Abstract
Purpose
The vast majority of patients with cancer at the end of life receive parenteral hydration in hospitals and no hydration in hospice, with limited evidence supporting either practice. In this randomized controlled trial, we determined the effect of hydration on symptoms associated with dehydration, quality of life, and survival in patients with advanced cancer.
Patients and Methods
We randomly assigned 129 patients with cancer from six hospices to receive parenteral hydration (normal saline 1 L per day) or placebo (normal saline 100 mL per day) daily over 4 hours. The primary outcome was change in the sum of four dehydration symptoms (fatigue, myoclonus, sedation and hallucinations, 0 = best and 40 = worst possible) between day 4 and baseline. Secondary outcomes included Edmonton Symptom Assessment Scale (ESAS), Memorial Delirium Assessment Scale (MDAS), Nursing Delirium Screening Scale (NuDESC), Unified Myoclonus Rating Scale (UMRS), Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F), Dehydration Assessment Scale, creatinine, urea, and overall survival. Intention-to-treat analysis was conducted to examine the change by day 4 ± 2 and day 7 ± 2 between groups.
Results
The hydration (n = 63) and placebo (n = 66) groups had similar baseline characteristics. We found no significant differences between the two groups for change in the sum of four dehydration symptoms (−3.3 v −2.8, P = .77), ESAS (all nonsignificant), MDAS (1 v 3.5, P = .084), NuDESC (0 v 0, P = .13), and UMRS (0 v 0, P = .54) by day 4. Results for day 7, including FACIT-F, were similar. Overall survival did not differ between the two groups (median, 21 v 15 days, P = .83).
Conclusion
Hydration at 1 L per day did not improve symptoms, quality of life, or survival compared with placebo.
INTRODUCTION
One controversial issue in the United States is whether patients with advanced cancer receiving hospice care should receive parenteral hydration when they are no longer able to maintain adequate fluid intake. Most patients with cancer will decrease their oral intake before death as a result of severe anorexia, nausea, dysphagia, and/or delirium. Dehydration in turn can cause or aggravate symptoms such as fatigue, myoclonus, and delirium.1,2 In addition, dehydration can result in accumulation of active metabolites of opioids that are commonly prescribed in this population, thereby producing severe sedation, agitation, or generalized myoclonus.
There are no established standards for hydration at end of life. Patients with advanced cancer with dehydration nearly always receive parenteral hydration in acute care facilities but almost never in hospice care. There is little scientific evidence to support either approach. The only double-blind randomized controlled study on this topic, which was conducted by our group, suggested that parenteral hydration decreases some symptoms associated with dehydration in patients with advanced cancer compared with placebo.3 In addition, several retrospective studies have suggested that hydration can reduce neuropsychiatric symptoms such as sedation, hallucinations, myoclonus, and fatigue.4,5
We conducted this randomized controlled trial to determine whether parenteral hydration was superior to placebo in improving symptoms associated with dehydration, delayed the onset and/or severity of delirium, and had an effect on quality of life (QoL) and survival in patients with advanced cancer receiving hospice care.
PATIENTS AND METHODS
Patients
Eligible patients were recruited between February 5, 2007, and April 16, 2011. Participating sites included Silverado Hospice, Odyssey Hospice, Vitas Hospice, Houston Hospice, and Christus VNA Hospice in the Greater Houston area. Inclusion criteria were a diagnosis of advanced cancer (ie, locally recurrent or metastatic disease), an age of 18 years or older, an admission to hospice, a reduced oral intake of fluids with evidence of mild or moderate dehydration as defined by decreased skin turgor in subclavicular region (> 2 seconds) and a score of ≥ 2 of 5 in the clinical dehydration assessment (see Study Assessments),6 an intensity of ≥ 1 on a 0 to 10 scale (0 = no symptom, 10 = the worst possible symptom) for fatigue and two of the three other target symptoms (hallucinations, sedation, and myoclonus), a life expectancy ≥ 1 week, availability of a primary caregiver, a Memorial Delirium Assessment Scale (MDAS) score less than 13, an ability to give written informed consent, and geographic accessibility (within 60 miles of The University of Texas MD Anderson Cancer Center). Patients were excluded if they had severe dehydration, defined as decreased blood pressure or low perfusion of limbs, decreased level of consciousness, or no urine output for 12 hours, history or clinical evidence of renal failure with creatinine more than 1.5× upper normal limit, history or clinical evidence of congestive heart failure, or history of bleeding disorders demonstrated by clinical evidence of active bleeding, hematuria, hematoma, ecchymoses, and petechiae. The institutional review board at MD Anderson Cancer Center approved this study. All patients provided written informed consent.
Study Design and Interventions
In this randomized, placebo-controlled, double-blind, multicenter study, eligible patients were stratified according to the accrual site. Using a computer-generated simple randomization scheme, the study pharmacist randomly assigned patients in a 1:1 ratio to receive either 1,000 mL (hydration group) or 100 mL (placebo group) of normal saline at home infused subcutaneously over 4 hours daily until the patient was unresponsive, developed progressive coma, or died. A volume of 1,000 mL per day was chosen on the basis of previous studies demonstrating that this amount was adequate to improve clinical outcomes in palliative care patients.3,7 Patients were approached and assessed by the research nurses at home on referral from the hospice team.
Both the patient and research nurse conducting the study assessments were blinded to the study intervention and the randomization sequence. Blinding was achieved by having a separate infusion research nurse who was aware of the treatment assignment, set the infusion at the appropriate rate, covered and placed the infusion pump in a locked backpack, and started the infusion at the patient's home each day. Blinding was further assured by the use of identical backpacks, counter weight (900 g) in the placebo backpack, and concealment of the rate of infusion on the infusion pump by a tape. At the end of study, we asked patients about their perception of study arm they have been assigned to (hydration, placebo, or do not know).
