Abstract
Purpose
Infusional chemotherapy is efficacious in patients with AIDS-related lymphoma, but it may be difficult to administer. We studied standard agents with rituximab plus pegylated liposomal doxorubicin (DR-COP) in an attempt to provide a more practical approach to therapy while ascertaining rates of response, potential infectious complications, and prognostic role of biologic markers.
Patients and Methods
We conducted a prospective, multi-institutional phase II trial, employing (day 1) pegylated liposomal doxorubicin 40 mg/m2, rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, vincristine 1.4 mg/m2 (not > 2 mg), and prednisone 100 mg orally on days 1 through 5, with concomitant antiretroviral therapy.
Results
In 40 evaluable patients, median CD4 cells was 114/μL (range, 5 to 1,026/μL), and median HIV-1 viral load (VL) was 25,000 copies/mL. High or intermediate/high age-adjusted International Prognostic Index was present in 28%. Overall response was 67.5%, with complete remission in 47.5% (95% CI, 31.5 to 63.9). Of 19 complete responders, 84% had extranodal disease, 47% had CD4 < 100/μL, and 47% had VL > 50,000 copies/mL; one relapsed. With 25.5-month median follow-up, 62% (95% CI, 44 to 75) of patients remain alive. Sixteen patients (40%) experienced 22 infections, with grade 4 in only two (5%). No patient died as a result of infection during treatment; one had opportunistic infection.
Conclusion
Profound immunodeficiency and high HIV-1 viral load do not preclude attainment of complete response after DR-COP with highly active antiretroviral therapy. The regimen is tolerable, and use of rituximab was not associated with death as a result of infection during treatment. This approach may be useful in patients in whom the more intensive infusional regimens are impractical.
INTRODUCTION
HIV infection has been altered by highly active antiretroviral therapy (HAART), leading to a substantial decrease in AIDS-defining conditions,1,2 including AIDS-related lymphoma (ARL).3,4 HAART has also been associated with a remarkable prolongation of survival in patients with ARL.5,6
Despite these advances, optimal therapy for ARL has not yet been defined. Although R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) is highly effective in HIV-negative patients with diffuse large B-cell lymphoma (DLBCL),7,8 outcome is inferior with HIV.9 This suboptimal response may be related to treatment delays resulting from intercurrent illnesses or to chemotherapy resistance, mediated by various mechanisms, including p-glycoprotein, the protein product of the multidrug resistance 1 gene (MDR-1).10–12 In HIV-negative lymphoma, MDR-1 expression is seen at diagnosis in < 20%, increasing to > 50% at time of relapse.13,14 By contrast, in 50 patients with ARL, 66% expressed MDR-1 at diagnosis, correlating with a lower rate of complete remission (CR) when compared with MDR-1–negative patients.15 Protease inhibitors, an important component of multiple HAART regimens, may serve as both substrates and inducers of MDR-1, providing a potential explanation for these differences.16,17
Infusional chemotherapy may overcome MDR-1 by providing continuous, intracellular entry of chemotherapeutic agents despite subsequent efflux. In this regard, the infusional EPOCH (etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin hydrochloride) regimen is quite effective in ARL.5,6,18,19 Nonetheless, EPOCH requires indwelling intravenous lines, infusion pumps, and either hospitalization or multiple outpatient visits each cycle for delivery of 4-day infusions.
Doxorubicin is one of the most active agents in DLBCL,20 but it is a substrate for p-glycoprotein. In vitro, liposomal encapsulation of doxorubicin can overcome excessive drug efflux resulting from MDR-1.21,22 In a phase I/II trial, liposomal doxorubicin together with standard agents was tested in 24 patients with newly diagnosed ARL, resulting in CR of 75%.23 The regimen was equally effective in MDR-1–positive and –negative patients, suggesting that efficacy could be related to the ability of liposome-encapsulated doxorubicin to overcome excessive MDR-1–induced drug efflux.
The current study was undertaken to determine if pegylated liposomal doxorubicin, added to standard agents, would result in efficacy similar to that reported with infusional regimens. If a CR rate of ≥ 60% was achieved, formal phase III testing against R-EPOCH would occur, and if found noninferior, the less complex regimen would likely be favored. We also assessed the potential toxicity of rituximab in terms of infections, as well as the prognostic significance of various biologic markers.
