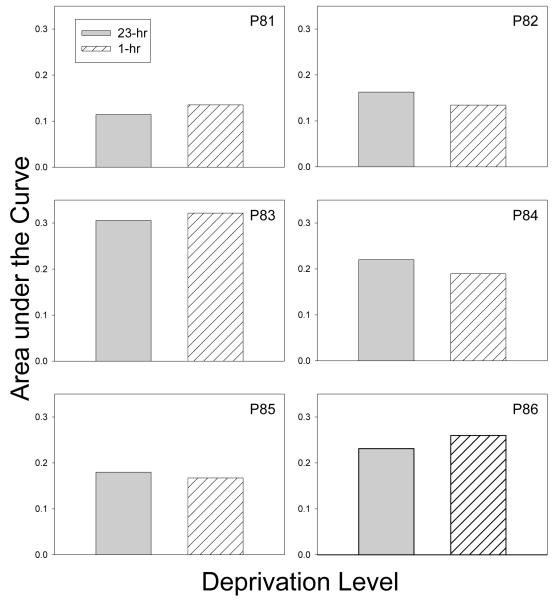
Finally, Figure 4 shows individual AuCs in the two deprivation phases. Again, no systematic differences in degree of discounting were observed between the two deprivation phases: group mean = .201 for 1-hr deprivation and .202 for 23-hr deprivation; t(5) < 1.0. Taken together with the results of the first experiment, the present findings suggest that degree of discounting is not significantly affected by level of deprivation, regardless of the way in which deprivation is manipulated, be it in terms of body weight (Experiment 1) or hours without food (Experiment 2).
Figure 4.
Area under the Curve for the 1-hr and 23-hr deprivation phases for each pigeon in Experiment 2.
General Discussion
Humans with lower incomes discount delayed monetary rewards more steeply than those with higher incomes (Green et al., 1996; Lawrance, 1991). One possible interpretation of that finding is that discounting is affected by level of deprivation, in that those with less money may be thought of as being more deprived than those with more money. The present study examined whether this interpretation is supported by results obtained when deprivation is experimentally manipulated, thereby eliminating the possible confounds that exist in comparable human data (e.g., individuals with lower income also tend to be less well educated). The results of the present study, however, provide no support for an interpretation of the Green et al. findings based on differences in deprivation.
In the present study, pigeons’ level of food deprivation was manipulated in two ways. In Experiment 1, subjects were studied under two body-weight levels (75-80% and 90-95% of free-feeding weight), whereas in Experiment 2, subjects were studied at the same body-weight (80-85% of free-feeding weight) and tested under 1-hr and 23-hr food deprivation. The degree to which the delayed food reinforcers were discounted was not affected by level of deprivation in either experiment, but discounting was for the most part well described by a simple hyperbola (Eq. 1 with s = 1.0) at both low and high levels of deprivation.
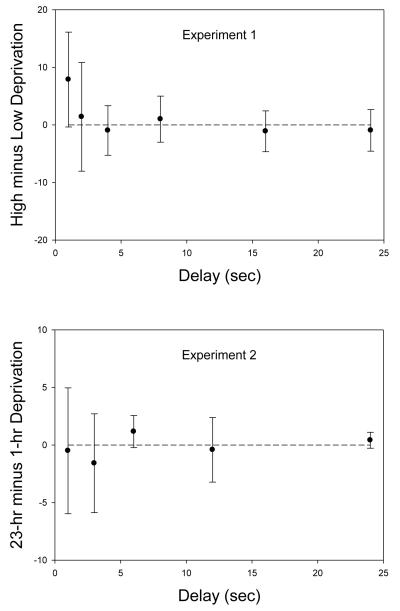
The logic of inferential statistics does not allow one to accept the null hypothesis based on experimental data. Nevertheless, null results are often important, and in the absence of statistical support, it is helpful to provide converging evidence using different analytical approaches that examine different aspects of the data. Accordingly, we conducted further analyses of the data from Experiments 1 and 2 based on the difference scores (number of food pellets at indifference in the high deprivation phase minus number of food pellets at indifference in the low deprivation phase) at each delay in each experiment. As may be seen in Figure 5, the confidence interval about the group mean difference scores included zero in every case, indicating a consistent failure to find a deprivation effect. These results are consistent with the absence of an effect of deprivation on discounting rate in other, related studies. Richards et al. (1997) examined the effect of water deprivation in rats and also found no systematic effect on discounting. Ostaszewski et al. (2003) examined the effect of restricting access to food on delay discounting in rats in two experiments and observed no consistent effect of deprivation.
Figure 5.
Group mean difference between the number of food pellets at the indifference points at each delay in the high and low deprivation phases of Experiment 1 (top panel) and Experiment 2 (bottom panel). Error bars represent the 95% confidence intervals about each mean difference.
It is possible that studies in which the smaller, sooner reinforcer is of one type and the larger, later reinforcer is of a different type, as they are in many everyday choice situations, would find effects of deprivation on discounting in animals when subjects are more deprived of one of the two types of reinforcer. Nevertheless, the issue of the difference between the present finding of no effect of deprivation on discounting and Green et al.’s (1996) finding that discounting is affected by income, remains because in both studies, subjects chose between smaller, immediate and larger, delayed reinforcers of the same type (i.e., food pellets for the pigeons in the present study, and hypothetical money for the humans in Green et al.).
