Abstract
Despite the widespread use of antiretroviral therapy that effectively limits viral replication, memory impairment remains a dilemma for HIV infected people. In the CNS, HIV infection of astrocytes leads to the production of the HIV-1 Nef protein without viral replication. Post mortem studies have found Nef expression in hippocampal astrocytes of people with HIV associated dementia suggesting that astrocytic Nef may contribute to HIV associated cognitive impairment even when viral replication is suppressed. To test whether astrocytic expression of Nef is sufficient to induce cognitive deficits, we examined the effect of implanting primary rat astrocytes expressing Nef into the hippocampus on spatial and recognition memory. Rats implanted unilaterally with astrocytes expressing Nef showed impaired novel location and novel object recognition in comparison with controls implanted with astrocytes expressing green fluorescent protein (GFP). This impairment was correlated with an increase in chemokine ligand 2 (CCL2) expression and the infiltration of peripheral macrophages into the hippocampus at the site of injection. Furthermore, the Nef exposed rats exhibited a bilateral loss of CA3 neurons. These results suggest that Nef protein expressed by the implanted astrocytes activates the immune system leading to neuronal damage and spatial and recognition memory deficits. Therefore, the continued expression of Nef by astrocytes in the absence of viral replication has the potential to contribute to HIV associated cognitive impairment.
Keywords: astrocyte, HAND, cognition, CD163, CCL2
Introduction
HIV/AIDS remains a global epidemic affecting over 33 million people, including 2.6 million new infections and 1.8 million deaths in 2009 (UNAIDS/WHO 2009 Global Summary). HIV infection is associated with a spectrum of neurological disorders and opportunistic infections in the CNS. The virus quickly invades the brain and establishes a foothold predominantly in microglia and infiltrating macrophages that support viral replication, and in astrocytes that produce a limited set of viral neurotoxins (Messam and Major, 2000; Tornatore et al., 1991; Wiley et al., 1986). In the beginning of the epidemic, 10–20% of the patients with HIV showed severe manifestations of neuropathology known as the AIDS dementia complex, characterized by low CD4, high viral loads, cognitive and motor impairments and behavioral changes (McArthur et al., 1993; Price et al., 1988). Since the advent of combination antiretroviral therapy (cART), the incidence of dementia has declined significantly; however, milder forms of neuropathology still affect as many as 50% of HIV positive persons (Heaton et al., 2011; McArthur et al., 2003; McArthur et al., 1999; Robertson et al., 2007). Even though viral replication is well controlled by cART, viral proteins persist in viral reservoir areas (Popovic et al., 2005) suggesting that neurotoxic viral proteins may contribute to the continued cognitive decline. Patients with HIV associated neurocognitive disorders (HAND) show deficits in attention, behavior changes and memory impairment (McArthur et al., 2003; McArthur et al., 1999; Sacktor et al., 2002). While it is known that HAND is a result of HIV infection in the brain, the roles of the various infected cell types remains an area of active investigation.
Replication in permissive cells in the brain such as microglia and perivascular macrophages induces the production of new virus and viral proteins, immune system activation and inflammation that result in neurotoxicity and cell death (Anderson et al., 2002). HIV crosses into the brain predominantly through infected mononuclear cells (Haase, 1986; Peluso et al., 1985) which release viral particles that infect permissive cells and produce latent infection of astrocytes (Conant et al., 1994; Thompson et al., 2001; Tornatore et al., 1994). Infection of astrocytes is widespread and the magnitude of astrocyte infection, particularly near perivascular macrophages, correlates with the severity of neuropathogenesis (Churchill et al., 2009). Astrocytes support viral persistence in the brain during suppressive cART (Dayton, 2008), since viral activity in astrocytes is not a target of antiretroviral treatment directed at replicating virus. Recent work showed TCF-4/β-catenin-mediated transcriptional repression of HIV-1 is crucial to restricted repression in astrocytes (Henderson et al., 2012). However, HIV-infected astrocytes do produce early viral proteins (Haughey and Mattson, 2002; Messam and Major, 2000; Van Marle et al., 2004), including Nef, which may constitute an important source of neurotoxins throughout the neurocognitive decline observed in cART-treated populations.
HIV Nef was found to be expressed in astrocytes of post mortem sub-cortical tissue from people with HIV associated dementia (Fiala et al., 2008; Ranki et al., 1995), suggesting that astrocytic expression of Nef may contribute to HIV associated cognitive deficits. Similarly, Nef mRNA was found post mortem in hippocampal tissue of patients who had suffered from HIV associated dementia (Torres-Munoz et al., 2001). In a non-human primate model of AIDS, rhesus macaques infected with a neurovirulent strain of the simian immunodeficiency virus (SIV) developed encephalitis. Post mortem analysis of this study found Nef expression in astrocytes of these monkeys (Overholser et al., 2003). These findings provide compelling evidence that Nef is present in astrocytes and in the hippocampus during HIV infection. However, it is unknown whether the Nef expressed by the astrocytes contributes to memory loss. We hypothesized that astrocytic Nef expression in the hippocampus could produce memory deficits. To test this hypothesis, we examined the effect of implanting astrocytes expressing Nef into the hippocampus on spatial and novel object recognition memory in rats.
Materials and Methods
Animals
Thirty-day-old, male Sprague Dawley rats were tested in the novel location recognition and novel object recognition tasks. We used three groups: rats implanted with astrocytes expressing Nef (N=14), green fluorescent protein (GFP, N=12), and a naive group (N=8). After surgery, rats were placed in an air purified biobubble and housed in pairs or three to a cage to avoid isolation stress. For pain and discomfort after surgery, a topical antibiotic with pain reliever (3.5 mg neomycin, 400 units of bacitracin, 85,000 units of polymixin, and 1% pramoxine hydrochloride) was put over the stitches for four consecutive days. Animals were kept on a 12 hour dark/light schedule and given free access to food and water. All the procedures were approved by the PSM Animal Care and Use Committee and were designed to minimize animal discomfort.
Primary astrocytes isolation
Primary astrocytes were obtained from three-month-old rats. Animals were anaesthetized with halothane and then decapitated for brain removal. The brain was placed in ice-cold Hank’s balanced salt solution for mincing and disruption with trypsin (Sigma, St. Louis, MO). After ten minutes, the trypsin was inactivated by the addition of Dulbecco’s Modified Eagle Media (DMEM) supplemented with 10% of fetal bovine serum, 10 mM of L-glutamine, 5% of non-essential amino acids, and 1% of streptomycin/penicillin. Cells were separated from debris by sedimentation. Astrocytes were grown in culture media and non-attached cells were removed by gentle shaking of the plate and washing. After three passages, the primary astrocytes were over 95% pure by immunofluorescence microscopic analysis of an astrocytic marker (glial fibrillary acidic protein, GFAP, BD Biosciences, 1:200), negative staining for microglial marker (Iba1/2, Santa Cruz Biotechnology 1:100), and nuclear counterstaining with DAPI. Western blots also confirmed the specificity and presence of GFAP and absence of Iba1/2 in these cultures. SVGA (Major et al., 1985), a human astrocytic cell line, cultures and total rat brain lysates were used for comparison.
Transient transfection of primary astrocytes
Nef expression vector, p96AM651 (NIH AIDS Reagent Program, Cat. #8677) or GFP control were introduced into cultured rat astrocytes by electroporation using the following standardized conditions: 4mm cuvette, 1.6 million cells, 5μg endotoxin free plasmid, 250V, 35msec time constant in 300μl serum free RPMI. Routine transfection efficiency by GFP expression was between 60–80% positive cells. After transfection, cells were washed and resuspended at a density of 200,000 cells/μL in artificial cerebral spinal fluid (126 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1 mM MgSO4, 26 mM NaHCO3, 20 mM glucose, and 2 mM CaCl2).