Study Assessments and End Points
Symptom burden, including fatigue, myoclonus, sedation, hallucinations, pain, nausea, depression, anxiety, drowsiness, shortness of breath, appetite, feelings of well-being, and sleep, was assessed using the Edmonton Symptom Assessment Scale (ESAS), which has been validated in the cancer population. Patients were asked to rate the severity of their symptoms over the previous 24 hours using a numerical rating scale of 0 to 10, with 0 meaning that the symptom is absent and 10 meaning the worst possible symptom.8–10 The primary outcome was change in the sum of four dehydration symptoms (fatigue, myoclonus, sedation, and hallucinations) between day 4 and baseline, which ranged from 0 to 40. Myoclonus was further assessed using the Unified Myoclonus Rating Scale (UMRS), a validated, videotape-assisted, clinical rating instrument.11 Sections 2 and 5 were used in this study. Section 2 assesses myoclonus at rest, and each item is rated on a scale of 0 to 4 (0 = “no jerks,” 4 = “≥ 10 jerks” in 10 seconds). Section 5 assesses performance of functional tests, and each item is rated on a scale of 0 to 4 (0 = “normal,” 4 = “cannot complete the task”).
Other secondary outcomes included delirium, QoL, and overall survival. The Memorial Delirium Assessment Scale (MDAS), the Richmond Agitation Sedition Scale (RASS), and the Nursing Delirium Screening Scale (NuDESC) were used to assess delirium. MDAS is a validated, clinician-rated rating scale. It consists of 10 items; each is scored from 0 to 3, with a diagnostic cutoff score of 7 of 30 or above supporting the presence of delirium.12 RASS is a one-item scale that measures the predominant features of delirium (agitation or sedation).13,14 NuDESC is a validated observational instrument conducted by research staff based on input from family caregivers. Five symptoms (disorientation, inappropriate behavior, inappropriate communication, illusions or hallucinations, and psychomotor retardation) are each give a score from 0 to 2, for a total score of 10.15
Patients also rated their QoL and fatigue during the last 7 days using the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F) questionnaire. The fatigue subscale consists of 13 items. Patients rate the intensity of fatigue and its related symptoms on a scale of 0 to 4 (0 = “not at all,” 4 = “very much”).16
Hydration status was assessed with the dehydration assessment scale on the basis of three physical findings, moisture on the mucous membranes of the mouth (0 = moist; 1 = somewhat dry; 2 = dry), axillary moisture (0 = moist; 1 = dry), and sunkenness of the eyes (0 = normal, 1 = slight sunken, 2 = sunken).6 These signs are selected on the basis of their significant correlations with biologic dehydration, as previously confirmed in elderly patients.17–19 The dehydration score (range, 0 to 7) is calculated as the total of these three scores. A higher score indicates a higher level of dehydration. We also collected electrolytes, creatinine, and urea.
All the preceding study assessments were conducted at baseline and day 4 ± 2 days for the first week and then every 3 to 5 days until the patient was off study, with the exception of FACIT-F and laboratory tests, which were only conducted at baseline and day 7, and the UMRS, which was not conducted at baseline.
Global symptom evaluation was used to estimate the minimal important difference in symptoms (fatigue, myoclonus, sedation, hallucinations, and delirium) before and after treatment. Patients were asked about their symptoms (worse, about the same, or better) after starting treatment. This tool has been used in a number of symptom researches.20
Overall survival was defined as time from study enrollment to last date of follow-up or death.
Statistical Analysis
A planned sample size of 150 patients (75 per arm) was powered to detect an effect size difference between the two study arms of 0.50 standard deviation units (ie, change of 4.2 on a 40-point scale), with an a two-tailed type I error rate of 0.05 and an 80% power assuming a maximum attrition rate of 15% by the 4-day follow-up. Because of funding limitations, this study was terminated after 129 patients were enrolled.
We summarized the baseline demographics and symptom profile using descriptive statistics, including medians, means, standard deviations, ranges, interquartile ranges, and frequencies.
We conducted a before-and-after analysis comparing between baseline and either day 4 ± 2 or day 7 ± 2 using the paired t test for continuous variables that were normally distributed (ie, ESAS) and the Wilcoxon rank sum test for continuous, nonparametric variables (eg, NuDESC). Intention-to-treat analysis was conducted to compare the change between the two arms using the t test for continuous variables that were normally distributed (ie, ESAS), the Mann-Whitney U test for continuous, nonparametric variables (eg, NuDESC), and the Fisher's exact test for categorical variables (eg, global symptom evaluation). A two-sided P value of less than .05 was considered statistically significant. We used the Kaplan-Meier method and log-rank test for survival analysis.
SPSS version 16.0 (SPSS, Chicago, IL) software was used for statistical analysis.
RESULTS
Patient Characteristics
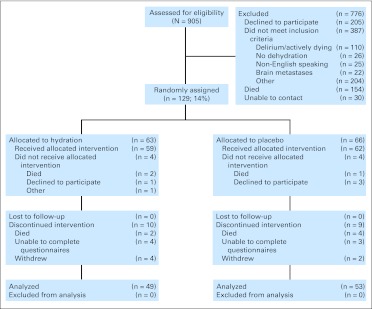
Figure 1 shows the study flowchart. Nine patients (7%) died before reaching the primary end point. Baseline patient characteristics were similar between the two study arms (Table 1). Eighty-eight percent of patients (n = 113) had Eastern Cooperative Oncology Group performance status of 3 to 4.
Fig 1.
CONSORT diagram.
Table 1.
