Abstract
Purpose
To establish the performance characteristics of annual transvaginal ultrasound and serum CA125 screening for women at high risk of ovarian/fallopian tube cancer (OC/FTC) and to investigate the impact of delayed screening interval and surgical intervention.
Patients and Methods
Between May 6, 2002, and January 5, 2008, 3,563 women at an estimated ≥ 10% lifetime risk of OC/FTC were recruited and screened by 37 centers in the United Kingdom. Participants were observed prospectively by centers, questionnaire, and national cancer registries.
Results
Sensitivity for detection of incident OC/FTC at 1 year after last annual screen was 81.3% (95% CI, 54.3% to 96.0%) if occult cancers were classified as false negatives and 87.5% (95% CI, 61.7% to 98.5%) if they were classified as true positives. Positive and negative predictive values of incident screening were 25.5% (95% CI, 14.3 to 40.0) and 99.9% (95% CI, 99.8 to 100) respectively. Four (30.8%) of 13 incident screen-detected OC/FTCs were stage I or II. Compared with women screened in the year before diagnosis, those not screened in the year before diagnosis were more likely to have ≥ stage IIIc disease (85.7% v 26.1%; P = .009). Screening interval was delayed by a median of 88 days before detection of incident OC/FTC. Median interval from detection screen to surgical intervention was 79 days in prevalent and incident OC/FTC.
Conclusion
These results in the high-risk population highlight the need for strict adherence to screening schedule. Screening more frequently than annually with prompt surgical intervention seems to offer a better chance of early-stage detection.
INTRODUCTION
Approximately 10%1–3 of ovarian cancers (OCs) are a result of familial/genetic predisposition, predominantly germline mutations in BRCA1 and BRCA2 and mismatch repair genes in Lynch syndrome (LS). The risk of OC (until age 70 years) varies between 3.4% to 33% in LS,4–6 11% to 37% in BRCA2 carriers, and 39% to 65% in BRCA1 carriers.7–10
Given the poor survival associated with OC,11 women with a known predisposing mutation or strong family history are offered risk-reducing bilateral salpingo-oophorectomy (RRSO) to prevent OC/fallopian tube cancers (FTCs).12 In premenopausal women, RRSO halves the risk of expected breast cancers13 but results in infertility and premature menopause, with associated increased cardiovascular14 and osteoporosis15 risks. Delaying surgery until age 50 years carries OC/FTC risks of 15% to 27% in BRCA1 and 0.4% to 4% in BRCA2 carriers.7–9,16 Screening might enable women to delay RRSO until menopause.
OC/FTC survival inversely correlates with stage.17 Although improved medium-term survival has been shown with general population screening,18 with a high proportion of early-stage cancers detected in the prevalence screen of an ongoing trial,19 recently another trial found no mortality benefit.20
Random assignment to a nonscreening arm is unacceptable to high-risk women and clinicians (United Kingdom Familial Ovarian Cancer Screening Study [UK FOCSS] consensus meeting, London, United Kingdom, 2004). Best evidence in this population will probably come from prospective cohort studies. Here we present the largest such study to date, to our knowledge, to define screening performance characteristics and investigate the impact of delayed screening interval and surgical intervention.
PATIENTS AND METHODS
Between May 6, 2002, and January 5, 2008, 3,563 women at an estimated minimum 10% lifetime OC risk were recruited, and data on screening and outcomes were collected prospectively. The study was designed to estimate sensitivity within ± 10% (expected 95% CI), assuming 0.5% annual OC incidence.
Entry Criteria
The inclusion criteria originally defined a minimum 10% lifetime OC risk (Appendix, online only) on the basis of family history or predisposing mutations, including LS-associated mutations. OC in the family was defined as epithelial OC, FTC, or primary peritoneal cancer (PPC). Borderline and nonepithelial OC were excluded. Women were excluded if they had undergone bilateral salpingo-oophorectomy, were age < 35 years, or were participating in other OC screening trials.
Recruitment
After ethical approval (Eastern Multicentre Research Ethics Committee 97/5/007), women were recruited by specialist nurses, clinical geneticists, or gynecologists at 37 regional centers in the United Kingdom. Before consenting, women were counseled that RRSO was recommended management, being the only method of preventing OC/FTC. The limitations of screening were highlighted. Documentation (death certificates, histopathology reports) of relevant familial cancers was required, and eligibility was confirmed by the coordinating center (CC). Recruiting centers forwarded screening results to the CC for database entry (UK FOCSS Trial Management System, developed in MS Visual Basic 6 and Classic ASP 3, Microsoft SQL Server 2000).
Screening
Phase I of UK FOCSS comprised annual transvaginal ultrasound scans (TVSs) and serum CA125 measurements, arranged and performed locally. Annual scans were performed by experienced National Health Service ultrasonographers, and follow-up scans for abnormalities were performed by expert gynecologists or radiologists. Where practical, scans were scheduled for menstrual cycle days 3 to 6. Collaborating centers were asked to complete datasheets describing ovarian volume and morphology, which were classified according to predetermined criteria (Appendix, online only). Guidelines for management of results were provided (Appendix Fig A1, online only), but management remained at the discretion of collaborating gynecologists. Serum CA125 was measured using preferred assays at collaborating clinical laboratories. We recommended cutoffs of 35 and 30 IU/mL in premenopausal and postmenopausal women, respectively.21 Between 2007 and 2009, phase II screening (once every 4 months) was introduced in response to concerns22 about the ability of annual screening to detect early-stage disease. Phase II is currently in follow-up and will be reported separately.
Documentation of Surgical Procedures and Diagnoses
Whenever women underwent salpingo-oophorectomy, the CC obtained documentation explaining surgical indication, whether CA125 and/or scan results had prompted surgery, the operation note, and histopathology and cytopathology reports. These were reviewed by a gynecologic oncologist (A.N.R.) and pathologist (E.B., N.S.). Serial sectioning of tubes/ovaries was not mandatory for RRSO specimens.
Criteria for Screening Performance Characteristics
Women undergoing salpingo-oophorectomy were only classified as having had RRSO if they were asymptomatic and had normal screening tests in the year before surgery and if the recruiting center indicated RRSO as the reason for withdrawal from the study. Cases in which abnormal screening results prompted surgery were true positive if invasive epithelial OC/FTC was diagnosed. All other diagnoses (including borderline/benign ovarian tumors) resulting from surgery prompted by abnormal test results were false positive. Cases in which a nonconcerning test result (eg, simple ovarian cysts, transiently raised CA125) had contributed to the decision to undergo surgery were classified as screening-related surgery to provide estimates of likely additional surgeries in any future screening program. True-negative patients were those in whom the last screen was normal, and no diagnosis of OC/FTC was made in the subsequent 365 days. Prevalent cases were those in which patients were diagnosed at first screen. Incident cases were those in which patients were diagnosed after subsequent screens.