Nef expression in vitro
After transfection with Nef or GFP plasmid, 100,000 astrocytes/well were plated in 48 well plates in 500μL of medium and collected each day across seven days for analysis of Nef expression. Cells were lysed in RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% Nonidet, 0.1% sodium deoxycholate, and 4 mM EDTA) and 25μg of each lysate was resolved in 15% SDS-PAGE gels. Proteins were transferred to PDVF membranes for western blotting as described (Silva et al., 1998) using a monoclonal anti-Nef antibody (NIH AIDS Research and Reference Reagent Program, cat. #3689; 1:2,000) or anti-actin (Sigma, St. Louis, MO; Cat. #A5060; 1:1,000) followed by chemiluminescent/ chemifluorescent detection (General Electric RJP 2332) using appropriate secondary antibodies. Nef expression in the cell lysates and in the cell supernatants was calculated using a standard curve of purified Nef protein from 2.0μg to 0.25μg (NIH AIDS Research and Reference Reagent Program, cat.#11478). ANOVA test was used to compare the Nef expression.
Infusion of transfected astrocytes in the hippocampus
Infusions were performed using a protocol modified from (Chauhan et al., 2003) and approved by the PSM-IACUC. Rats were anesthetized with ketamine (1 cc/kg). The astrocytes expressing Nef or GFP were infused into the hippocampus using the following coordinates (anterior posterior −0.28 mm, midlateral ±0.17mm, and dorsoventral −.037). 100,000 cells (0.5μL) were infused over a one minute period. The needle was kept in place for five minutes prior to retracting to prevent backflow of infused cells. Accuracy of cell infusion was confirmed by histological analysis.
Novel location recognition and novel object recognition
Novel location and object recognition tasks were performed after infusion of astrocytes expressing Nef or GFP using a modification of a published protocol (Acevedo et al., 2006; Benice and Raber, 2008; Benice et al., 2006). Naive animals were used as a control to validate the behavioral paradigm. Behavior testing was performed in an arena (wood covered with formica, 36 × 36 × 18 inches) located in a small, designated room (7.5 × 7.5 feet) with fixed spatial cues on the walls. A video camera was place above the arena. The objects used in novel location and novel object recognition were made in hard plastic. All the objects were fixed in a plastic square platform of (2.5 × 2.5 inches). The objects were positioned five and half inches away from adjacent corners. The distance between objects was eighteen and half inches. The protocol was performed as shown in Figure 2A. After two days of recovery from the surgery, animals habituated to the arena for 5 minutes over three consecutive days. Twenty-four hours after habituation was completed (day 6), three objects, snowmen (square), cup (triangle), and baby (circle), were placed in three corners of the box. Animals were put in the box for three 10 minute trials with an interval of 1 hour between trials for acclimation and to learn the object locations. Between each trial, the box and objects were cleaned with 70% ethanol. After 24 hours, the snowmen object (square) was relocated to the opposite corner (see Figure 2A). Rats were placed into the box to explore the objects for 10 minutes to test for spatial memory. Then the snowmen object (square) and cup (triangle) were removed. A new object (star) was put in the place of the cup as a novel object to test for recall of object recognition memory. Animals were put in the box and allowed to explore the old and the new object for 10 minutes. Ethovision software (Noldus Information Technology Inc, Leesburg, VA) was used to track the animal. The software tracks three body points (nose, center, and tail). We used the nose point for the exploration of the objects (Benice and Raber, 2008), the center point of the animal to measure locomotion and time spent in the center of the open field as a measure of stress/anxiety.
Figure 2.
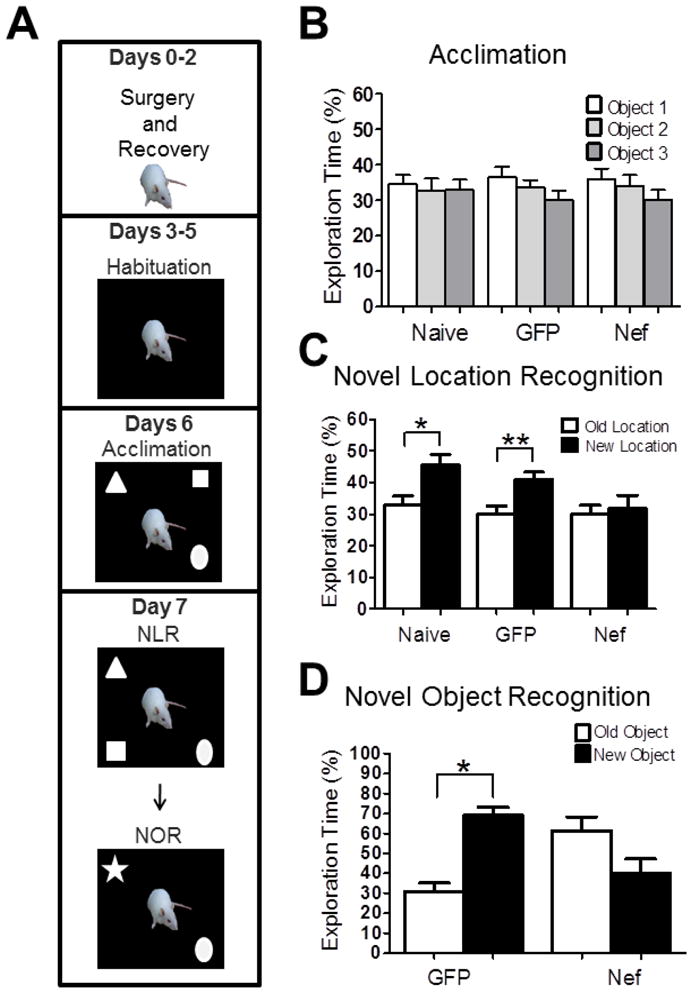
Nef expression unilaterally in the hippocampus causes learning impairment in spatial and non-spatial memory. (A) A schematic chart of the novel location recognition (NLR) and novel object recognition (NOR) tasks. Average exploration time of rats from the three groups (Naïve, n=8; GFP, n=12; or Nef, n=14): (B) for each object during acclimation; (C) for the test object prior to (open bars) and after moving the location (closed bars); and (D) for the test object prior to (open bars) and after replacing with a novel object (closed bars). All error bars represent standard error. *p<0.05; **p<0.005.
Nef and CD163 immunohistochemistry
After the novel location test, animals were euthanized with an overdose of pentobarbital and perfused with saline solution followed by 4% paraformaldehyde. The brains were removed and stored in 30% sucrose in 4% paraformaldehyde. After brain saturation, brain slices (25 μm) were cut using a cryostat and mounted on slides. Slides were treated with xylene and washed with graded ethanol followed by 3% hydrogen peroxide prior to antigen retrieval (citrate buffer pH 6.0 at 95° for 45 minutes). Slides were then incubated with the primary antibody (Nef from AIDS Reagent Program, (1:100); CD163 from Cell Sciences from Canton, MA (1:10) in a humidified chamber at 4° C. A control reaction was performed without the primary antibody. Detection was by fluorescent conjugated multilink secondary antibody (Biogenex Cat. # LP000-ULE) or a secondary antibody (Invitrogen Co. Cat. No. A31619) with 0.28μM DAPI (Invitrogen) for or hematoxilin counterstaining. Analysis of CD163 immunoreactivity was done using NIS-Elements software (Nikon). Immunostaining was quantified by taking images of each slice using a constant light and exposure setting by a blinded observer. Digital images were analyzed by quantifying the total number of pixels above constant predefined threshold intensity. Pixel numbers were converted into an area and the stained area was report as the percent of the total image area that was stained above threshold.