Baseline Patient Characteristics
| Characteristic | Hydration (n = 63) |
Placebo (n = 66) |
Total (N = 129) |
|||
|---|---|---|---|---|---|---|
| No.* | %* | No.* | %* | No.* | %* | |
| Age, years | ||||||
| Median | 67 | 67 | 67 | |||
| Range | 43-92 | 41-92 | 41-92 | |||
| Female sex | 25 | 40 | 36 | 55 | 61 | 47 |
| Race | ||||||
| White | 36 | 57 | 41 | 62 | 77 | 60 |
| Black | 15 | 24 | 18 | 27 | 33 | 26 |
| Hispanic | 10 | 16 | 7 | 11 | 17 | 13 |
| Other | 2 | 3 | 0 | 0 | 2 | 1 |
| Cancer | ||||||
| Breast | 2 | 3 | 6 | 9 | 8 | 6 |
| GI | 25 | 40 | 22 | 33 | 47 | 36 |
| Genitourinary | 11 | 18 | 8 | 12 | 19 | 15 |
| Gynecologic | 5 | 8 | 4 | 6 | 9 | 7 |
| Head and neck | 1 | 1 | 3 | 5 | 4 | 3 |
| Hematologic | 2 | 3 | 2 | 3 | 4 | 3 |
| Lung | 11 | 18 | 13 | 20 | 24 | 19 |
| Other | 6 | 9 | 8 | 12 | 14 | 11 |
| ECOG performance status | ||||||
| 2 | 8 | 13 | 6 | 9 | 14 | 11 |
| 3 | 32 | 53 | 34 | 52 | 66 | 52 |
| 4 | 21 | 34 | 26 | 39 | 47 | 37 |
| ESAS† | ||||||
| Pain | 4 | 2–6 | 5 | 1–6 | 4 | 2–6 |
| Fatigue | 7 | 5–8 | 7 | 5–9 | 7 | 5–8 |
| Nausea | 0 | 0–5 | 0 | 0–5 | 0 | 0–5 |
| Depression | 2 | 0–5 | 2 | 0–5 | 2 | 0–5 |
| Anxiety | 3 | 0–5 | 2 | 0–5.5 | 3 | 0–5 |
| Drowsiness | 5 | 4–7 | 6 | 4–8 | 5 | 4–7 |
| Dyspnea | 3 | 0–5 | 3 | 0–5 | 3 | 0–5 |
| Appetite | 8 | 5–9 | 7 | 5–9 | 7 | 5–9 |
| Well-being | 5 | 3–8 | 5 | 4–7 | 3 | 0–5 |
| Hallucinations | 3 | 0–4.5 | 2.5 | 1–5 | 5 | 3–7 |
| Myoclonus | 3 | 1–5 | 3 | 1–5 | 3 | 1–5 |
| Composite outcome (fatigue, drowsiness, hallucinations, myoclonus)† | 18 | 15–22 | 18 | 15–22 | 18 | 15–22 |
| Opioid use | ||||||
| Morphine | 35 | 59 | 37 | 58 | 72 | 59 |
| Hydromorphone | 6 | 10 | 7 | 11 | 13 | 11 |
| Oxycodone | 2 | 3 | 4 | 6 | 6 | 5 |
| Fentanyl | 3 | 5 | 5 | 8 | 8 | 7 |
| Methadone | 18 | 31 | 14 | 22 | 32 | 26 |
| Hydrocodone | 9 | 15 | 8 | 13 | 17 | 14 |
| Propoxyphene | 3 | 5 | 2 | 3 | 5 | 4 |
| None | 6 | 10 | 8 | 13 | 14 | 11 |
| RASS† | 0 | −1-0 | 0 | −1-0 | 0 | −1-0 |
| MDAS† | 5 | 3–8 | 6 | 4–9 | 6 | 3–9 |
| NuDESC, day† | 1 | 0–2 | 1 | 0–3 | 1 | 0–3 |
| NuDESC, evening† | 1 | 0–3 | 1 | 0–3 | 1 | 0–3 |
| NuDESC, night† | 1 | 0–2 | 1 | 0–2 | 1 | 0–2 |
| FACT–G† | 57 | 52–64 | 61 | 50–71 | 59 | 51–66 |
| FACIT–F† | 74 | 62–82 | 68 | 58–88 | 72 | 59–84 |
| Dehydration assessment scale† | 3 | 3–4 | 3 | 2.8–4 | 3 | 3–4 |
| Creatinine† | 0.78 | 0.60–1.1 | 0.9 | 0.62–1.1 | 0.8 | 0.60–1.1 |
| Osmolality† | 290 | 281–298 | 292 | 282–302 | 290 | 281–299 |
| BUN† | 16 | 12–22 | 18 | 13–31 | 18 | 12–27 |
| Sodium† | 135 | 132–141 | 137 | 134–140 | 136 | 133–140 |
| Calcium† | 9 | 8–9 | 9 | 8–9 | 9 | 8–9 |
| BNP† | 230 | 2–521 | 170 | 3–519 | 220 | 84–518 |
Abbreviations: BNP, brain natriuretic peptide; BUN, blood urea nitrogen; ECOG, Eastern Cooperative Oncology Group; ESAS, Edmonton Symptom Assessment Scale; FACIT-F, Functional Assessment of Chronic Illness Therapy–Fatigue; FACT-G, Functional Assessment of Cancer Therapy–General; MDAS, Memorial Delirium Assessment Scale; NuDESC, Nursing Delirium Screening Scale; RASS, Richmond Agitation Sedition Scale; UMRS, Unified Myoclonus Rating Scale.
Unless otherwise specified.
Median and interquartile range.