Cancers diagnosed > 365 days after a woman's last screen are reported but not included in the analyses of annual screening performance. PPC (defined according to recognized pathologic criteria23) is unlikely to be amenable to early-stage detection using current techniques; however, data are presented both including and excluding PPC from the screening performance analysis.
Interval cancers (false negatives) were those presenting clinically < 365 days after the last screen. Occult cancers found in RRSO specimens < 365 days after the last annual screen can be classified as either false negative or true positive, because they might have been missed or detected at the next annual screen had RRSO not been performed. We therefore report screening performance using both these scenarios, on the assumption that the true sensitivity of screening in a population not undergoing RRSO falls between these two estimates.
Follow-Up
Collaborators notified the CC when women withdrew from the study. In December 2006, all women were invited to join phase II of the study and to confirm they still had one or more ovaries/fallopian tubes. All women were flagged with the relevant national cancer registry (National Health Service Information Centre for Health and Social Care, General Registrar Office for Scotland, and Northern Ireland Cancer Registry).
For women who withdrew, data was censored 365 days after withdrawal date. Details of OC/FTCs occurring after censoring are reported but not included in the analyses of annual incidence or screening performance. OC/FTCs diagnosed within 365 days of a woman's last screen were included in the analyses. Of women in phase I of UK FOCSS, 66.2% transferred to phase II of the study and subsequently underwent CA125 testing once every 4 months and annual TVS. For these women, withdrawal date from phase I was the date of their first screen on phase II (ie, > 4 months after their last annual phase I screen), and data were censored 365 days after withdrawal date. Because no cancers occurred within 1 year of a woman transferring to phase II, sensitivity was not artificially increased by the introduction of screening once every 4 months.
To investigate potentially avoidable delays (which could influence stage at detection), we analyzed screening delays in women diagnosed with OC/FTC and the interval between abnormal test results and surgical investigation. Detection screens were defined as an abnormal TVS and/or elevated CA125 result found at an annual screen leading to surgery/diagnostic biopsy resulting in diagnosis of OC/FTC. Delays in annual screens were defined as any detection screen (CA125 or TVS) performed > 365 days after previous normal annual screen. Delay was calculated as days between detection screen and prior normal annual screen minus 365. Interval from screen to diagnosis was calculated to the date of surgery/diagnostic biopsy. Composite delay was calculated as the sum of screening delay and screen to diagnosis interval. To investigate any effect of delayed screening, we analyzed International Federation of Gynecology and Obstetrics stage, optimal debulking (< 1 cm residual disease), and overall and disease-specific survival from diagnosis (irrespective of whether cancers were screen detected), comparing women screened in the year before diagnosis with those not screened in the year before diagnosis. We excluded LS-associated cases from these analyses to avoid contaminating the predominant BRCA-associated cases.
RESULTS
The median age of participants at recruitment was 44.6 years (range, 35 to 81 years). Table 1 lists indications for inclusion. One thousand thirty-four women (29.0% of the cohort) had undergone mutation testing before censoring. Six hundred three women (65.2% of those in whom test results were known; 16.9% of the cohort) were known mutation carriers.
Table 1.
Indication for Inclusion and Mutation Status of Study Participants
| Indication for Inclusion or Mutation Status | No. | % |
|---|---|---|
| Indication for Inclusion | ||
| Known mutation in family and/or proband | 867 | 24.4 |
| Breast/ovarian family history; no known mutation | 1,499 | 42.1 |
| Ovarian only family history; no known mutation | 889 | 25.0 |
| Lynch syndrome family history; no known mutation | 25 | 0.7 |
| Not fitting standard inclusion criteria but deemed high risk by recruiting center and study clinical geneticist (J.M.) | 283* | 7.9 |
| Total | 3,563 | 100 |
| Mutation status of proband at censor date | ||
| BRCA1 | 282 | 7.9 |
| BRCA2 | 250 | 7.0 |
| BRCA1 and BRCA2 | 6 | 0.2 |
| MLH1 | 28 | 0.8 |
| MSH2 | 33 | 0.9 |
| MSH6 | 4 | 0.1 |
| PMS1 | 0 | 0.0 |
| PMS2 | 0 | 0.0 |
| Tested negative | 322 | 9.0 |
| Tested but result pending | 109 | 3.1 |
| Untested | 2,529 | 71.0 |
| Total | 3,563 | 100 |
NOTE. Documentation (death certificates or histopathology reports) of relevant cancers in the family was required. This was available for 63.9% of women included for reasons other than a predisposing mutation in themselves or first-degree relative.
Nine were possible Lynch syndrome families, and 271 were breast/ovarian cancer families.
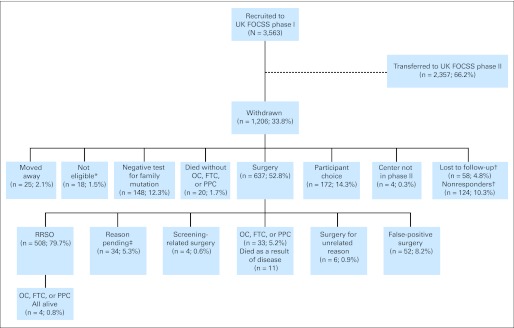
The study accumulated 11,366 women-years of screening (mean, 3.2 years per woman). Figure 1 shows the flow of participants. Although 182 women (5.1%) were lost to direct follow-up by the CC, they remained flagged by the cancer registries. The commonest reason for withdrawal was RRSO (14.3% of the study population), but an additional 4.2% withdrew because they were subsequently found not to carry their family's predisposing mutation.
Fig 1.
Flow of participants through study. All percentages refer to proportion of population defined in preceding row. UK FOCSS, United Kingdom Familial Ovarian Cancer Screening Study; FTC, fallopian tube cancer; OC, ovarian cancer; PPC, primary peritoneal cancer; RRSO, risk-reducing salpingo-oophorectomy. (*) Ineligible on basis of new information regarding diagnoses in family history becoming available subsequent to recruitment. (†)Lost to follow-up: unable to establish current whereabouts; nonresponders: failed to respond despite confirmation of correct contact details. (‡) Reason for surgery pending, but known not to have had OC, FTC, or PPC.
Index Cancers
Table 2 shows cancers occurring during screening and follow-up according to whether cancers were detected at prevalence or incidence screens, screen negative, occult, or PPC. Twenty-six primary invasive epithelial OC/FTC and one PPC were observed during 11,366 women screen–years before censoring (annual OC/FTC/PPC incidence, 0.24%). An additional 10 cancers occurred beyond censoring 365 days after a last screen (median, 539 days; range, 382 to 1369).
Table 2.