Nissl Staining
Brain slices were cut and mounted on positively charged glass slides followed by dehydration with graded ethanol (50, 70, 95, and 100%), incubation in chloroform and ethanol (1:1) for a period of 30 minutes and rehydrated with graded ethanol (100, 95, 70, 50%). Slides were incubated in distilled water for 5 minutes and stained for 5 seconds in the thionin solution (3.6 % NAOH, 10.2% acetic acid, 0.25% thionin) prior to rinse with distilled water and incubation in 50% ethanol for 1 minute. Slices were submerged in 70% ethanol solution for 7 minutes followed by 95% acetic acid for 3 minutes. For the last step, slides were immersed in 95% ethanol for 2 minutes and two incubations of 100% ethanol solution. The slides were dipped in xylene twice for 5 minutes each. Five drops of permount and a cover slide were added. After 24 hrs, photos were taken using NIS Elements software and an Olympus microscope. Images were identified with a random number and three blinded observers counted the number of cells in the CA fields. Neuron counts in CA3 were performed for 11 or 12 rats in each group (Nef and GFP, respectively) with 2–3 sections counted for each rat. Counts for each field were averaged to obtain a single value for each rat which was then used to obtain the group average across the animals in each treatment.
qPCR of CCL2 messenger RNA
Total RNA was extracted using an Allprep DNA/RNA/Protein from Qiagen CO. (Valencia, CA) and analyzed using an Experion Automated Electrophoresis station from Bio-Rad to verify the quality and concentration of the sample. RNA (1μg) was reverse transcribed to complementary DNA (cDNA) using Iscript cDNA synthesis from Bio-Rad. CCL2 amplification was done using Sybr Green Super mix from Bio-Rad and CCL2 PCR primers from Qiagen (Valencia, CA). To calculate the relative expression of the control (GFP group or cells transfected with GFP) and the experimental control (Nef group or cells transfected with Nef), we used the 2−ΔΔCT method normalized using a housekeeping gene, beta actin. We report the relative expression of the CCL2 compared to the GFP group.
Statistical Analysis
Statistical analysis was performed using Graph Pad Prism version 5.02. One-way ANOVA with Tukey’s post-hoc tests were used to compare protein expression, preference among the objects, speed, distance, and time spent in the center. Student t test was used to measure the significance of old location versus new location in the novel location and novel object tasks, CD163 immunoreactivity, the number of neurons in the CA3 field, and CCL2 PCR. P values less than or equal to 0.05 were considered significant.
Results
Nef expression in primary astrocytes is maintained for seven days
We developed a model system to provide expression of the HIV-1 Nef protein in astrocytes to assess if endogenous production of Nef protein by astrocytes is sufficient to cause learning deficits. We extracted primary astrocytes from Sprague Dawley rats, cultured them in vitro and verified the predominance of astrocytes in the cultures by western blot and immunofluorescent analysis of GFAP reactivity. Figure 1A shows three independent isolations of primary rat astrocytes (lanes 1–3) as well as an astrocyte cell line, SVGA (lane 4) are positive for GFAP. The second panel indicates each of the three extractions as well as the SVGA cells are negative for Iba1/2 indicating no microglial contamination, while whole brain extraction (WB) shows specific staining for microglia. Immunofluorescence analysis supports the findings in the western blot in the final panel of Figure 1A as the cells are positive for GFAP (green) with no detectable Iba1/2.
Figure 1.
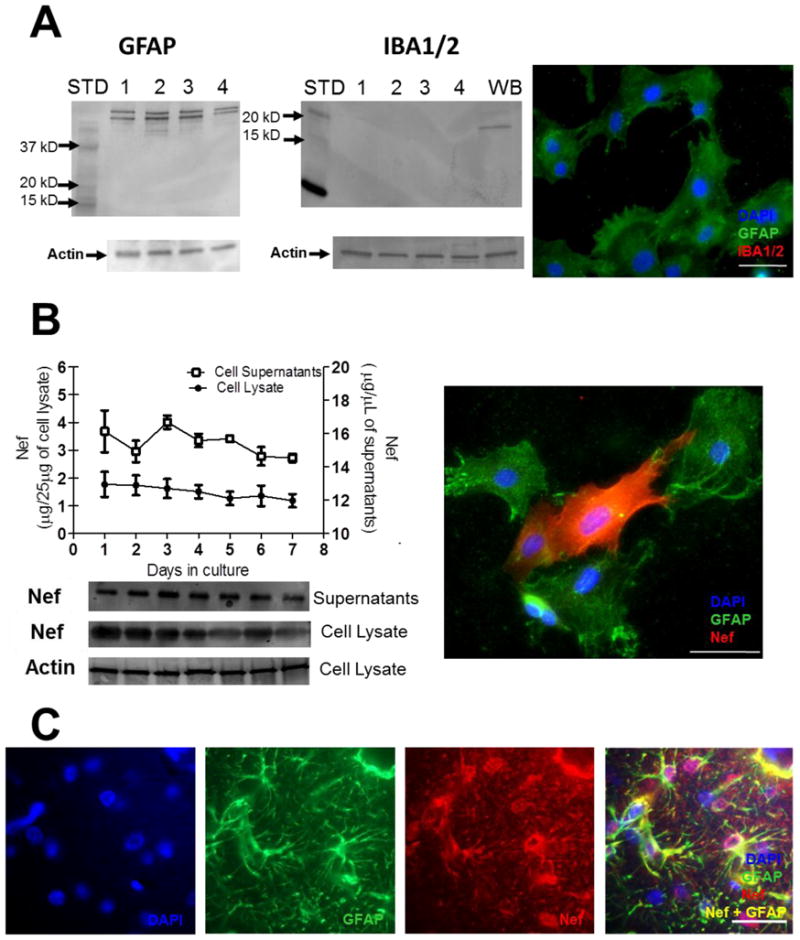
Nef is expressed for seven days after transfection of primary rat astrocytes in vitro and in vivo from infused cells. (A) Primary culture composition was assessed for astrocytes (GFAP) and microglia (Iba1/2). Western blot of three, independent primary cell extractions (lanes 1–3) and SVGA astrocytes (lane 4) show positive for GFAP and negative for Iba1/2. Positive control for microglia in whole brain (WB) tissue shows positive reaction with Iba1/2. Molecular weight markers are labeled (STD). In the third panel, immunofluorescence for GFAP (green) and Iba1/2 (red) show cultures are astrocytes. DAPI was used for nuclear counterstaining. (B) Cells and culture supernatants were collected each day for seven days after transfection with Nef plasmid. Western blots, lower part, measured Nef protein expression. Densitometry of western blots was used to quantify (upper part) Nef from cell lysates and supernatants from the same cell cultures. Concentrations of Nef were estimated using a Nef protein standard (refer to methods). Actin served as a loading control for cell lysates. Immunofluorescence indicates Nef (red) expression from GFAP positive (green) astrocytes. (C) Immunostaining of hippocampal tissue proximal to infusion site: left to right, DAPI, GFAP (green), and Nef (red) in split channel, and merged image at seven days post-surgery. Scale bars = 25μM.