We asked patients and caregivers to guess the treatment assignment to check the effectiveness of blinding. A large proportion of patients (67%) and caregivers (42%) reported that they did not know which study arm the patients had been assigned to, and only 17% of patients and 29% of caregivers correctly guessed the study intervention that the patient received, suggesting that blinding was effective.
Change in Symptom Profile
By day 4, both hydration (mean, −3.3; P = .004) and placebo groups (mean, −2.8; P = .03) demonstrated significant improvement in the sum of four dehydration symptoms as compared with baseline (Table 2). Furthermore, the hydration group demonstrated improvements in hallucinations (P = .002) and myoclonus (P = .01), whereas the placebo group demonstrated improvements in pain (P = .02), depression (P = .04), anxiety (P = .002), and myoclonus (P = .03). However, there were no significant differences between groups for the change in the sum of four dehydration symptoms or any individual symptoms (Table 2).
Table 2.
Change in Symptom Profile, Delirium, Quality of Life, and Clinical Hydration Assessments at Day 4 and Day 7
| Assessment | Change Between Day 4 and Baseline |
Change Between Day 7 and Baseline |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydration (n = 49) Change From Baseline |
Placebo (n = 51) Change From Baseline |
Hydration v Placebo, P† | Hydration (n = 44) Change From Baseline |
Placebo (n = 49) Change From Baseline |
Hydration v Placebo, P† | |||||||||
| Mean | SD | P* | Mean | SD | P* | Mean | SD | P* | Mean | SD | P* | |||
| ESAS | ||||||||||||||
| Pain | −0.8 | 3.4 | .09 | −1.0 | 3.0 | .02 | .73 | −0.1 | 3.5 | .18 | −1.2 | 2.6 | < .001 | .12 |
| Fatigue | −0.6 | 3.2 | .19 | −0.6 | 2.8 | .16 | .91 | −1.1 | 3.5 | .12 | −0.6 | 3.0 | .43 | .49 |
| Nausea | −0.2 | 2.4 | .47 | −0.3 | 3.0 | .56 | > .99 | −0.9 | 3.0 | < .001 | −1.0 | 3.4 | .02 | .89 |
| Depression | 0.04 | 2.7 | .92 | −1.0 | 3.6 | .04 | .09 | −1.1 | 2.8 | < .001 | −1.0 | 3.5 | .008 | .83 |
| Anxiety | −0.3 | 3.3 | .55 | −1.6 | 3.6 | .002 | .06 | −0.14 | 3.3 | .04 | −1.5 | 3.9 | .15 | .86 |
| Drowsiness | −0.3 | 3.8 | .60 | −0.4 | 3.6 | .40 | .86 | −1.2 | 3.7 | .72 | −0.6 | 3.6 | .95 | .47 |
| Dyspnea | −0.3 | 2.5 | .42 | −0.6 | 3.8 | .25 | .62 | −0.9 | 2.2 | < .001 | −1.4 | 3.5 | .07 | .45 |
| Appetite | −0.7 | 3.5 | .17 | −0.3 | 3.9 | .55 | .62 | 0 | 3.4 | .03 | 0.3 | 4.1 | .85 | .70 |
| Well-being | −0.9 | 3.4 | .08 | −0.1 | 4.2 | .90 | .30 | −0.7 | 3.7 | .18 | 0.1 | 3.8 | .88 | .34 |
| Hallucinations | −1.2 | 2.6 | .002 | −0.9 | 3.7 | .10 | .55 | −1.1 | 3.3 | .36 | −1.1 | 3.1 | .13 | .94 |
| Myoclonus | −1.1 | 3.0 | .01 | −1.0 | 3.1 | .03 | .79 | −1.5 | 2.7 | .005 | −1.6 | 2.8 | .006 | .85 |
| Composite outcome (fatigue, drowsiness, hallucinations, myoclonus)‡ | −3.3 | −1.1 to −5.4 | .004 | −2.8 | −0.2 to −5.3 | .03 | .77 | −4.9 | −2.2 to −7.7 | .22 | −3.8 | −1.1 to −6.4 | .44 | .54 |
| RASS∥ | 0 | −1-0 | .007§ | 0 | −2-0 | < .001§ | .07¶ | 0 | −1-0 | .002§ | −1 | −2-0 | < .001§ | .35¶ |
| MDAS∥ | 1 | −2-5.8 | .01§ | 3.5 | −0.3-14.5 | < .001§ | .08¶ | 2 | −2-10 | .003§ | 2.5 | −1-14 | < .001§ | .44¶ |
| NuDESC∥ | ||||||||||||||
| Day | 0 | −1-1 | .41§ | 0 | −1-2 | .15§ | .13¶ | 0 | 0-0 | .76§ | 0 | 0-1 | .21§ | .36¶ |
| Evening | 0 | −1-1 | .73§ | 0 | −1-2 | .11§ | .40¶ | 0 | −1-1 | .70§ | 0 | −1-3 | .10§ | .39¶ |
| Night | 0 | −1-0 | .27§ | 0 | −1-2 | .02§ | .03¶ | 0 | −1-1 | .31§ | 0 | −1-1 | .15§ | .79¶ |
| UMRS∥# | 0 | 0-3 | — | 0 | 0-8 | — | .54¶ | 1 | 0-5.8 | — | 0 | 0-5.5 | — | .76¶ |
| FACT-G | — | — | — | — | — | 6.7 | 11.2 | .02 | 2.6 | 16.7 | .07 | .31 | ||
| FACIT-F | — | — | — | — | — | 9.1 | 17.9 | .008 | 1.4 | 25.6 | .09 | .23 | ||
| Dehydration assessment scale | −0.8 | 1.5 | < .001 | −0.6 | 1.4 | .001 | .38 | −1.0 | 1.7 | .76 | −0.5 | 1.4 | .010 | .13 |
| BUN∥ | — | — | — | — | — | −2 | −7-3 | .12§ | 2 | −1-8 | .10§ | .02¶ | ||
| Creatinine∥ | — | — | — | — | — | −0.1 | −0.2-0 | .049§ | −0.1 | −0.1-0.1 | .53§ | .25¶ | ||
| Osmolality | — | — | — | — | — | 2.7 | 10.2 | < .001 | 5.7 | 19 | .07 | .52 | ||
| Sodium | — | — | — | — | — | 1.9 | 5.0 | .001 | 0.7 | 5.0 | .001 | .36 | ||
| Calcium | — | — | — | — | — | −0.1 | 0.7 | .002 | 2.7 | 14.4 | .82 | .33 | ||
| BNP∥ | — | — | — | — | — | 90 | −59-291 | .07 | 31 | 1.5-91 | .02 | .53† | ||
Abbreviations: BNP, brain natriuretic peptide; BUN, blood urea nitrogen; ESAS, Edmonton Symptom Assessment Scale; FACIT-F, Functional Assessment of Chronic Illness Therapy–Fatigue; FACT-G, Functional Assessment of Cancer Therapy–General; MDAS, Memorial Delirium Assessment Scale; NuDESC, Nursing Delirium Screening Scale; RASS, Richmond Agitation Sedition Scale; SD, standard deviation; UMRS, Unified Myoclonus Rating Scale.