Ovarian, Tubal, and Peritoneal Cancers Occurring During Screening and Follow-Up
| FIGO Stage | No. of Patients | Tumor |
Gene Mutation | Age (years) | CA125 at Diagnosis (u/mL) | Imaging Modalitya | Delay in Annual Screen at Detection (days)b | Interval Between Abnormal Test and Surgery (days) | Composite Interval (days)c | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substage | Grade | Histotype | Site | |||||||||
| Prevalent cancers | 9 | |||||||||||
| I | 5 | Ia | G3 | Clear cell | OC | MSH2 | 35 | 24 | TVS | NA | 141 | NA |
| Ic | G3 | Serous | OC | MLH1 | 60 | 128 | TVS | NA | 36 | NA | ||
| Ic | G3 | Serous | OC | BRCA2 | 51 | 22 | TVS | NA | 126 | NA | ||
| Ic | G2 | Serous | FTC | BRCA1 | 55 | 21 | TVS | NA | 20 | NA | ||
| Ic | G3 | Clear celld | OC | MSH2 | 49 | 94 | TVS | NA | 36 | NA | ||
| II | 1 | IIb | G3 | Serous | FTC | BRCA1 | 47 | 103 | TVS | NA | 79 | NA |
| III | 3 | IIIa | G3 | Serous | OC | BRCA2 | 53 | 48 | TVS | NA | 96 | NA |
| IIIb | G3 | Serous | OC | BRCA1 | 48 | 88 | TVS | NA | 92 | NA | ||
| IIIc | G3 | Serous | OC | BRCA1 | 57 | 223 | TVS | NA | 74 | NA | ||
| Incident screen-detected cancers | 13 | |||||||||||
| I | 2 | Ia | G1 | Endometrioid | OC | Untestede | 45 | 21 | TVS | 49 | 19 | 68 |
| Ia | G2 | Serous | FTC | BRCA1 | 43 | 11 | TVS | −52 | 79 | 27 | ||
| II | 2 | IIc | G3 | Adenocarcinoma/endometrioid | OC | BRCA1 | 55 | 39 | TVS | 737 | 138 | 875 |
| IIc | G3 | Serous | OC | BRCA1 | 52 | 192 | TVS | 231 | 21 | 252 | ||
| III | 9 | IIIa | G3 | Endometrioid | OC | BRCA1 | 45 | 124 | TVS | 27 | 184 | 211 |
| IIIb | G2 | Serous | OC | BRCA1 | 46 | 73 | TVS | −1 | 69 | 68 | ||
| IIIb | G2 | Serous | OC | Untestedf | 42 | 45 | TVS | 102 | 107 | 209 | ||
| IIIb | G3 | Serous | OC | BRCA1 | 48 | 3,874 | TVS | −50 | 15 | −35 | ||
| IIIb | G3 | Adenocarcinoma/mucinous | OC/FTC | BRCA1 | 57 | 4 | TVS | 30 | 147 | 177 | ||
| IIIc | G2 | Endometrioid | OC | BRCA1 | 52 | 246 | TVS | 78 | 20 | 98 | ||
| IIIc | G3 | Serous/endometrioid | OC/FTC | BRCA1 | 49 | 323 | TVS | 98 | 32 | 130 | ||
| IIIc | G3 | Serous | FTC | BRCA2 | 60 | 17 | TVS | 236 | 177 | 413 | ||
| IIIc | G3 | Serous | FTC | VUSg | 58 | 166 | TVS | 6 | 96 | 102 | ||
| FIGO Stage | No. of Patients | Tumor |
Gene Mutation | Age (years) | CA125 at Diagnosis (u/mL) | Imaging Modalitya | Last Screen to Diagnosis Interval (days) | Presentation | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Substage | Grade | Histotype | Site | ||||||||
| Screen-negative cancers | 8 | ||||||||||
| I | 1 | Ich | G3 | Serous/endometrioid | OC | BRCA1 | 67 | Not donei | Not donei | 221 | Ovarian cyst torsion |
| III | 5 | IIIc | G3 | Serous | OC | BRCA1 | 65 | 582 | CT | 382 | GI symptoms |
| IIIc | G3 | Serous | OC | BRCA1 | 67 | 527 | TVS | 421 | GI symptoms | ||
| IIIc | G3 | Serous | FTC/OC | BRCA1 | 74 | 278 | TVS | 1,369 | Abdominopelvic pain | ||
| IIIc | G3 | Serous | OCj | BRCA1 | 60 | 724 | TVS | 593 | Postmenopausal bleeding | ||
| IIIc | G3 | Serous | OC | BRCA2 | 62 | 550 | CT | 1,073 | Breast cancer restaging | ||
| IV | 2 | IV | G2 | Serous | OC | BRCA1 | 62 | 1,513 | TVS | 327 | Postmenopausal bleeding |
| IV | G3 | Small cell | OC | BRCA2 | 58 | 560 | CT | 982 | GI symptoms | ||
| Occult and primary peritoneal cancers | 7 | ||||||||||
| I | 2 | Ia | G2 | Serous | FTC | BRCA1 | 53 | Not donek | Not donek | 76 | Occult found at RRSO |
| Ic | G3 | Serous/mucinous | OC | BRCA1 | 38 | Not done | Not done | 539 | Occult found at RRSO | ||
| II | 1 | IIc | G2 | Serous | OC/FTC | BRCA1 | 60 | Not donel | Not donel | 106 | Occult found at RRSO |
| III | 4 | IIIb | G3 | Serous | Peritoneal | BRCA1 | 61 | 404 | CT | 497 | Screen detected |
| IIIc | G2 | Serous | Peritoneal | Untestedm | 60 | 1173 | CT | 255 | GI symptoms | ||
| IIIc | G2 | Serous | Peritoneal | BRCA1 | 40 | Not done | Not done | 411 | Occult found at RRSO | ||
| IIIc | G3 | Serous | Peritoneal | BRCA2 | 57 | 613 | CT | 678 | CA125 taken approximately 1 year after RRSO | ||
Abbreviations: CT, computed tomography (instead of ultrasound); FTC, fallopian tube cancer; NA, not applicable; OC, ovarian cancer; RRSO, risk-reducing salpingo-oophorectomy; TVS, transvaginal ultrasound; VUS, variant of unknown significance.
Imaging was abnormal in all patients for whom it was performed.
Negative number denotes annual screen scheduled early.
Sum of delay in annual screening and interval from abnormal test to surgery (negative number results from annual screen scheduled early).
Occurring against a background of endometriosis.
Confirmed diagnoses of bowel cancer in paternal cousin (at age 61 years), paternal grandmother (at age 50 years), and sister (at age 51 years, with synchronous OC) and possibly breast cancer in maternal cousin (at age 35 years). Family has tested negative for BRCA1, BRCA2, and immunohistochemical Lynch syndrome markers. Patient herself had previous unilateral oophorectomy for endometriosis.
Confirmed OC diagnoses in mother at age 52 years and maternal grandmother at age 73 years.
VUS in BRCA1 (5313-12 G>A).
Incompletely staged.