Next, astrocytes were transiently transfected with a plasmid expressing HIV-1 Nef. We followed Nef expression in vitro over seven days to mimic the duration of our in vivo implantation and behavioral testing paradigm. Primary rat astrocytes expressing Nef were collected and cell lysates were prepared daily for a week. Western blots were performed on these lysates and the approximate quantity of Nef produced in these cells was calculated to be 1μg/25μg total cell lysate each day using densitometric comparison against a purified Nef protein standard (Figure 1B). We also detected Nef protein in the cell culture medium using western blotting (Figure 1B). The high level of Nef detected extracellularly suggests that transfected astrocytes secrete Nef, although our experiments do not rule out a contribution from cell lysis. Protein levels of Nef were stable in the cytoplasm (p=0.81, n=3) and in the culture supernatants (p=0.26, n=3) over seven days. Immunofluorescence of transfected primary astrocytes shows strong Nef immunoreactivity in positive transfectants (Figure 1B, right panel).
To verify that transfected astrocytes would continue to express Nef for the duration of the behavioral testing after being implanted into the hippocampus, we infused astrocytes transfected with either Nef or GFP unilaterally into the right dorsal hippocampus of 30-day-old male Sprague Dawley rats. After seven days, animals were sacrificed and brain sections surrounding the infusion site were cut and stained for Nef and GFAP immunoreactivity. Our results show that Nef continued to be strongly expressed seven days after implantation and was highly associated with GFAP positive cells (Figure 1C).
Rats implanted with astrocytes expressing Nef show memory impairment
To determine whether the astrocytic expression of Nef affected hippocampal-dependent memory, rats were tested for spatial and object recognition memory (testing paradigm shown in Figure 2A). After recovering from surgery rats were habituated to the behavioral testing environment. Six days after astrocyte implantation, rats were allowed to explore and learn three objects in an open field for three ten-minute trials. Rats did not show any significant preference among the objects during the acclimation phase (Naïve, n = 8, GFP, n = 12, Nef, n = 14; p=0.85; Figure 2B). Twenty-four hours later, rats were tested for novel location recognition by moving one object to a new location. Nef-treated rats showed no preference for the object in the new location, indicating a failure to recall the original position of the moved object (p =0.64; Figure 2C). In contrast, control rats that were either not implanted (naïve) or implanted with astrocytes expressing GFP preferentially explored the moved object indicating they recognized that the object was in a new location (Naive, p=0.01; GFP, p < 0.001; Figure 2C). After completion of the novel location recognition task, GFP- and Nef-treated rats were tested for novel object recognition by removing two of the objects and replacing one with a novel object. Nef-treated rats also failed to recognize the novel object and actually tended to prefer exploring the familiar object (n= 5, p = 0.06; Figure 2D). The memory deficit was not due to implantation of astrocytes since the rats implanted with astrocytes expressing GFP spent more time exploring the new object (n= 5, p=0.01; Figure 2D). These data demonstrate that Nef expression by the astrocytes produced memory impairment in two different learning tasks.
Rats implanted with Nef-expressing astrocytes showed normal motor function and anxiety levels
To rule out effects on motor function or anxiety levels as possible explanations for the Nef-induced memory deficits, the speed, the distance traveled, and the percent of time the rats spent in the center of the open field during all days of testing were assessed. Animals from the three groups moved at the same average velocity (p=0.79; Figure 3A) and traveled equal distances (p=0.79; Figure 3B) in the testing phases. Rats implanted with Nef expressing astrocytes, GFP expressing astrocytes, or without surgery spent the same amount of time in the center of the open field (p=0.78; Figure 3C). These results suggest that neither locomotor nor anxiety behavior differences contributed to the memory deficits caused by Nef.
Figure 3.
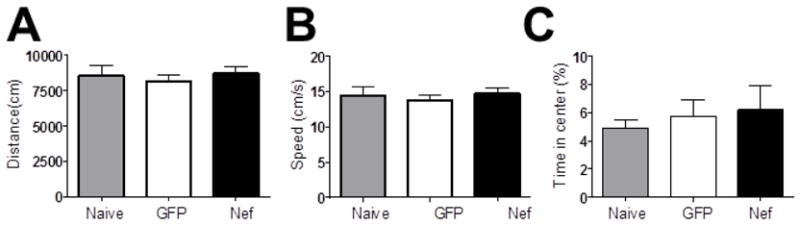
Neither Nef exposure nor surgery caused widespread behavioral changes. Locomotor and anxiety behavior for each group during the acclimation phase are presented (mean plus standard error) as representative data. (A) Total distance traveled and (B) velocity were assessed for locomotor function. (C) Time spent in the center served as a measure of anxiety.
Astrocytic Nef expression induces bilateral loss of CA3 neurons
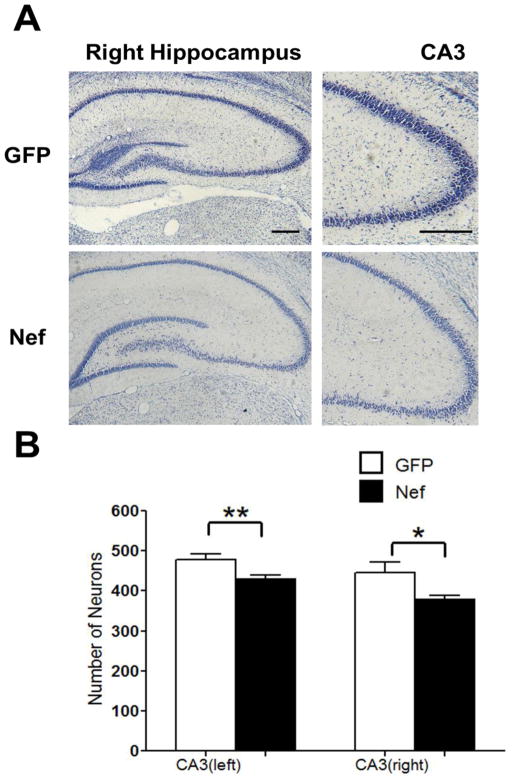
The addition of Nef in vitro causes neuronal death (Trillo-Pazos et al., 2000; Van Marle et al., 2004). Therefore the memory impairment induced by implantation of Nef-expressing astrocytes could be secondary to neuronal death. To examine whether astrocytic Nef expression caused a loss of hippocampal neurons, animals were sacrificed after behavioral testing, brain slices were Nissl stained, and the number of neurons in the CA3 region of the hippocampus were counted. Nissl staining of hippocampal brain slices from rats implanted with astrocytes expressing Nef showed lighter staining than GFP controls (Figure 4A, region counted shown at higher magnification in right panels). Quantitative analysis showed animals in the Nef group contained fewer neurons in CA3 when compared to brain slices from rats implanted with GFP expressing astrocytes (GFP n=12 rats, Nef n=11 rats, 2–3 sections per rat; GFP left hemisphere, 479±14; Nef left hemisphere, 430±10, p=0.01; GFP right hemisphere, 446±25; Nef right hemisphere, 378± 10, p=0.04; Figure 4B) indicating that Nef expression by the astrocytes promoted loss of hippocampal neurons.
Figure 4.
Rats in the Nef group had fewer hippocampal neurons in CA3. (A) Neurons were detected by Nissl staining. Representative right hippocampal sections at 100x (left) and 400x (right) magnification from rats implanted with astrocytes expressing GFP or Nef. CA3 panels indicate region counted for lower graph. (B) Neurons were counted in CA3 and mean (plus standard error) counts for Nef and GFP groups were calculated in both hemispheres. Graph shows average number of neurons per animal per CA3 region. *p<0.05, **p<0.01. Scale bars = 100μM.