Paired t test unless otherwise specified.
t test unless otherwise specified.
Mean and 95% CI.
The Mann-Whitney U test was used to compare between hydration and placebo groups.
Median and interquartile range.
The Wilcoxon rank sum test was used to compare between hydration and placebo groups.
UMRS was only conducted on day 4 ± 2 and day 7 ± 2.
By day 7, we found no significant improvements in the sum of four dehydration symptoms from baseline in either groups. Both groups demonstrated significant improvements in nausea, depression, and myoclonus, as compared with baseline (P < .05, Table 2). However, we found no significant differences between the two groups for the change in the sum of four dehydration symptoms or for any individual symptoms (Table 2).
On the global symptom evaluation, a majority of patients (≥ 50%) in both study groups described improvement with their symptoms from baseline at days 4 and 7 (Table 3). However, there was no significant difference in improvement between the two groups.
Table 3.
Global Symptom Evaluation Between the Hydration Group and Placebo Group
| Evaluation | Change Between Day 4 and Baseline |
Change Between Day 7 and Baseline |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hydration (n = 36) |
Placebo (n = 39) |
P* | Hydration (n = 44) |
Placebo (n = 49)* |
P* | |||||
| No. | % | No. | % | No. | % | No. | % | |||
| Better | 20 | 56 | 21 | 54 | .79 | 9 | 50 | 12 | 57 | .45 |
| Same | 14 | 39 | 17 | 44 | 9 | 50 | 7 | 33 | ||
| Worse | 2 | 6 | 1 | 3 | 0 | 0 | 2 | 10 | ||
Fisher's exact test.
Delirium Outcomes
MDAS and RASS scores significantly worsened from baseline in both groups at days 4 and 7 (P < .001; Table 2). There was a trend for less deterioration in the hydration group as compared with the placebo group (RASS, P = .065; MDAS, P = .085). By day 4, the placebo group showed significantly more deterioration from baseline in night-time NuDESC scores as compared with the hydration group (P = .028; Table 2).
QoL
We observed significant improvements in Functional Assessment of Cancer Therapy–General (mean 6.7, P = .021) and FACIT-F (mean 9.1, P = .008) scores for the hydration group at day 7, and a trend for improvement for the placebo group (mean 2.6, P = .07 and mean 1.4, P = .086, respectively) (Table 2). However, there was no significant difference in the change in Functional Assessment of Cancer Therapy–General and FACIT-F scores from baseline between the hydration and placebo groups (P > .05).
Hydration Status
The dehydration assessment scale improved from baseline in both hydration and placebo groups at day 4, although this was not observed at day 7 (Table 2). There was no significant difference between groups for the change in dehydration assessment scale from baseline at day 4 or day 7. By day 7, the hydration group had a significantly lower blood urea nitrogen level (change from baseline −2 v 2; P = .02). The change in creatinine level did not differ (P = .25).
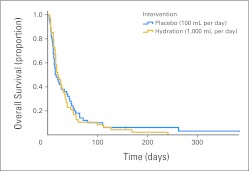
Survival
The overall median survival was 17 days (95% CI, 13 to 21 days). As shown in Figure 2, there was no significant difference in median survival between the hydration and placebo groups (21 v 15 days; P = .83).
Fig 2.
Overall survival by hydration status. The median survival was 21 days (range, 13 to 29 days) for the hydration group (gold) and 15 days (range, 12 to 18 days) for the placebo group (blue), with a P value of .83 (log-rank test).
DISCUSSION
Our study did not find parenteral hydration at 1,000 mL per day to be superior to placebo (100 mL per day) in improving symptoms associated with dehydration, QoL, or survival in terminally ill patients with cancer receiving home hospice care. Both groups reported similar degree of improvement in their symptoms. We also observed a trend for less deterioration in mental status in the hydration group.
One concern regarding the lack of observed difference between groups is that the volume of hydration may have been too low. This volume was specifically chosen based on our understanding that terminally ill patients with cancer require lower hydration volume as a result of weight loss, decreased insensible losses, decreased clearance of free water, and advanced age.2,7,21,22 We previously found that larger volumes were unnecessary in terminally ill patients with cancer.7 Indeed, we observed a statistically significant improvement in the urea level in the hydration arm, suggesting that hydration had taken place. Rather, the short duration of hydration and a large placebo effect may account for the lack of observed differences. Another limitation of this study is the fact that we excluded patients with severe dehydration (eg, hemodynamic instability, altered mentation) because these individuals tend to be acutely ill and in severe distress, making it difficult to obtain informed consent, deliver the study interventions, and keep them on study. Because some of these patients may have benefited the most from parenteral hydration, future studies may consider inclusion of this subgroup.