Last screen 221 days before emergency surgery; ovaries not seen on scan because of bowel gas; no CA125 taken; prior screen (scan and CA125) normal 574 days before presentation.
This patient had a synchronous stage II grade 2 endometrioid endometrial cancer.
Last screen 76 days before RRSO; CA125, 18 u/mL; normal scan.
Last screen 106 days before RRSO; CA125, 21 u/mL; one normal ovary seen on scan (other obscured by bowel gas).
Personal history of bilateral breast cancer at ages 49 and 52 years; one sister had breast cancer at age 47 years; another sister had OC at age 71 years and breast cancer at age 63 years; another sister had OC at age 53 years.
Twenty-nine (78.4%) of 37 cancers contained serous carcinoma; the remainder were predominantly endometrioid. Two clear-cell carcinomas occurred in LS mutation carriers. The median age of diagnosis was 53 years (range, 35 to 74 years), and 15 (40.5%) of 37 were premenopausal. Thirty-three (89.2%) of 37 cancers occurred in pathogenic mutation carriers. Of these, 24 (72.7%) were BRCA1 mutation carriers, six (18.2%) were BRCA2 mutation carriers, and three (9.1%) were carriers of LS mutations MLH1 (one patient) and MSH2 (two patients). An additional woman had a BRCA1 variant of unknown significance. Three women (8.1% of all OC/FTC/PPC) had not undergone mutation testing (Table 2). Twenty-two (66.7%) of 33 women with a pathogenic mutation knew their mutation status before diagnosis of OC/FTC/PPC, and 13 women (35.1%) with OC/FTC/PPC had a prior diagnosis of breast cancer (11 women) or ductal carcinoma in situ (two women).
Of prevalent OC/FTCs, six (66.7%; 95% CI, 35.1% to 88.2%) of nine were International Federation of Gynecology and Obstetrics stage I or II. Four (30.8%; 95% CI, 12.4% to 58.0%) of 13 incident screen-detected OC/FTCs were stage I or II. When LS cases were excluded, six (85.7%; 95% CI, 42.1% to 99.6%) of seven OC/FTCs in women not screened in the year before diagnosis were stage IIIc or higher, compared with six (26.1%; 95% CI, 10.2% to 48.4%) of 23 women who were screened in the year before diagnosis (Fisher's test P = .009). Four (57.1%; 95% CI, 18.4% to 90.1%) of seven women not screened in the year before diagnosis underwent optimal debulking surgery (residual < 1 cm), compared with 21 (91.3%; 95% CI, 72.0% to 98.9%) of 23 women who were screened in the year before diagnosis (Fisher's test P = .068). Both mean overall and disease-specific survival (to March 31, 2011) were 48.4 months (95% CI, 39.4 to 57.4) in women not screened in the year before diagnosis, compared with 71.9 months (95% CI, 60.7 to 83.2) in those who were screened in the year before diagnosis (log-rank [Mantel-Cox] P = .233); all deaths resulted from OC/FTC.
Screening Performance
Annual screening performance characteristics are listed in Table 3. Two women were diagnosed with interval OCs within 365 days of normal screens. Four women had occult OC/FTC/PPC found at RRSO (prevalence, 0.8%; 95% CI, 0.2% to 2.0%). Two of these were within 365 days of normal screens and were includable as false negatives or true-positives in the sensitivity analysis. Two of the eight screen-negative OC/FTCs were diagnosed < 365 days of an annual screen and so were included as false negatives. Fifteen (68.2%) of 22 of all screen-detected cancers had a raised CA125 at detection (median, 80.5 IU/mL; range, 4 to 3,874) according to the predetermined cutoffs. All screen-detected cancers had abnormal TVS at detection.
Table 3.
Screening Performance Characteristics
| Screen Type | Total Screened Population (N = 3,563) |
BRCA1 and BRCA2 Mutation Carriers (n = 538) |
Unknown Mutation Status at Enrollment (n = 3,065) |
LS Mutation or Family History (n = 99) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalent |
Incident* |
Incident† |
Prevalent |
Incident‡ |
Prevalent |
Incident* |
Incident† |
Prevalent |
Incident |
|||||||||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Sensitivity | ||||||||||||||||||||
| Occult FN | 90.0 | 55.5 to 99.8 | 81.3 | 54.3 to 96.0 | 76.5 | 50.1 to 93.2 | 85.7 | 42.1 to 99.6 | 76.9 | 46.2 to 95.0 | NA§ | 91.7 | 61.5 to 99.8 | 75.0 | 19.4 to 99.4 | 100 | 29.2 to 100 | 100 | 2.5 to 100 | |
| Occult TP | 100 | 69.2 to 100 | 87.5 | 61.7 to 98.5 | 82.4 | 56.6 to 96.2 | 100 | 59.0 to 100 | 91.7 | 61.5 to 99.8 | NA§ | 100 | 73.5 to 100 | 75.0 | 19.4 to 99.4 | 100 | 29.2 to 100 | 100 | 2.5 to 100 | |
| Specificity∥ | 99.7 | 99.5 to 99.9 | 98.9 | 98.5 to 99.2 | 98.9 | 98.5 to 99.2 | 99.2 | 98.0 to 99.8 | 99.2 | 97.9 to 99.8 | 99.7 | 99.4 to 99.8 | 99.8 | 98.3 to 99.2 | 99.8 | 98.3 to 99.2 | 100 | 96.1 to 100 | 100 | 96.1 to 100 |
| PPV | ||||||||||||||||||||
| Occult FN | 42.9 | 21.8 to 66.0 | 25.5 | 14.3 to 40.0 | 25.5 | 14.3 to 40.0 | 60.0 | 26.2 to 87.8 | 71.4 | 41.9 to 91.6 | NA§ | 23.9 | 12.6 to 38.8 | 23.9 | 12.6 to 38.8 | 100 | 29.2 to 100 | 100 | 2.5 to 100 | |
| Occult TP | 45.4 | 24.4 to 67.8 | 27.0 | 15.6 to 41.0 | 27.0 | 15.6 to 41.0 | 63.6 | 30.1 to 89.1 | 73.3 | 44.9 to 92.2 | NA§ | 25.5 | 14.0 to 40.3 | 25.5 | 14.0 to 40.3 | 100 | 29.2 to 100 | 100 | 2.5 to 100 | |
| NPV | ||||||||||||||||||||
| Occult FN | 100 | 99.8 to 100 | 99.9 | 99.8 to 100 | 99.9 | 99.7 to 100 | 99.8 | 98.9 to 100 | 99.4 | 98.2 to 99.9 | 99.9 | 99.8 to 100 | 99.9 | 99.7 to 100 | 100 | 99.8 to 100 | 100 | 96.1 to 100 | 100 | 96.1 to 100 |
| Occult TP | 100 | 99.9 to 100 | 99.9 | 99.8 to 100 | 99.9 | 99.8 to 100 | 100 | 99.3 to 100 | 99.6 | 98.5 to 100 | 99.9 | 99.8 to 100 | 100.00 | 99.9 to 100 | 100 | 99.8 to 100 | 100 | 96.1 to 100 | 100 | 96.1 to 100 |
| Screen Type | Prior Study for Comparison24¶
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Occult Status | No. of Cancers |
Sensitivity |
Specificity |
PPV |
NPV |
||||||
| Total | Prevalent | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | ||
| Incident plus prevalent | Occult excluded | 7 | 2 | 42 | 14 to 70 | 99 | 98 to 99 | 23 | 5 to 40 | 99 | 99 to 100 |
| Incident plus prevalent | Occult FN | 12 | 2 | 71 | 38 to 11 | 99 | 98 to 99 | 23 | 5 to 40 | 100 | 100 to 100 |
Abbreviations: FN, false negative; LS, Lynch syndrome; NA, not applicable; NPV, negative predictive value; PPC, primary peritoneal cancer; PPV, positive predictive value; TP, true positive.