Nef-treated animals showed upregulated expression of CCL2 and macrophage infiltration at the site of the injection
In vitro studies demonstrated that Nef increased expression of chemokine ligand 2 (CCL2) in a human glioblastoma cell line (Lehmann et al., 2006). In addition, patients with HAD showed higher CCL2 in the cerebral spinal fluid compared with the control group (Kelder et al., 1998). Consistent with these previous findings, our astrocyte cultures showed a 22-fold induction in CCL2 mRNA expression by Nef compared to astrocytes expressing GFP 48 hrs after transfection (p=0.04; Figure 5A). To determine whether the Nef-expressing astrocytes continued to express CCL2 after implantation, we measured CCL2 expression at the site of implantation in both Nef- and GFP-treated rats. We found a 4-fold increase in CCL2 expression in rats implanted with astrocytes expressing Nef (p=0.05; Figure 5B).
Figure 5.
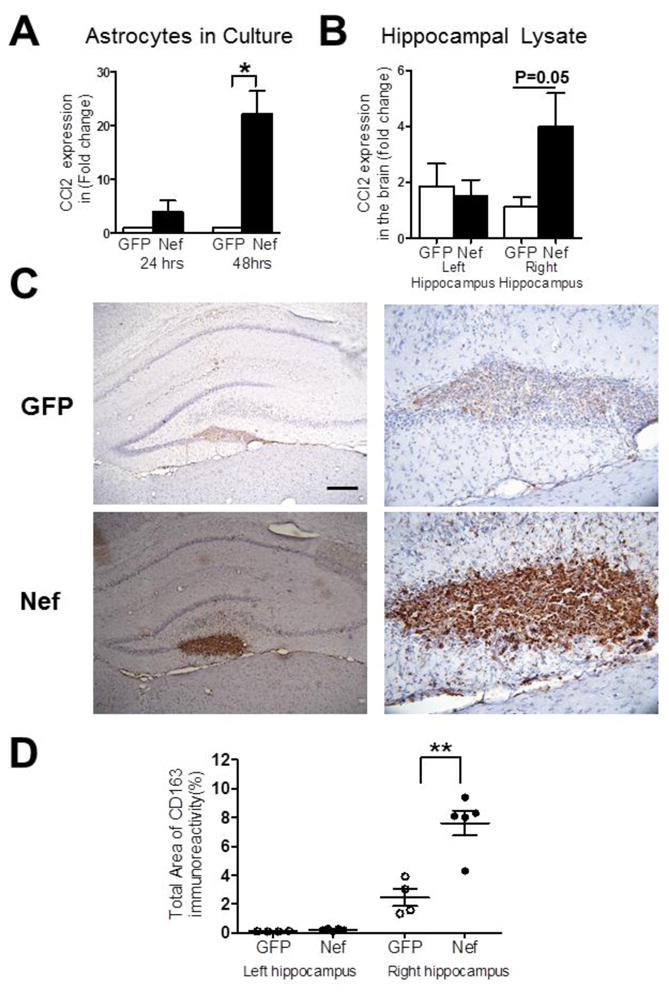
The Nef group shows CCL2 induction and enhanced infiltration of CD163-positive macrophages proximal to injection site compared to GFP controls. Real time, quantitative RT-PCR was used to measure CCL2 expression in: (A) cultures of transfected primary astrocytes expressing GFP (open bars) or Nef (closed bars) or (B) hippocampal tissue implanted with GFP-expressing (open bars) or Nef-expressing (closed bars) astrocytes. Data are presented for both non-infused (left) and infused (right) hemispheres. (C) Representative tissue sections immunostained for macrophage infiltration using the CD163 marker, GFP group (top) and Nef group (bottom) at 100x (left) and 400x (right) magnification. (D) Densitometric analysis was used to quantify CD163 staining. Scatter plot shows mean (+/− standard error) for the GFP (open circles) and Nef closed circles) groups. Scale bar = 100μM. *p<0.05; **p<0.005.
Since CCL2 is a potent monocyte chemoattractant, we next examined whether there was infiltration of peripheral macrophages in the rats implanted with Nef expressing astrocytes. To distinguish between resident microglia and peripheral macrophages, we used the CD163 marker, which is expressed by activated peripheral macrophages but not microglia (Fischer-Smith et al., 2008a; Fischer-Smith et al., 2008b). We found that rats implanted with Nef-expressing astrocytes had significantly higher expression of CD163 at the site of implantation compared to rats implanted with GFP-expressing astrocytes (p=0.002; Figure 5C, D). As with expression of CCL2, both groups showed similar levels of CD163 immunoreactivity in the left hippocampus (p=0.06; Figure 5D). In sum these data suggest the Nef transfected astrocytes express CCL2, which recruits peripheral macrophages to infiltrate the hippocampus at the site of injection.
Discussion
While several properties of Nef including direct neurotoxicity (Trillo-Pazos et al., 2000) are well characterized in vitro, the functional importance of HIV-1 Nef protein in the development and progression of neuropathology, especially in the era of cART, is less clear. To begin to address this issue, we created an animal model that mimics the astrocytic expression of Nef found in patients with HIV-associated dementia. We found that the implantation of primary astrocytes expressing HIV-1 Nef into the hippocampus of rats disrupts spatial and recognition memory. The memory loss was associated with astrocytic expression of Nef, induction of CCL2 and infiltration of CD163 positive mononuclear cells. Rats in the Nef-expressing group also showed a reduced number of neurons in the CA3 region of the hippocampus. Our study demonstrates that production of Nef from astrocytes alone is sufficient to produce loss of neurons in the hippocampus resulting in deficits in spatial and recognition memory. Since infiltration of activated macrophages was not found in the contralateral hippocampus, the loss of neurons distal to the site of infusion was likely the result of the release of a soluble neurotoxic substance from the Nef-expressing astrocytes or from the activated macrophages. In support of this, several model systems show that Nef-expressing astrocytes release pro-inflammatory molecules including CCL2 (Lehmann et al., 2006; Van Marle et al., 2004).
Interestingly, we found evidence for both intracellular and extracellular Nef during seven days in culture. Other work has shown that Nef secretion occurs from infected T cells in vitro and extracellular Nef, found in the serum of HIV infected people, has been linked to the depletion of uninfected T-cells suggesting cytotoxic effects of extracellular Nef (Fujii et al., 1996; Huang et al., 2004). Although the expression of Nef by the implanted astrocytes for the duration of the behavioral testing could indicate that disruption of astrocytic functions led to memory impairment, the detection of Nef in supernatants in vitro suggests that the infusion of Nef expressing astrocytes in vivo may have directly exposed the neurons to Nef. In vitro studies have demonstrated that Nef protein alone is directly neurotoxic (Trillo-Pazos et al., 2000). Immunostaining for Nef at day 7 in vivo demonstrated maintenance of Nef expression from the infused cells. Costaining with GFAP in Figure 1C showed the strongest Nef signal in astrocytes with more diffuse staining between cells indicating there may be release of Nef from the transfected cells as we find in vitro. Thus our results leave open the possibility that Nef contributes directly to the neuronal loss we observed in our model. Extracellular Nef could also directly contribute to the cell infiltration observed in our model system, since intracranial injection of Nef can disrupt the blood brain barrier and promote leukocyte infiltration into the brain (Koedel et al., 1999; Sporer et al., 2000). Our in vivo detection of increased macrophage infiltration uniquely in the infused hemisphere favors the role of a soluble factor from astrocytes or extracellular Nef in the bilateral loss of neurons.