Although we observed no significant between-group differences in the primary outcome, both groups demonstrated significant improvements in before-and-after comparison and similar levels of perceived overall benefit. These findings are similar to our previous hydration study3 and suggest that independent of the amount of hydration received, frequent visits and assessments by research nurses may result in significant improvement in the perception of overall benefit, to the extent that it may even overshadow the biomedical effect of hydration. One possible way to circumvent the potential dramatic effect of nursing interventions may be by training family members to administer fluids and limiting the frequency of phone calls and assessments to the first and last day of study. In addition to our clinical findings reported here, we were also able to collect patient and family perceptions of hydration, which we published recently.23 In that study, we found the overwhelming majority of patients and families found parenteral hydration as clinically useful and helped enhance their comfort, dignity, and QoL. Our group has also observed a large placebo effect in other types of symptom control studies.24–28
One critical argument related to hydration in terminally ill patients concerns the relationship between delirium and hydration status. Delirium is common and progressive at the end of life and causes immense distress to patients and families.29–33 Delirium has multiple contributing etiologies, such as end organ failure, dehydration, and medications.29,34,35 Preliminary studies conducted in advanced cancer and elderly patients suggest that hydration may help in delirium prevention5,36,37 or its reversal when delirium is attributed to dehydration or opioid toxicities.38–40 We observed a significant deterioration in MDAS and RASS scores for both groups. This is not surprising given the trajectory of illness and the fact that we excluded patients with delirium upfront (ie, limited room for improvement). Interestingly, we observed a trend for lesser decline in the hydration group and significant worsening of nighttime NuDesc scores in the placebo group. Future studies may need to include patients with delirium, who may actually benefit the most from hydration. This could be done by either obtaining informed consent before this complication occurs or by using proxy consent.
The overall median survival for study participants was 17 days, which is similar to the life expectancy of hospice patients across the United States.41 We did not observe any significant differences in survival between the two study arms. Consistent with other studies,6,42–46 our study suggests that patients with a short life expectancy may not benefit from hydration in regard to survival.
Our study suggests that placebo-controlled interventional trials are feasible in the hospice setting. Supportive care trials are rarely done in this setting,47 out of the ethical concern that these trials may be too burdensome for patients and families and that many would decline enrollment.47 In our study, the majority of patients and families who we approached expressed willingness to participate. Although current hospice regulations precludes patients who elect hospice from participating in disease-modifying clinical trials, supportive care studies with the potential to improve symptoms and QoL should be encouraged.48
Our study had several limitations. First, our planned sample size was 150 patients, but because of funding issues, we terminated the study after 129 patients. The power to detect statistical significance given the found values and sample sizes was 4.8%; with 64 per group this would only have increased to 5.2%. Second, we conducted testing for multiple outcomes. Thus P values close to .05 should be interpreted with caution. Third, we excluded patients with severe dehydration, as discussed previously. Fourth, we did not monitor oral fluid intake during the study and therefore were unable to account for the potential cointervention effect of oral intake as a result of home visits by research nurses and patient/family education regarding benefits of hydration. Future studies should include careful measurement of oral intake.
In conclusion, our results suggest that in patients with advanced cancer who are mildly to moderately dehydrated and within days to weeks of death, parenteral hydration at 1,000 mL per day does not improve symptoms associated with dehydration, QoL, or survival as compared with placebo. Our study supports current hospice practice of not administering hydration routinely. Further studies are required to determine whether any subgroups, such as delirious patients or those with longer survival, would benefit from parenteral hydration.
Supplementary Material
Acknowledgment
We thank Janet Williams for her assistance with database management.
Footnotes
Supported by Grant No. R01CA122292-01 from the National Cancer Institute (E.B.). This study is also supported in part by National Institutes of Health Grants No. RO1NR010162-01A1 (E.B.) and RO1CA124481-01 (E.B.), the MD Anderson Cancer Center Support Grant (Grant No. CA 016672), an institutional startup fund (D.H.), and the National Cancer Institute (Grants No. 3R01CA122292-03S1 and 1K01CA151785-01, I.T.V.).
Presented in part at the 48th Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2012, Chicago, IL.