Excluding PPC.
Including PPC.
No PPCs in BRCA carriers occurred within 1 year of prior screen.
Not applicable because no prevalent cancers occurred in unknown mutation status group.
Occult cancers not applicable (specificity does not depend on false-negative or true-positive rate).
Only large prior study to our knowledge with data reported in a fashion that allows some comparison of performance characteristics with our study.
Fifty-two women (1.5%) underwent false-positive surgery prompted at least in part by abnormal screening test results. Five of these women (9.6%) had raised CA125 alone, 43 (82.7%) had a suspicious scan alone, and four (7.7%) had abnormal results in both tests. Four (7.7%) of these 52 women had gynecologic pathology (one benign teratoma, one mucinous borderline tumor, one serous borderline tumor, one fibroid). All these lesions were detected on TVS, and only the fibroid had raised CA125. An additional four women (0.1%) underwent surgery after equivocal ultrasound results or transient nonconcerning small rises in CA125. Because documentation from the collaborating center indicated that test results had contributed to the woman's decision to undergo RRSO, these cases were classified as screening-related surgery.
Intervals in Screening and Surgical Investigation
Table 4 shows delays in annual screens detecting incident OC/FTCs and intervals between abnormal results and diagnosis in prevalent and incident cases. Reasons for delays included: temporarily leaving the United Kingdom, assuming abnormal results were the result of endometriosis, and women's reluctance to undergo surgery.
Table 4.
Screening and Surgery Intervals in Screen-Detected Cancers
| Screen Type | No. of Cancers | Screen Delay (days) |
Surgery Interval (days) |
Composite Interval (days) |
|||
|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | Median | Range | ||
| Prevalent | 9 | NA* | 79 | 20–141 | NA* | ||
| Incident screen detected | 13 | 88 | 6–737 | 79 | 15–184 | 154 | 27–875 |
| Stage I/II | 4 | 231 | 49–737 | 50 | 19–138 | 160 | 27–875 |
| Stage III | 9 | 78 | 6–236 | 96 | 15–184 | 154 | 68–413 |
Abbreviation: NA, not applicable.
Prevalent screen is the first screen and cannot be delayed. Calculation of composite delay for prevalent cases is not appropriate.
DISCUSSION
The present study is the first large prospective high-risk population screening study reported to our knowledge. The other large ongoing studies are the US Cancer Genetics Network and Gynaecologic Oncology Group 19925 studies. The strengths of our study are its size, reliable follow-up via multiple routes, and analysis of intervals in screening and surgical investigation not previously studied in this context. Its limitations are the lack of an enforced screening/management protocol, incomplete documentation confirming relatives' cancers, and incomplete screening results for those not undergoing surgery. This prevents reliable estimates of repeat testing rates. Finally, there was no mandatory pathology protocol for RRSO specimens, possibly explaining the low occult cancer prevalence (0.8%).
Previously, a meta-analysis22 suggested that annual screening might not provide adequate sensitivity for early-stage disease. However, when duplicate cases were excluded, 14 (45.2%) of 31 detected cases were stage I or II.26 Subsequently, additional cohort studies were reported separately24,27–31 and as a pooled analysis.32 We reanalyzed these data33 and found a borderline significant (P = .046) improvement in the stage distribution of screen-detected BRCA1/2-associated cancers (52.6% of incident v 19.0% of prevalent cancers were stage I or II). Our study suggests that screening in the year before diagnosis reduces the proportion of patients diagnosed at stage ≥ IIIc but does not increase the proportion diagnosed at stage I. This is consistent with the hypothesis that high-grade serous OC undergoes early transcoelomic spread.34 The nonsignificantly higher optimal debulking rate and nonsignificant trend to increased survival in those screened in the year before diagnosis suggest that screening might have an effect on survival, but they do not prove screening will reduce mortality. In particular, there could be a lead-time effect, with longer survival resulting solely from earlier diagnoses rather than screening efficacy. In addition, this analysis compares small nonrandomized groups determined by screening interval, so there could be other important differences between them.
We found 66.7% of the prevalent cases were early stage. This could be chance, or it could reflect the fact that three of five stage I cancers occurred in LS mutation carriers. The prognosis of LS OC is better than that associated with BRCA1/2 mutations,35 possibly because presentation occurs earlier.36 We therefore speculate that LS-associated tumors have a longer sojourn time, explaining the high proportion of early-stage disease in the prevalence screen. Given that 33 of 37 OC/FTC/PPCs occurred in women with a predisposing mutation, clearly mutation carriers are at highest cancer risk. However, first-degree relatives of cancer-affected individuals from untested high-risk families should also be considered high risk and counseled accordingly.37 Where an unaffected relative is the only family member tested, and she is mutation negative, then her risk would be considerably lower than that of a mutation carrier, but not as low as that of a woman testing negative for a relative's known pathogenic mutation. We are not aware of any data on OC incidence in mutation-negative women from otherwise untested high-risk families. However, given our findings, we speculate that they may not be at sufficiently high risk to justify familial OC screening.
The performance characteristics of annual screening were encouraging. The incident sensitivity (> 80%) was higher than that previously reported24 (Table 3). However, the proportion of early-stage disease was disappointing (two of 13 incident screen-detected cases were stage I), supporting the current assertion in the National Comprehensive Cancer Network guideline38 that annual screening is ineffective in high-risk women. The high incident PPV (similar to that previously reported24) means that only four women underwent surgery for each case of cancer detected. As expected, the PPV was greater in mutation carriers than in those of unknown mutation status. Only 0.6% underwent screening-related surgery prompted by nonnormal but clinically nonsuspicious screening results.
The high negative predictive value is relevant to this population of women, who may undergo screening to delay RRSO to complete childbearing or delay surgically induced menopause. Although much of the high negative predictive value derives from the low annual incidence of OC/FTC even in BRCA1/BRCA2 mutation carriers, the knowledge that normal test results provide 99.9% (99.4% in known mutation carriers) probability a woman will not be diagnosed with OC in the next year should help decision making regarding timing of RRSO.