Our findings are consistent with a previous study that reported spatial learning deficits in rats implanted with macrophages transformed to express Nef in the hippocampus (Mordelet et al., 2004). As in our studies, they found infiltration of peripheral macrophages as a result of Nef expression (Mordelet et al., 2004). Interestingly, they did not detect neuronal loss in contrast to the reduced neuron counts we observe using astrocytes to produce the Nef. In a separate mouse model, endogenous Nef expression was induced in striatal neurons using an alphavirus vector. The mice demonstrated astrocytic and microglial hypertrophy, neuronal loss, inflammation and alterations in motor function (Van Marle et al., 2004). In contrast to our observation of neuron loss distal to the site of surgery, neuronal loss was predominantly at the site of infusion, perhaps reflecting a difference in the cells expressing Nef or the sensitivity of neurons in the striatum.
Our results complement and extend these earlier studies by showing that the exclusive expression of Nef in astrocytes, which mimics histological observations in post-mortem HIV dementia patients and SIV infected macaques (Fiala et al., 2008; Overholser et al., 2003; Ranki et al., 1995; Saito et al., 1994), is sufficient to cause neuroinflammation, neurotoxicity, and memory impairment. Our results are also consistent with the findings that people with HAND show increased expression of CCL2 in cerebral spinal fluid and plasma (Kelder et al., 1998), which is associated with increases in perivascular macrophages and multinucleated giant cells found in autopsies of patients succumbing to HIV dementia. The model described here offers a unique system to isolate the effects of endogenous Nef expression in discrete regions of the brain. As well, the system is suited for testing agents to ameliorate the effects of HIV neurotoxins and thus serve as potential complementary therapy to current antiretrovirals to improve outcomes.
One such target for complementary therapy includes neurotransmitter systems and circuits. Several reports suggest neuropathology in HIV infection is mediated by disruption of neurotransmitters, receptors and transporters during infection with HIV or SIV (Gelman et al., 2012; Gelman et al., 2004). While our work was focused on the hippocampus and no specific neurotransmitter, several other studies have dealt with other brain regions and particular neurotransmitter systems. For example, dopamine levels are reduced in demented AIDS patients and those with milder forms of HAND (Kumar et al., 2009; Sardar et al., 1996). The progressive dopamine loss, rather than HIV RNA levels, correlated with degree of impairment (Kumar et al., 2011). The lack of dopamine in substantia nigra, which unlike viral replication is not a direct target of therapy, correlated with poor neuropsychological performance in HIV positive patients (Kumar et al., 2011). Studies in macaques show dopamine reduction even during very early infection with SIV (Scheller et al., 2005), while use of the HIV-1 transgenic rat, which expresses viral proteins but not viral replication, indicates very early alterations in dopaminergic systems that manifest as a greater sensitivity to methamphetamine (Moran et al., 2012). Tat protein itself, which is among the proteins expressed in the transgenic rat, alters dopamine transport by reducing uptake in striatal synaptosomes in a PKC-dependent manner (Midde et al., 2012) and by destroying dopamine terminals and inhibiting monoamine transporter in rat striatum (Theodore et al., 2012; Theodore et al., 2006). Minocycline, which has antioxidant activities, offers a protective effect against nigrostriatal loss of dopamine if administered early in macaques (Meulendyke et al., 2012). Recently, a transcriptional analysis of components of the dopaminergic circuits in the prefontal cortex of HIV infected subjects indicated the potential utility of a pharmacological approach for synaptic modulation to improve neurological outcomes in HIV/AIDS (Gelman et al.).
In addition to dopamine alterations, disruption of cholinergic systems or glutamate levels by HIV or viral proteins is also a factor in neuronal damage during infection. SIV infected macaques showed reduced choline acetyltransferase and vesicular acetylcholine transporter levels in hippocampus and basal forebrain (Depboylu et al., 2012; Koutsilieri et al., 2000). These changes were accompanied by a decrease in cyclooxygenase-1 expression. Interestingly, although treatment of the macaques with a nucleoside reverse transcriptase inhibitor effectively reduced brain viral load, these neurochemical were maintained indicating they may be irreversible (Depboylu et al., 2012). Overproduction of glutaminase in mitochondria of HIV infected macrophages and microglia leads to neurotoxicity (Huang et al., 2011; Tian et al.). Astrocyte glutamate transporter function is also compromised by HIV infection and the gp120 neurotoxin, which exacerbates the excitotoxicity of excess production (Wang et al., 2003). Since glutamate is the predominant excitatory neurotransmitter in the hippocampus and excess levels lead to neurotoxicity, our model may be useful in future studies for elucidating the contribution of glutamate disruption by Nef to HAND.
Conclusion
We have demonstrated that Nef expression from astrocytes in the hippocampus is sufficient to produce spatial memory deficits from a short exposure of seven days. Based on our findings and reports from the literature, we propose the following model (Figure 6). Astrocytes expressing Nef in the hippocampus secrete CCL2 and promote the transmigration of the CD163-positive mononuclear cells into the brain. Inflammatory proteins are known to be released from Nef-expressing astrocytes (Lehmann et al., 2006; Van Marle et al., 2004) or from activated macrophages (Fischer-Smith et al., 2008b; Guillemin et al., 2001) leading to neuronal toxicity and cognitive impairment. In addition, extracellular Nef released from the astrocytes by cell lysis or a regulated process may also contribute to inflammation by disruption of the blood brain barrier (Koedel et al., 1999; Sporer et al., 2000) or direct neurotoxicity (Trillo-Pazos et al., 2000). Future studies will be critical to delineate the relative roles and contributions of Nef directly and indirectly, such as by promoting cell infiltration and inflammation or modulating neurotransmitter circuits, to the memory impairment in this system as well as to understand the mechanisms and identify potential treatments for preventing the cognitive deficits that contribute to the morbidity of HIV infection.
Figure 6.
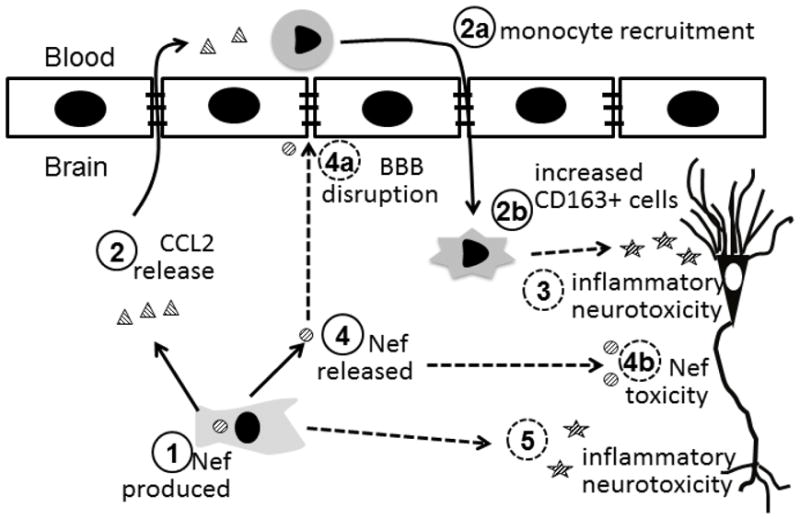
Proposed components of learning impairment caused by astrocytic Nef expression. Nef protein is produced in astrocytes (1) resulting in induction of CCL2 (2). The secreted CCL2 serves as a powerful attractant to peripheral monocytes that migrate to the brain (2a) and differentiate to macrophages expressing CD163 (2b). These macrophages contribute to the local inflammatory environment resulting in the loss of neurons (3). Nef released from astrocytes (4) may compromise the blood brain barrier (4a) and cause direct neurotoxicity (4b). Finally, Nef production in astrocytes can promote release of proinflammatory molecules (5) further contributing to the neurotoxic environment. The loss of neuron numbers and function results in learning impairment. Solid lines indicate findings from this study; dashed lines are based on other published data.