The sponsor of the study had no role in study design, data collection, analysis, interpretation, or writing of the report.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00423722.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Eduardo Bruera, J. Lynn Palmer
Financial support: Eduardo Bruera
Administrative support: Eduardo Bruera, Julio Allo, Susan Frisbee-Hume
Provision of study materials or patients: Joseph Trumble, Joseph Roosth, Susan Krauter, Carol Strickland, Kenneth Unger
Collection and assembly of data: Eduardo Bruera, Joseph Trumble, Joseph Roosth, Susan Krauter, Carol Strickland, Kenneth Unger, Julio Allo, Susan Frisbee-Hume, Kenneth Tarleton
Data analysis and interpretation: Eduardo Bruera, David Hui, Shalini Dalal, Isabel Torres-Vigil, J. Lynn Palmer
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Dalal S, Del Fabbro E, Bruera E. Is there a role for hydration at the end of life? Curr Opin Support Palliat Care. 2009;3:72–78. doi: 10.1097/SPC.0b013e32832531a5. [DOI] [PubMed] [Google Scholar]
- 2.Dalal S, Bruera E. Dehydration in cancer patients: To treat or not to treat. J Support Oncol, 483. 2004;2:467–479. [PubMed] [Google Scholar]
- 3.Bruera E, Sala R, Rico MA, et al. Effects of parenteral hydration in terminally ill cancer patients: A preliminary study. J Clin Oncol. 2005;23:2366–2371. doi: 10.1200/JCO.2005.04.069. [DOI] [PubMed] [Google Scholar]
- 4.de Stoutz ND, Bruera E, Suarez-Almazor M. Opioid rotation for toxicity reduction in terminal cancer patients. J Pain Symptom Manage. 1995;10:378–384. doi: 10.1016/0885-3924(95)90924-c. [DOI] [PubMed] [Google Scholar]
- 5.Bruera E, Franco JJ, Maltoni M, et al. Changing pattern of agitated impaired mental status in patients with advanced cancer: Association with cognitive monitoring, hydration, and opioid rotation. J Pain Symptom Manage. 1995;10:287–291. doi: 10.1016/0885-3924(95)00005-J. [DOI] [PubMed] [Google Scholar]
- 6.Morita T, Hyodo I, Yoshimi T, et al. Association between hydration volume and symptoms in terminally ill cancer patients with abdominal malignancies. Ann Oncol. 2005;16:640–647. doi: 10.1093/annonc/mdi121. [DOI] [PubMed] [Google Scholar]
- 7.Bruera E, Belzile M, Watanabe S, et al. Volume of hydration in terminal cancer patients. Support Care Cancer. 1996;4:147–150. doi: 10.1007/BF01845764. [DOI] [PubMed] [Google Scholar]
- 8.Philip J, Smith WB, Craft P, et al. Concurrent validity of the modified Edmonton Symptom Assessment System with the Rotterdam Symptom Checklist and the Brief Pain Inventory. Support Care Cancer. 1998;6:539–541. doi: 10.1007/s005200050212. [DOI] [PubMed] [Google Scholar]
- 9.Strömgren AS, Groenvold M, Pedersen L, et al. Symptomatology of cancer patients in palliative care: Content validation of self-assessment questionnaires against medical records. Eur J Cancer. 2002;38:788–794. doi: 10.1016/s0959-8049(01)00470-1. [DOI] [PubMed] [Google Scholar]
- 10.Moro C, Brunelli C, Miccinesi G, et al. Edmonton symptom assessment scale: Italian validation in two palliative care settings. Support Care Cancer. 2006;14:30–37. doi: 10.1007/s00520-005-0834-3. [DOI] [PubMed] [Google Scholar]
- 11.Frucht SJ, Leurgans SE, Hallett M, et al. The Unified Myoclonus Rating Scale. Adv Neurol. 2002;89:361–376. [PubMed] [Google Scholar]
- 12.Lawlor PG, Nekolaichuk C, Gagnon B, et al. Clinical utility, factor analysis, and further validation of the memorial delirium assessment scale in patients with advanced cancer: Assessing delirium in advanced cancer. Cancer. 2000;88:2859–2867. [PubMed] [Google Scholar]
- 13.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 14.Chester JG, Beth Harrington M, Rudolph JL. Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med. 2012;7:450–453. doi: 10.1002/jhm.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudreau JD, Gagnon P, Harel F, et al. Impact on delirium detection of using a sensitive instrument integrated into clinical practice. Gen Hosp Psychiatry. 2005;27:194–199. doi: 10.1016/j.genhosppsych.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 17.Eaton D, Bannister P, Mulley GP, et al. Axillary sweating in clinical assessment of dehydration in ill elderly patients. BMJ. 1994;308:1271. doi: 10.1136/bmj.308.6939.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross CR, Lindquist RD, Woolley AC, et al. Clinical indicators of dehydration severity in elderly patients. J Emerg Med. 1992;10:267–274. doi: 10.1016/0736-4679(92)90331-m. [DOI] [PubMed] [Google Scholar]
- 19.McGee S, Abernethy WB, 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281:1022–1029. doi: 10.1001/jama.281.11.1022. [DOI] [PubMed] [Google Scholar]
- 20.Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: A comparison of two techniques. J Clin Epidemiol. 1996;49:1215–1219. doi: 10.1016/s0895-4356(96)00206-5. [DOI] [PubMed] [Google Scholar]
- 21.Fainsinger RL, MacEachern T, Miller MJ, et al. The use of hypodermoclysis for rehydration in terminally ill cancer patients. J Pain Symptom Manage. 1994;9:298–302. doi: 10.1016/0885-3924(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 22.Reiff TR. Water and aging. Clin Geriatr Med. 1987;3:403–411. [PubMed] [Google Scholar]
- 23.Cohen MZ, Torres-Vigil I, Burbach BE, et al. The meaning of parenteral hydration to family caregivers and patients with advanced cancer receiving hospice care. J Pain Symptom Manage. 2012;43:855–865. doi: 10.1016/j.jpainsymman.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruera E, Roca E, Cedaro L, et al. Action of oral methylprednisolone in terminal cancer patients: A prospective randomized double-blind study. Cancer Treat Rep. 1985;69:751–754. [PubMed] [Google Scholar]