To what may one attribute the discrepancy between the results obtained with humans and those obtained with pigeons and rats? One obvious possibility is that it represents a species difference. Before reaching this conclusion, however, one would have to rule out some other, equally obvious possibilities. As already noted, Green et al. (1996) studied discounting of hypothetical, monetary rewards whereas the present study, like those of Ostaszewski et al. (2003) and Richards et al. (1997), examined discounting of real, directly consumable reinforcers (i.e., food and water). There is growing evidence for differences in the discounting of various types of outcomes, even within the same species (for a review, see Green & Myerson, in press), and the different rewards/reinforcers used in the human and animal studies considered here may account for the difference in results. It also is possible, of course, that differences in degree of food and water deprivation simply are not analogous to differences in level of income.
Another relevant finding is that drug-dependent humans show steeper discounting when they are drug-deprived than when they are not (Field et al., 2006; Giordano et al., 2002). As in the Green et al. (1996) study of income, however, the types of rewards studied (cigarettes, heroin, and money) in these experiments were different than those in the studies of deprivation in animals, and future studies of discounting using more comparable rewards (e.g., real foods or liquids in humans, or drugs in animals) might shed light on this issue.
The present finding that pigeons’ discounting is not affected by deprivation is consistent with the view, exemplified by the matching law (Herrnstein, 1970), that pigeons’ choices are controlled by the relative, rather than the absolute, value of reinforcers. That is, assume that on a discounting task, deprivation results in (proportionally) equivalent changes in the value of both the immediate and delayed reinforcers, at least when both are the same commodity. Then, if choices are made on the basis of relative value, they should be unaffected by whether the immediate and delayed reinforcers are both of lower value or both of higher value.
McSweeney (1975) studied the effects of food deprivation on pigeons’ choice on concurrent VI schedules and found that as body weight increased, overall response rates decreased but choice, as measured by relative rates of responding, did not change. Logue and Peña-Correal (1985) examined the effects of deprivation on pigeons’ choices in a situation more similar to the present one (discrete trials with smaller, sooner and larger, later reinforcers), and they also found that changes in deprivation did not affect choice, whereas other aspects of behavior were affected (e.g., latencies to eat when food reinforcement was delivered). The results of both of these studies support our hypothesis that food deprivation results in proportionally equivalent changes in the value of alternative reinforcers, leaving preference between these alternatives unchanged, which would explain why deprivation did not affect discounting rates in either experiment of the present study.
Other evidence consistent with the idea that discounting is controlled by relative value is the finding that when rats chose between immediate and delayed amounts of the same reinforcer, the degree to which they discounted was the same regardless of the quality of the reinforcers involved (Calvert et al., 2010). That is, although the rats in the Calvert et al. study strongly preferred saccharin-flavored water to quinine-flavored water, their choices between immediate and delayed liquids were the same regardless of whether both were saccharin-flavored or quinine-flavored. The relative choice view also is consistent with the fact that in both pigeons and rats the subjective value of a reinforcer available after a given delay, when expressed as a proportion of the amount of the delayed reinforcer, is approximately the same regardless of the delayed amount (Green et al., 2004; Richards et al., 1997). In addition to providing further support for the relative choice view as applied to discounting, the present findings contribute to the growing evidence for the generality of the hyperboloid model by showing that it provides an equally good description of pigeons’ discounting of delayed food reinforcers under both low and high deprivation conditions.
Acknowledgements
The research was supported by Grant RO1 MH055308 from the National Institutes of Health. Luís Oliveira was supported by a graduate fellowship (SFRH/BD/61164/2009) from the Foundation for Science and Technology (FCT, Portugal). We thank the members of the Psychonomy Cabal, and in particular Amy Baum, for assistance with the running of the experiments.