Highlights.
Astrocyte HIV-1 Nef production in hippocampus produces learning impairment in rats.
Nef expression in astrocytes stimulates CCL2 production.
CCL2 induced by Nef stimulates peripheral monocyte infiltration into hippocampus.
Neuronal loss is associated with Nef expression, CCL2 induction and monocyte infiltration.
Acknowledgments
This research was supported by funds from NIH grants DA026722 and GM008239 to RJN, RCMI Behavioral Facility and RCMI Molecular Biology Core Laboratory (RR003050, MD007579), and MBRS RISE Program (R25GM082406). Special thanks to Janet Colon (western blots and immunofluorescence) ; Maria Colon, Samary Mendez, Anitza Hernandez, Ana Lopez, Michael Manoharan, and Eliezer Ruiz (surgery and behavioral experiments); and Myrella Cruz, Alcira Benitez, Angelica Perez-Burgos, and Tirtsa Porrata-Doria (immunohistochemistry and cell count).
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo SF, Ohtsu H, Benice TS, Rizk-Jackson A, Raber J. Age-dependent measures of anxiety and cognition in male histidine decarboxylase knockout (Hdc−/−) mice. Brain Res. 2006;1071:113–23. doi: 10.1016/j.brainres.2005.11.067. [DOI] [PubMed] [Google Scholar]
- Anderson E, Zink W, Xiong H, Gendelman HE. HIV-1-associated dementia: a metabolic encephalopathy perpetrated by virus-infected and immune-competent mononuclear phagocytes. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S43–54. doi: 10.1097/00126334-200210012-00004. [DOI] [PubMed] [Google Scholar]
- Benice TS, Raber J. Object recognition analysis in mice using nose-point digital video tracking. J Neurosci Methods. 2008;168:422–30. doi: 10.1016/j.jneumeth.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–23. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Turchan J, Pocernich C, Bruce-Keller A, Roth S, Butterfield DA, Major EO, Nath A. Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J Biol Chem. 2003;278:13512–13519. doi: 10.1074/jbc.M209381200. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR. Extensive astrocyte infection is prominent in human immunodeficiency virus–associated dementia. Annals of Neurology. 2009;66:253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- Conant K, Tornatore C, Atwood W, Meyers K, Traub R, Major EO. In vivo and in vitro infection of the astrocyte by HIV-1. Adv Neuroimmunol. 1994;4:287–9. doi: 10.1016/s0960-5428(06)80269-x. [DOI] [PubMed] [Google Scholar]
- Dayton AI. Hitting HIV where it hides. Retrovirology. 2008;5:15. doi: 10.1186/1742-4690-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depboylu C, Weihe E, Eiden LE. Lentiviral Infection of Rhesus Macaques Causes Long-Term Injury to Cortical and Hippocampal Projections of Prostaglandin-Expressing Cholinergic Basal Forebrain Neurons. Journal of Neuropathology & Experimental Neurology. 2012;71:15–27. doi: 10.1097/NEN.0b013e31823cfac5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M, Singer EJ, Commins D, Mirzapoiazova T, Verin A, Espinosa A, Ugen K, Bernas M, Witte M, Weinand M, Lossinsky AS. HIV-1 Antigens in Neurons of Cocaine-Abusing Patients. Open Virol J. 2008;2:24–31. doi: 10.2174/1874357900802010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Bell C, Croul S, Lewis M, Rappaport J. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J Neurovirol. 2008a;14:318–26. doi: 10.1080/13550280802132857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Tedaldi EM, Rappaport J. CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS Res Hum Retroviruses. 2008b;24:417–21. doi: 10.1089/aid.2007.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y, Otake K, Tashiro M, Adachi A. Soluble Nef antigen of HIV-1 is cytotoxic for human CD4+ T cells. FEBS Lett. 1996;393:93–6. doi: 10.1016/0014-5793(96)00859-9. [DOI] [PubMed] [Google Scholar]
- Gelman B, Lisinicchia J, Chen T, Johnson K, Jennings K, Freeman D, Soukup V. Prefrontal Dopaminergic and Enkephalinergic Synaptic Accommodation in HIV-associated Neurocognitive Disorders and Encephalitis. Journal of NeuroImmune Pharmacology. 2012:1–15. doi: 10.1007/s11481-012-9345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Soukup VM, Schuenke KW, Keherly MJ, Holzer C, Richey FJ, Lahart CJ. Acquired neuronal channelopathies in HIV-associated dementia. J Neuroimmunol. 2004;157:111–119. doi: 10.1016/j.jneuroim.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Kerr SJ, Smythe GA, Smith DG, Kapoor V, Armati PJ, Croitoru J, Brew BJ. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem. 2001;78:842–53. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- Haase AT. Pathogenesis of lentivirus infections. Nature. 1986;322:130–6. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S55–61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- Heaton R, Franklin D, Ellis R, McCutchan J, Letendre S, LeBlanc S, Corkran S, Duarte N, Clifford D, Woods S, Collier A, Marra C, Morgello S, Mindt M, Taylor M, Marcotte T, Atkinson J, Wolfson T, Gelman B, McArthur J, Simpson D, Abramson I, Gamst A, Fennema-Notestine C, Jernigan T, Wong J, Grant I, Charter ft, Groups H. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of NeuroVirology. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LJ, Narasipura SD, Adarichev V, Kashanchi F, Al-Harthi L. Identification of novel TCF-4 binding sites on the HIV LTR which associate with TCF-4, β-catenin and SMAR1 to repress HIV transcription. Journal of Virology. 2012 doi: 10.1128/JVI.00486-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MB, Jin LL, James CO, Khan M, Powell MD, Bond VC. Characterization of Nef-CXCR4 interactions important for apoptosis induction. J Virol. 2004;78:11084–11096. doi: 10.1128/JVI.78.20.11084-11096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zhao L, Jia B, Wu L, Li Y, Curthoys N, Zheng JC. Glutaminase Dysregulation in HIV-1-Infected Human Microglia Mediates Neurotoxicity: Relevant to HIV-1-Associated Neurocognitive Disorders. The Journal of Neuroscience. 2011;31:15195–15204. doi: 10.1523/JNEUROSCI.2051-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- Koedel U, Kohleisen B, Sporer B, Lahrtz F, Ovod V, Fontana A, Erfle V, Pfister HW. HIV Type 1 Nef Protein Is a Viral Factor for Leukocyte Recruitment into the Central Nervous System. J Immunol. 1999;163:1237–1245. [PubMed] [Google Scholar]
- Koutsilieri E, Czub S, Scheller C, Sopper S, Tatschner T, Stahl-Hennig C, Meulen Vt, Riederer P. Brain choline acetyltransferase reduction in SIV infection. An index of early dementia? Neuroreport. 2000;11:2391–2393. doi: 10.1097/00001756-200008030-00011. [DOI] [PubMed] [Google Scholar]
- Kumar A, Ownby R, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. Journal of NeuroVirology. 2011;17:26–40. doi: 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Fernandez J, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, Kumar M. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. Journal of NeuroVirology. 2009;15:257–274. doi: 10.1080/13550280902973952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann MH, Masanetz S, Kramer S, Erfle V. HIV-1 Nef upregulates CCL2/MCP-1 expression in astrocytes in a myristoylation- and calmodulin-dependent manner. J Cell Sci. 2006;119:4520–30. doi: 10.1242/jcs.03231. [DOI] [PubMed] [Google Scholar]