Many cancers had long intervals between annual screens and/or between abnormal results and diagnosis. The United Kingdom mandates a 62-day maximum acceptable interval from suspected cancer referral to treatment.39 However, this limit was not consistently delivered nationally until 2006,40 when UK FOCSS had been running for 4 years. In this high-risk population, it is essential that screening is not delayed, that abnormal results are assumed to represent possible cancer, and that the threshold for rapid follow-up tests or surgery is set much lower than in the general population. Stricter protocols may increase the false-positive rate, but the PPV achieved was sufficiently high to remain acceptable even if some increase in false positives occurs.
Given the delays we observed, we speculate that rigorous adherence to screening schedules and swifter action on abnormal results might result in earlier stage at diagnosis. Phase II of UK FOCSS has implemented the following modifications: one, the screening frequency has been increased to once every 4 months; two, the threshold for and timing of repeat tests is protocol driven; three, CA125 is assayed in a single laboratory to reduce interassay variability; four, serial CA125 values are analyzed by a risk of OC algorithm,41 which has demonstrated superior performance to CA125 when used as a cutoff42; and five, collaborators are prompted to organize scans and referrals via an Internet-based database, modeled on the successful UKCTOCS (United Kingdom Collaborative Trial of Ovarian Cancer Screening) database.43 It is hoped that these changes will optimize early-stage OC detection in the high-risk population. If this is achieved, and if UKCTOCS demonstrates reduced general population OC mortality, then high-risk women wishing to delay RRSO can be offered a risk-minimizing screening strategy before surgery. Until then, RRSO remains the only proven method of preventing mortality from OC/FTC.
Acknowledgment
Presented in part at the 15th Annual Meeting of the European Society of Gynaecological Oncology, Berlin, Germany, October 15-November 1, 2007; the British Gynaecological Cancer Society Annual Meeting, Dublin, Ireland, November 26-27, 2009; the 4th International Conference on Ovarian Cancer Screening, London, United Kingdom, November 29-30, 2011; and the Annual UK Cancer Genetics Group Winter Meeting, London, United Kingdom, December 2, 2011.
We are grateful to the women throughout the United Kingdom who participated in the study and to all the medical, nursing, sonography, and administrative staff who work on United Kingdom Familial Ovarian Cancer Screening Study. We thank Lisa Hinton, Michelle Johnson, and Lisa Perreault for their clerical support and Robert Liston and Andy Ryan for database design and support. We are also grateful to the collaborating center lead clinicians and the Trial Steering and Data Monitoring Committee members (Appendix Table A1, online only).
Appendix
Family History/Mutation Eligibility Criteria
Eligibility was determined as follows: Participants were known carriers of one of the OC predisposing genes (BRCA1, BRCA2, MLH1, MSH2, MSH6, PMS1, PMS2) or first-degree relatives (mother, sister, daughter) of an affected member of a high-risk family. High-risk families were those fulfilling any of the following criteria:
The family contained two or more individuals with ovarian cancer (OC) who were first-degree relatives
The family contained one individual with OC and one individual with breast cancer diagnosed at age < 50 years who were first-degree relatives
The family contained one individual with OC and two individuals with breast cancer diagnosed at age < 60 years who were connected by first-degree relationships
The family contained an affected individual with a mutation of one of the known OC predisposing genes (BRCA1, BRCA2, MLH1, MSH2, MSH6, PMS1, PMS2)
The family contained three individuals with colorectal cancer, at least one of whom was diagnosed at age < 50 years as well as one individual with OC, and all of these individuals were connected by first-degree relationships
The first three criteria could be modified where paternal transmission occurred (ie, families in which affected relatives were related by second degree through an unaffected intervening male relative and in which the proband had an affected sister were eligible)
In addition, when women did not fall within these inclusion criteria, but the recruiting center felt that they had a lifetime risk of OC of ≥ 10%, the study clinical geneticist (J.M.) reviewed the pedigree and documentation of diagnoses to determine eligibility.
UItrasound Classification System
Ovarian size.
Ovarian diameter was measured in three dimensions and used to calculate ovarian volume using the formula for an ellipsoid (d1 × d2 × d3 × 0.523).
Ovarian morphology.
Ovarian echogenicity was assessed for the presence of cysts, cyst septae, solid areas, and papillations. Morphology was classified as follows:
Normal: uniform ovarian echogenicity or one or both ovaries not visualized despite a good view of the pelvic side wall (ie, iliac vessels visualized)
Equivocal: single or multiple simple cysts (ie, cysts with no septae or papillations and thin walls with a regular internal outline) and polycystic ovaries with classical scan features of small peripheral cysts and increased stromal echogenicity
Suspicious: all complex morphology (nonuniform ovarian echogenicity) excluding that described under equivocal
All suspicious scans and those with an ovarian volume > 60 mL were considered abnormal.
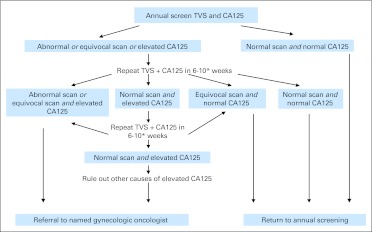
Fig A1.
Annual transvaginal ultrasound scan (TVS) and CA125 screening. Normal, equivocal, and abnormal scan definitions provided in Appendix. Elevated CA125: ≥ 35 and > 30 IU/mL in premenopausal and postmenopausal women, respectively. (*) Earlier intervention if clinical suspicion warrants. These represent guidelines only; clinical management of the women remained at the discretion of collaborating local gynecologists. When an elevated CA125 or non-normal scan was found at an annual screen, it was the local gynecologist's decision whether the test should be repeated before surgical investigation. If the test was repeated, the interval before repeat testing was also at his or her discretion. The study guidelines recommended that intervention be considered earlier if there was clinical suspicion of cancer to discourage unnecessary delays when screening test results were clearly concerning.
Table A1.