References
- Bradshaw CM, Szabadi E. Choice between delayed reinforcers in a discrete-trials schedule: The effect of deprivation level. Quarterly Journal of Experimental Psychology. 1992;44B:1–16. doi: 10.1080/02724999208250599. [DOI] [PubMed] [Google Scholar]
- Calvert A, Green L, Myerson J. Delay discounting of qualitatively different reinforcers in rats. Journal of the Experimental Analysis of Behavior. 2010;93:171–184. doi: 10.1901/jeab.2010.93-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen-Szalanski JJJ, Goldberg AD, Anderson ME, Mitchell TR. Deprivation, delay of reinforcement and the selection of behavioral strategies. Animal Behavior. 1980;28:341–346. [Google Scholar]
- Eisenberger R, Masterson FA, Lowman K. Effects of previous delay of reward, generalized effort, and deprivation on impulsiveness. Learning and Motivation. 1982;13:378–389. [Google Scholar]
- Field M, Santarcangelo M, Sumnall H, Goudie A, Cole J. Delay discounting and the behavioural economics of cigarette purchases in smokers: the effects of nicotine deprivation. Psychopharmacology. 2006;186:255–263. doi: 10.1007/s00213-006-0385-4. [DOI] [PubMed] [Google Scholar]
- Fisher I. The rate of interest: its nature, determination and relation to economic phenomena. Macmillan; New York: 1907. [Google Scholar]
- Freeman KB, Nonnemacher JE, Green L, Myerson J, Woolverton WL. Delay discounting in rhesus monkeys: Equivalent discounting of more and less preferred sucrose concentrations. Learning & Behavior. 2012;40:54–60. doi: 10.3758/s13420-011-0045-3. [DOI] [PubMed] [Google Scholar]
- Giordano LA, Bickel WK, Loewenstein G, Jacobs EA, Marsch L, Badger GJ. Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology. 2002;163:174–182. doi: 10.1007/s00213-002-1159-2. [DOI] [PubMed] [Google Scholar]
- Grace RC. The matching law and amount-dependent exponential discounting as accounts of self-control choice. Journal of the Experimental Analysis of Behavior. 1999;71:27–44. doi: 10.1901/jeab.1999.71-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace RC, Sargisson RJ, White KG. Evidence for a magnitude effect in temporal discounting with pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 2012;38:102–108. doi: 10.1037/a0026345. [DOI] [PubMed] [Google Scholar]
- Green L, Fry AF, Myerson J. Discounting of delayed rewards: A life-span comparison. Psychological Science. 1994;5:33–36. [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychological Bulletin. 2004;130:769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J. Experimental and correlational analyses of delay and probability discounting. In: Madden GJ, Bickel WK, editors. Impulsivity: The behavioral and neurological science of discounting. American Psychological Association; Washington, DC: 2010. pp. 67–92. [Google Scholar]
- Green L, Myerson J. How many impulsivities? A discounting perspective. Journal of the Experimental Analysis of Behavior. doi: 10.1002/jeab.1. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Holt DD, Slevin JR, Estle SJ. Discounting of delayed food rewards in pigeons and rats: Is there a magnitude effect? Journal of the Experimental Analysis of Behavior. 2004;81:39–50. doi: 10.1901/jeab.2004.81-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Lichtman D, Rosen S, Fry A. Temporal discounting in choice between delayed rewards: The role of age and income. Psychology and Aging. 1996;11:79–84. doi: 10.1037//0882-7974.11.1.79. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, McFadden E. Rate of temporal discounting decreases with amount of reward. Memory & Cognition. 1997;25:715–723. doi: 10.3758/bf03211314. [DOI] [PubMed] [Google Scholar]
- Hastjarjo T, Silberberg A. Effects of reinforcer delays on choice as a function of income level. Journal of the Experimental Analysis of Behavior. 1992;57:119–125. doi: 10.1901/jeab.1992.57-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman JA. Individual discount rates and the purchase and utilization of energy-using durables. The Bell Journal of Economics. 1979;10:33–54. [Google Scholar]
- Herrnstein RJ. On the law of effect. Journal of the Experimental Analysis of Behavior. 1970;13:243–266. doi: 10.1901/jeab.1970.13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Myerson J, Hilgard J, Braver TS, Green L. Are people really more patient than other animals? Evidence from human discounting of real liquid rewards. Psychonomic Bulletin & Review. 2009;16:1071–1075. doi: 10.3758/PBR.16.6.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. Journal of the Experimental Analysis of Behavior. 2002;77:129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney RL, Raiffa H. Decisions with multiple objectives: Preferences and value tradeoffs. Wiley; New York: 1993. [Google Scholar]
- Lawrance EC. Poverty and the rate of time preference: Evidence from panel data. Journal of Political Economy. 1991;99:54–77. [Google Scholar]
- Logue AW, Peña-Correal TE. The effect of food deprivation on self-control. Behavioural Processes. 1985;10:355–368. doi: 10.1016/0376-6357(85)90036-1. [DOI] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative analyses of behavior. Vol. 5. The effect of delay and of intervening events on reinforcement value. Erlbaum; Hillsdale, NJ: 1987. pp. 55–73. [Google Scholar]
- McSweeney FK. Concurrent schedule responding as a function of body weight. Animal Learning & Behavior. 1975;3:264–270. [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostaszewski P, Karzel K, Szafrańska P. Changes in discounting rates as adaptation to food deprivation in rats: the role of age and deprivation level. Polish Psychological Bulletin. 2003;34:203–211. [Google Scholar]
- Richards JB, Mitchell SH, de Wit H, Seiden LS. Determination of discount functions in rats with an adjusting-amount procedure. Journal of the Experimental Analysis of Behavior. 1997;67:353–366. doi: 10.1901/jeab.1997.67-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman M. Optimal prey selection: The effects of food deprivation. Behaviour Analysis Letters. 1983;3:359–369. [Google Scholar]
- Weatherly JN. Altering participants’ hypothetical annual income can alter their rates of discounting the same delayed monetary outcome. The Journal of General Psychology. 2012;139:42–54. doi: 10.1080/00221309.2011.652236. [DOI] [PubMed] [Google Scholar]