- Major EO, Miller AE, Mourrain P, Traub RG, de Widt E, Sever J. Establishment of a line of human fetal glial cells that supports JC virus multiplication. Proc Natl Acad Sci U S A. 1985;82:1257–1261. doi: 10.1073/pnas.82.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Selnes OA, Jacobson LP, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43:2245–52. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Sacktor N, Selnes O. Human immunodeficiency virus-associated dementia. Semin Neurol. 1999;19:129–50. doi: 10.1055/s-2008-1040831. [DOI] [PubMed] [Google Scholar]
- Messam CA, Major EO. Stages of restricted HIV-1 infection in astrocyte cultures derived from human fetal brain tissue. Journal of neurovirology. 2000;6(Suppl 1):S90–4. [PubMed] [Google Scholar]
- Meulendyke K, Pletnikov M, Engle E, Tarwater P, Graham D, Zink M. Early Minocycline Treatment Prevents a Decrease in Striatal Dopamine in an SIV Model of HIV-Associated Neurological Disease. Journal of NeuroImmune Pharmacology. 2012;7:454–464. doi: 10.1007/s11481-011-9332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde NM, Gomez AM, Zhu J. HIV-1 Tat Protein Decreases Dopamine Transporter Cell Surface Expression and Vesicular Monoamine Transporter-2 Function in Rat Striatal Synaptosomes. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2012 doi: 10.1007/s11481-012-9369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Aksenov MY, Booze RM, Webb KM, Mactutus CF. Adolescent HIV-1 Transgenic Rats: Evidence for Dopaminergic Alterations in Behavior and Neurochemistry Revealed by Methamphetamine Challenge. Current HIV research. 2012 doi: 10.2174/157016212802138788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordelet E, Kissa K, Cressant A, Gray F, Ozden S, Vidal C, Charneau P, Granon S. Histopathological and cognitive defects induced by Nef in the brain. Faseb J. 2004;18:1851–1861. doi: 10.1096/fj.04-2308com. [DOI] [PubMed] [Google Scholar]
- Overholser ED, Coleman GD, Bennett JL, Casaday RJ, Zink MC, Barber SA, Clements JE. Expression of simian immunodeficiency virus (SIV) nef in astrocytes during acute and terminal infection and requirement of nef for optimal replication of neurovirulent SIV in vitro. J Virol. 2003;77:6855–6866. doi: 10.1128/JVI.77.12.6855-6866.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R, Haase A, Stowring L, Edwards M, Ventura P. A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology. 1985;147:231–6. doi: 10.1016/0042-6822(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Popovic M, Tenner-Racz K, Pelser C, Stellbrink HJ, van Lunzen J, Lewis G, Kalyanaraman VS, Gallo RC, Racz P. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2005;102:14807–12. doi: 10.1073/pnas.0506857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Ranki A, Nyberg M, Ovod V, Haltia M, Elovaara I, Raininko R, Haapasalo H, Krohn K. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS. 1995;9:1001–8. doi: 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–21. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Saito Y, Sharer LR, Epstein LG, Michaels J, Mintz M, Louder M, Golding K, Cvetkovich TA, Blumberg BM. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994;44:474–81. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- Sardar AM, Czudek C, Reynolds GP. Dopamine deficits in the brain: the neurochemical basis of parkinsonian symptoms in AIDS. Neuroreport. 1996;7:910–2. doi: 10.1097/00001756-199603220-00015. [DOI] [PubMed] [Google Scholar]
- Scheller C, Sopper S, Jenuwein M, Neuen-Jacob E, Tatschner T, Grunblatt E, ter M, Riederer P, Koutsilieri E. Early impairment in dopaminergic neurotransmission in brains of SIV-infected rhesus monkeys due to microglia activation. J Neurochem. 2005;95:377–387. doi: 10.1111/j.1471-4159.2005.03373.x. [DOI] [PubMed] [Google Scholar]
- Silva W, Maldonado H, Chompre G, Mayol N. Caveolae a new subcellular transport organelle. Bol Asoc Med P R. 1998;90:30–3. [PubMed] [Google Scholar]
- Sporer B, Koedel U, Paul R, Kohleisen B, Erfle V, Fontana A, Pfister HW. Human immunodeficiency virus type-1 Nef protein induces blood-brain barrier disruption in the rat: role of matrix metalloproteinase-9. J Neuroimmunol. 2000;102:125–130. doi: 10.1016/s0165-5728(99)00170-8. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Dwoskin LP, Maragos WF. HIV-1 protein Tat inhibits vesicular monoamine transporter-2 activity in rat striatum. Synapse. 2012;66:755–7. doi: 10.1002/syn.21564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Maragos WF. Methamphetamine and human immunodeficiency virus protein Tat synergize to destroy dopaminergic terminals in the rat striatum. Neuroscience. 2006;137:925–935. doi: 10.1016/j.neuroscience.2005.10.056. [DOI] [PubMed] [Google Scholar]
- Thompson KA, McArthur JC, Wesselingh SL. Correlation between neurological progression and astrocyte apoptosis in HIV-associated dementia. Ann Neurol. 2001;49:745–52. doi: 10.1002/ana.1011. [DOI] [PubMed] [Google Scholar]
- Tian C, Sun L, Jia B, Ma K, Curthoys N, Ding J, Zheng J. Mitochondrial Glutaminase Release Contributes to Glutamate-Mediated Neurotoxicity during Human Immunodeficiency Virus-1 Infection. Journal of NeuroImmune Pharmacology. 2012:1–10. doi: 10.1007/s11481-012-9364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatore C, Meyers K, Atwood W, Conant K, Major E. Temporal patterns of human immunodeficiency virus type 1 transcripts in human fetal astrocytes. J Virol. 1994;68:93–102. doi: 10.1128/jvi.68.1.93-102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatore C, Nath A, Amemiya K, Major EO. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J Virol. 1991;65:6094–6100. doi: 10.1128/jvi.65.11.6094-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Munoz J, Stockton P, Tacoronte N, Roberts B, Maronpot RR, Petito CK. Detection of HIV-1 gene sequences in hippocampal neurons isolated from postmortem AIDS brains by laser capture microdissection. J Neuropathol Exp Neurol. 2001;60:885–92. doi: 10.1093/jnen/60.9.885. [DOI] [PubMed] [Google Scholar]
- Trillo-Pazos G, McFarlane-Abdulla E, Campbell IC, Pilkington GJ, Everall IP. Recombinant nef HIV-IIIB protein is toxic to human neurons in culture. Brain Res. 2000;864:315–326. doi: 10.1016/s0006-8993(00)02213-7. [DOI] [PubMed] [Google Scholar]
- Van Marle G, Henry S, Todoruk T, Sullivan A, Silva C, Rourke SB, Holden J, McArthur JC, Gill MJ, Power C. Human immunodeficiency virus type 1 Nef protein mediates neural cell death: a neurotoxic role for IP-10. Virology. 2004;329:302–318. doi: 10.1016/j.virol.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, Rothstein JD, Volsky DJ. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology. 2003;312:60–73. doi: 10.1016/s0042-6822(03)00181-8. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]