- 25.Bruera E, Moyano JR, Sala R, et al. Dexamethasone in addition to metoclopramide for chronic nausea in patients with advanced cancer: A randomized controlled trial. J Pain Symptom Manage. 2004;28:381–388. doi: 10.1016/j.jpainsymman.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Bruera E, Strasser F, Palmer JL, et al. Effect of fish oil on appetite and other symptoms in patients with advanced cancer and anorexia/cachexia: A double-blind, placebo-controlled study. J Clin Oncol. 2003;21:129–134. doi: 10.1200/JCO.2003.01.101. [DOI] [PubMed] [Google Scholar]
- 27.Bruera E, Chadwick S, Brenneis C, et al. Methylphenidate associated with narcotics for the treatment of cancer pain. Cancer Treat Rep. 1987;71:67–70. [PubMed] [Google Scholar]
- 28.Bruera E, El Osta B, Valero V, et al. Donepezil for cancer fatigue: A double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2007;25:3475–3481. doi: 10.1200/JCO.2007.10.9231. [DOI] [PubMed] [Google Scholar]
- 29.Lawlor PG, Gagnon B, Mancini IL, et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: A prospective study. Arch Intern Med. 2000;160:786–794. doi: 10.1001/archinte.160.6.786. [DOI] [PubMed] [Google Scholar]
- 30.Breitbart W, Gibson C, Tremblay A. The delirium experience: Delirium recall and delirium-related distress in hospitalized patients with cancer, their spouses/caregivers, and their nurses. Psychosomatics. 2002;43:183–194. doi: 10.1176/appi.psy.43.3.183. [DOI] [PubMed] [Google Scholar]
- 31.Namba M, Morita T, Imura C, et al. Terminal delirium: Families' experience. Palliat Med. 2007;21:587–594. doi: 10.1177/0269216307081129. [DOI] [PubMed] [Google Scholar]
- 32.Morita T, Hirai K, Sakaguchi Y, et al. Family-perceived distress from delirium-related symptoms of terminally ill cancer patients. Psychosomatics. 2004;45:107–113. doi: 10.1176/appi.psy.45.2.107. [DOI] [PubMed] [Google Scholar]
- 33.Bruera E, Bush SH, Willey J, et al. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer. 2009;115:2004–2012. doi: 10.1002/cncr.24215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morita T, Tei Y, Tsunoda J, et al. Increased plasma morphine metabolites in terminally ill cancer patients with delirium: An intra-individual comparison. J Pain Symptom Manage. 2002;23:107–113. doi: 10.1016/s0885-3924(01)00392-x. [DOI] [PubMed] [Google Scholar]
- 35.Lawlor PG. The panorama of opioid-related cognitive dysfunction in patients with cancer: A critical literature appraisal. Cancer. 2002;94:1836–1853. doi: 10.1002/cncr.10389. [DOI] [PubMed] [Google Scholar]
- 36.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 37.Seymour DG, Henschke PJ, Cape RD, et al. Acute confusional states and dementia in the elderly: The role of dehydration/volume depletion, physical illness and age. Age Ageing. 1980;9:137–146. doi: 10.1093/ageing/9.3.137. [DOI] [PubMed] [Google Scholar]
- 38.Gagnon P, Allard P, Mâsse B, et al. Delirium in terminal cancer: A prospective study using daily screening, early diagnosis, and continuous monitoring. J Pain Symptom Manage. 2000;19:412–426. doi: 10.1016/s0885-3924(00)00143-3. [DOI] [PubMed] [Google Scholar]
- 39.Maddocks I, Somogyi A, Abbott F, et al. Attenuation of morphine-induced delirium in palliative care by substitution with infusion of oxycodone. J Pain Symptom Manage. 1996;12:182–189. doi: 10.1016/0885-3924(96)00050-4. [DOI] [PubMed] [Google Scholar]
- 40.Tuma R, DeAngelis LM. Altered mental status in patients with cancer. Arch Neurol. 2000;57:1727–1731. doi: 10.1001/archneur.57.12.1727. [DOI] [PubMed] [Google Scholar]
- 41.National Hospice and Palliative Care Organization (NHPCO): ed 2011. Alexandria, VA: NHPCO; 2012. Jan, NHPCO Facts and Figures: Hospice Care in America—National Data Set 2010. [Google Scholar]
- 42.Cerchietti L, Navigante A, Sauri A, et al. Hypodermoclysis for control of dehydration in terminal-stage cancer. Int J Palliat Nurs. 2000;6:370–374. doi: 10.12968/ijpn.2000.6.8.9060. [DOI] [PubMed] [Google Scholar]
- 43.Morita T, Tei Y, Tsunoda J, et al. Underlying pathologies and their associations with clinical features in terminal delirium of cancer patients. J Pain Symptom Manage. 2001;22:997–1006. doi: 10.1016/s0885-3924(01)00360-8. [DOI] [PubMed] [Google Scholar]
- 44.Morita T, Tei Y, Inoue S. Agitated terminal delirium and association with partial opioid substitution and hydration. J Palliat Med. 2003;6:557–563. doi: 10.1089/109662103768253669. [DOI] [PubMed] [Google Scholar]
- 45.Andrews M, Bell ER, Smith SA, et al. Dehydration in terminally ill patients. Is it appropriate palliative care? Postgrad Med. 1993;93:201–203. doi: 10.1080/00325481.1993.11701584. 206-208. [DOI] [PubMed] [Google Scholar]
- 46.Waller A, Hershkowitz M, Adunsky A. The effect of intravenous fluid infusion on blood and urine parameters of hydration and on state of consciousness in terminal cancer patients. Am J Hosp Palliat Care. 1994;11:22–27. doi: 10.1177/104990919401100607. [DOI] [PubMed] [Google Scholar]
- 47.Casarett DJ, Karlawish J, Hirschman KB. Are hospices ready to participate in palliative care research? Results of a national survey. J Palliat Med. 2002;5:397–406. doi: 10.1089/109662102320135289. [DOI] [PubMed] [Google Scholar]
- 48.Casarett D, Ferrell B, Kirschling J, et al. NHPCO Task Force Statement on the Ethics of Hospice Participation in Research. J Palliat Med. 2001;4:441–449. doi: 10.1089/109662101753381566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.