UK FOCSS Collaborators, Data Monitoring Committee, and Trial Steering Committee Members
| Center | Collaborators |
|---|---|
| Belfast | Patrick Morrison, Hans Nagar |
| Birmingham | James Nevin |
| Bristol | Robert Anderson, John Murdoch |
| Cambridge | Robin Crawford |
| Cardiff | Jonathon Gray, Mark Rogers |
| Cheltenham and Gloucester | Robert Gornall |
| Chester | Sharon Rowe |
| Cumberland | Sheila Pearson |
| Derby | Ian Scott, Howard Jenkins |
| Durham | Partha Sengupta |
| Dundee | David Goudie |
| East Kent | Andy Nordin |
| Edinburgh | Mary Porteous |
| Gateshead | Richard Edmondson |
| Glasgow | Rosemary Davidson |
| Guys | Gabriella Pichert, Chris Jacobs |
| Hammersmith | Sadaf Ghaem-Maghami |
| Hull | Mike Lind, David Poole |
| Kettering | Robert Haughney |
| Leeds | Carol Chu, Roger Rand, Richard Hutson, Ian Beck, Cheng Choy |
| Leicester | Richard Trembath, Quentin Davies |
| Lincoln | Martin Lamb |
| Liverpool | Carol Bejamin |
| London Northwest | Huw Dorkins |
| London UCLH | Usha Menon, Michelle Johnson |
| Manchester | Gareth Evans |
| Mid Essex | Colin Partington, Christopher Goodfellow |
| Milton Keynes | Christopher B. Lynch |
| Newcastle | Fiona Douglas |
| North England | Paul Brennan, Mary George, John McDonald |
| North Staffordshire | Vijay Menon |
| North Wales | Alex Murray, Philip Banfield, Simon Leeson, Philip Toon |
| Northampton | Sue Price, Alistair Duncan |
| Nottingham | Susan Ritchie, Karin Williamson |
| Oxford | Cyril Chapman, Anneke Lucassen, Lucy Side, Lisa Walker |
| Peninsula | Carole Brewer, Tony Falconer, Tito Lopes |
| Sheffield | Jackie Cook |
| Somerset West | Robert Fox |
| Southampton | Diana Eccles |
| St George's London | Shirley Hodgson |
| Surrey | Gareth Beynon |
| Swansea | Alex Murray, Omar Freites |
| The Royal Marsden, London | Rosalind Eeles |
| Wycombe/Stoke | Damien Eustace |
| West Kent | Andreas Papadopoulos |
| Data Monitoring Committee | Shehla Mohammed, Mahesh Parmar (chair), Karina Reynolds |
| Trial Steering Committee | Louise Bayne (lay member), Kate Brain, Derek Cruikshank, Stephen Duffy, Diana Eccles, Lindsay Fraser, Ian Jacobs, Usha Menon, Julietta Patnick (chair), Adam Rosenthal, Steve Skates |
Abbreviations: UK FOCSS, United Kingdom Familial Ovarian Cancer Screening Study; UCLH, University College London Hospitals.
Footnotes
See accompanying editorial on page 8
Written on behalf of the United Kingdom Familial Ovarian Cancer Screening Study collaborators (listed in Appendix Table A1, online only).
Supported by Cancer Research UK (Grants No. C315/A2621 and C1005/A6383), the UK Department of Health, and the Eve Appeal and in part by the National Cancer Institute Early Detection Research Network (Grants No. CA152990 and CA086381) and the National Institute for Health Research University College London (UCL) Hospitals/UCL Comprehensive Biomedical Research Centre (research team at UCL coordinating center).
Clinical trial information: ISRCTN32794457.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Usha Menon, Abcodia (C) Consultant or Advisory Role: Ian J. Jacobs, Beckton Dickinson (C), Abcodia (C), Women's Health Specialists (C) Stock Ownership: Usha Menon, Abcodia; Ian J. Jacobs, Abcodia Honoraria: Adam N. Rosenthal, Fujirebio Diagnostics; Ranjit Manchanda, Abbott Research Funding: Adam N. Rosenthal, Cancer Research UK, Eve Appeal, UK Department of Health, Bupa Foundation; Lindsay Fraser, Cancer Research UK; Steven J. Skates, Fujirebio Diagnostics; Usha Menon, Medical Research Council, Cancer Research UK, National Institute for Health Research, National Institutes of Health; Ian J. Jacobs, Medical Research Council, Cancer Research UK, Beckton Dickinson Expert Testimony: D. Gareth Evans, Marie DuPlessis (C) Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: James Mackay, Usha Menon, Ian J. Jacobs
Collection and assembly of data: Adam N. Rosenthal, Lindsay Fraser, Ranjit Manchanda, Philip Badman, Susan Philpott, Jessica Mozersky, Richard Hadwin, D. Gareth Evans, Diana M. Eccles, Ian J. Jacobs
Data analysis and interpretation: Adam N. Rosenthal, Ranjit Manchanda, Philip Badman, Fay H. Cafferty, Elizabeth Benjamin, Naveena Singh, D. Gareth Evans, Steven J. Skates, James Mackay, Ian J. Jacobs
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Rubin SC, Blackwood MA, Bandera C, et al. BRCA1, BRCA2 and hereditary nonpolyposis colorectal cancer gene mutations in an unselected ovarian cancer population: Relationship to family history and implications for genetic testing. Am J Obstet Gynecol. 1998;178:670–677. doi: 10.1016/s0002-9378(98)70476-4. [DOI] [PubMed] [Google Scholar]
- 2.Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700–710. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807–2816. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 4.Barrow E, Robinson L, Alduaij W, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: A report of 121 families with proven mutations. Clin Genet. 2009;75:141–149. doi: 10.1111/j.1399-0004.2008.01125.x. [DOI] [PubMed] [Google Scholar]
- 5.Cederquist K, Emanuelsson M, Wiklund F, et al. Two Swedish founder MSH6 mutations, one nonsense and one missense, conferring high cumulative risk of Lynch syndrome. Clin Genet. 2005;68:533–541. doi: 10.1111/j.1399-0004.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- 6.Vasen HF, Stormorken A, Menko FH, et al. MSH2 mutation carriers are at higher risk of cancer than MLH1 mutation carriers: A study of hereditary nonpolyposis colorectal cancer families. J Clin Oncol. 2001;19:4074–4080. doi: 10.1200/JCO.2001.19.20.4074. [DOI] [PubMed] [Google Scholar]
- 7.Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families: The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in series unselected for family history: A combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans DG, Shenton A, Woodward E, et al. Penetrance estimates for BRCA1 and BRCA2 based on genetic testing in a clinical cancer genetics service setting: Risks of breast/ovarian cancer quoted should reflect the cancer burden in the family. BMC Cancer. 2008;8:155. doi: 10.1186/1471-2407-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S, Iversen ES, Friebel T, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol. 2006;24:863–871. doi: 10.1200/JCO.2005.03.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman MP, Forman D, Bryant H, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the International Cancer Benchmarking Partnership): An analysis of population-based cancer registry data. Lancet. 2011;377:127–138. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finch A, Beiner M, Lubinski J, et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 mutation. JAMA. 2006;296:185–192. doi: 10.1001/jama.296.2.185. [DOI] [PubMed] [Google Scholar]
- 13.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michelsen TM, Pripp AH, Tonstadd S, et al. Metabolic syndrome after risk-reducing salpingooophorectomy in women at high risk for hereditary breast ovarian cancer: A controlled observational study. Eur J Cancer. 2009;45:82–89. doi: 10.1016/j.ejca.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Tuppurainen M, Kröger H, Honkanen R, et al. Risks of perimenopausal fractures: A prospective population-based study. Acta Obstet Gynecol Scand. 1995;74:624–628. doi: 10.3109/00016349509013475. [DOI] [PubMed] [Google Scholar]
- 16.Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers: Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56:265–271. [PMC free article] [PubMed] [Google Scholar]
- 17.Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary: FIGO 26th Annual Report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(suppl 1):S161–S192. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs IJ, Skates SJ, Macdonald N, et al. Screening for ovarian cancer: A pilot randomised controlled trial. Lancet. 1999;353:1207–1210. doi: 10.1016/S0140-6736(98)10261-1. [DOI] [PubMed] [Google Scholar]
- 19.Menon U, Gentry-Maharaj A, Hallett R, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: Results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Lancet Oncol. 2009;10:327–340. doi: 10.1016/S1470-2045(09)70026-9. [DOI] [PubMed] [Google Scholar]
- 20.Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening randomized controlled trial. JAMA. 2011;305:2295–2303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 21.Pittaway DE, Fayez JA. Serum CA-125 antigen levels increase during menses. Am J Obstet Gynecol. 1987;156:75–76. doi: 10.1016/0002-9378(87)90207-9. [DOI] [PubMed] [Google Scholar]
- 22.Hogg R, Friedlander M. Biology of epithelial ovarian cancer: Implications for screening women at high genetic risk. J Clin Oncol. 2004;22:1315–1327. doi: 10.1200/JCO.2004.07.179. [DOI] [PubMed] [Google Scholar]
- 23.Bloss JD, Liao SY, Buller RE, et al. Extraovarian peritoneal serous papillary carcinoma: A case control retrospective comparison to papillary adenocarcinoma of the ovary. Gynecol Oncol. 1993;50:347–351. doi: 10.1006/gyno.1993.1223. [DOI] [PubMed] [Google Scholar]
- 24.Hermsen BB, Olivier RI, Verheijen RH, et al. No efficacy of annual gynaecological screening in BRCA1/2 mutation carriers: An observational follow-up study. Br J Cancer. 2007;96:1335–1342. doi: 10.1038/sj.bjc.6603725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Cancer Institute: Ovarian cancer prevention and early detection study. http://ovariancancer.gog199.cancer.gov/index.html.
- 26.Jacobs I. Screening for familial ovarian cancer: The need for well-designed prospective studies. J Clin Oncol. 2005;23:5443–5445. doi: 10.1200/JCO.2005.03.909. [DOI] [PubMed] [Google Scholar]
- 27.Gaarenstroom KN, van der Hiel B, Tollenaar RA, et al. Efficacy of screening women at high risk of hereditary ovarian cancer: Results of an 11-year cohort study. Int J Gynecol Cancer. 2006;16(suppl 1):S54–S59. doi: 10.1111/j.1525-1438.2006.00480.x. [DOI] [PubMed] [Google Scholar]
- 28.Dørum A, Heimdal K, Løvslett K, et al. Prospectively detected cancer in familial breast/ovarian cancer screening. Acta Obstet Gynecol Scand. 1999;78:906–911. [PubMed] [Google Scholar]
- 29.Stirling D, Evans DG, Pichert G, et al. Familial ovarian cancer screening: Current protocols are ineffective in detecting early stage ovarian malignancy. J Clin Oncol. 2005;23:5588–5596. doi: 10.1200/JCO.2005.05.097. [DOI] [PubMed] [Google Scholar]
- 30.Vasen HF, Tesfay E, Boonstra H, et al. Early detection of breast and ovarian cancer in families with BRCA mutations. Eur J Cancer. 2005;41:549–554. doi: 10.1016/j.ejca.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 31.Munkarah A, Chatterjee M, Tainsky MA. Update on ovarian cancer screening. Curr Opin Obstet Gynecol. 2007;19:22–26. doi: 10.1097/GCO.0b013e328011ec99. [DOI] [PubMed] [Google Scholar]
- 32.Evans G, Gaarenstroom K, Stirling D, et al. Screening for familial ovarian cancer: Poor survival in BRCA 1/2 related cancers. J Med Genet. 2009;46:593–597. doi: 10.1136/jmg.2008.058248. [DOI] [PubMed] [Google Scholar]
- 33.Manchanda R, Rosenthal A, Burnell M, et al. Change in stage distribution observed with annual screening for ovarian cancer in BRCA carriers. J Med Genet. 2009;46:423–424. doi: 10.1136/jmg.2009.067462. [DOI] [PubMed] [Google Scholar]
- 34.Brown PO, Palmer C. The preclinical natural history of serous ovarian cancer: Defining the target for early detection. PLoS Med. 2009;6:e1000114. doi: 10.1371/journal.pmed.1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grindedal EM, Renkonen-Sinisalo L, Vasen H, et al. Survival in women with MMR mutations and ovarian cancer: A multicentre study in Lynch syndrome kindreds. J Med Genet. 2010;47:99–102. doi: 10.1136/jmg.2009.068130. [DOI] [PubMed] [Google Scholar]
- 36.Ketabi Z, Bartuma K, Bernstein I, et al. Ovarian cancer linked to lynch syndrome typically presents as early-onset, non-serous epithelial tumors. Gynecol Oncol. 2011;121:462–465. doi: 10.1016/j.ygyno.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Manchanda R, Abdelraheim A, Johnson M, et al. Outcome of risk-reducing salpingo-oophorectomy in BRCA carriers and women of unknown mutation status. BJOG. 2011;118:814–824. doi: 10.1111/j.1471-0528.2011.02920.x. [DOI] [PubMed] [Google Scholar]
- 38.National Comprehensive Cancer Network clinical practice guidelines in oncology: Genetic/familial high-risk assessment—Breast and ovarian, version 1.2012 [Google Scholar]
- 39.National Health Service: Cancer reform strategy 2007. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_081006.
- 40.National Health Service: Cancer improvement. http://www.improvement.nhs.uk/cancer/GoingFurtheronCancerWaits/tabid/62/Default.aspx.
- 41.Skates SJ, Menon U, MacDonald N, et al. Calculation of the risk of ovarian cancer from serial CA-125 values for preclinical detection in postmenopausal women. J Clin Oncol. 2003;21(suppl 10):s206–s210. doi: 10.1200/JCO.2003.02.955. [DOI] [PubMed] [Google Scholar]
- 42.Menon U, Skates SJ, Lewis S, et al. Prospective study using the risk of ovarian cancer algorithm to screen for ovarian cancer. J Clin Oncol. 2005;23:7919–7926. doi: 10.1200/JCO.2005.01.6642. [DOI] [PubMed] [Google Scholar]
- 43.Menon U, Gentry-Maharaj A, Ryan A, et al. Recruitment to multicentre trials: Lessons from UKCTOCS—Descriptive study. BMJ. 2008;337:a2079. doi: 10.1136/bmj.a2079. [DOI] [PMC free article] [PubMed] [Google Scholar]