PATIENTS AND METHODS
Treatment Regimen
Pegylated liposomal doxorubicin 40 mg/m2 (Doxil; Manufactured for Centocor Biotech Products [Raritan, NJ] by Ben Venue Labs [Bedford, OH]) was administered intravenously (IV) on day 1, with IV rituximab 375 mg/m2, IV cyclophosphamide 750 mg/m2, IV vincristine 1.4 mg/m2 (not > 2 mg), and 100 mg of oral prednisone, on days 1 through 5 of each cycle, repeated every 21 to 28 days. CNS prophylaxis was mandated in patients with involvement of bone marrow, testis, sinuses, or epidural regions and with stage IV and/or ≥ two extranodal sites, with specific regimen left to physician discretion.
Supportive Therapy
Granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, or pegfilgrastim was started on day 3 of each cycle and continued until beyond nadir of blood counts. Erythropoietic growth factor support occurred at physician discretion. Prophylaxis against Pneumocystis carinii was required. Oral quinalones were required with CD4 cell counts ≤ 100/μL at entry or during treatment and with absolute neutrophil count < 500/μL. HAART was required, with specific regimen left to physician discretion. Zidovudine was prohibited.24
Inclusion Criteria
Patients were HIV infected, age ≥ 18 years, had Karnofsky performance status of ≥ 50% or Eastern Cooperative Oncology Group score of 0, 1, or 2, and had previously untreated, histologically documented, CD20+ B-cell lymphoma as diagnosed at the treating site, including: follicular large-cell (grade 3), DLBCL, immunoblastic, plasmablastic, or primary effusion lymphoma. Burkitt's lymphoma, primary CNS, and leptomeningeal lymphoma were excluded.
All stages were allowed, with adequate organ function and no history of myocardial infarction. Patients with history of cutaneous or mucocutaneous disorders, causing hospitalization or inability to eat or drink for ≥ 2 days, were excluded because of risk of cutaneous reactions to rituximab.25 Women had negative pregnancy tests. Institutional review board approval was required, as was signed consent.
Baseline and Follow-Up Evaluations
Medical history, physical examination, ECG, HIV-1 RNA level, CD4 and CD8 counts, routine chemistries, and complete blood count were required at baseline and before every cycle, and quantitative immunoglobulins and assessment for hepatitis C and B viruses were required every other cycle. Computed tomography (CT) scan or magnetic resonance imaging (MRI) of chest, abdomen, and pelvis was required at baseline and every two cycles. Bone marrow biopsy or aspirate was required. Positron emission tomography (PET) or PET/CT was not required. One month after completion of chemotherapy, these studies were repeated to confirm response.
Chemotherapy was administered two cycles beyond documentation of CR. Patients attaining partial remission (PR) after six cycles or stable disease (SD) after four cycles were withdrawn. Patients with progressive disease (PD) were withdrawn at PD and then observed for 12 weeks for safety. After treatment, interim history, physical examination, and blood work were performed every 2 months (year 1) and every 6 months (for 2 more years), with CT or MRI every 6 months.
Definition of Response
Radiographic responses were based on CT or MRI. CR required disappearance of all evidence of disease. PR required ≥ 50% decrease in the sum of the greatest diameters of the six largest masses, no increase in other nodes, liver, or spleen, and regression of splenic or hepatic nodules by ≥ 50%, without new disease. SD was less than PR, without progression. PD required 50% increase from baseline or nadir in total tumor size of previous masses and/or appearance of new lesions. Recurrence or relapse was appearance of lymphoma after CR. Time to progression was time from chemotherapy initiation to first progression. Response duration was time from first response to first progression.
Pathology and Immunohistochemistry
Central pathology review was performed, along with review of all site reports. Patients were eligible with a diagnosis of DLBCL from the treating institution. As per protocol guidelines, data from all 40 eligible patients were included in response and toxicity assessments, even if central pathologic review did not confirm the original diagnosis. Immunohistochemical studies were performed as previously described, using monoclonal antibodies to CD-10, BCL-2, BCL-6, MUM-1, and FOXP1.26 PRDM1/BLIMP-1 (3H2E8; Santa Cruz Biotech, Santa Cruz, CA) and Ki-67 (MIB-1; DakoCytomation, Carpenteria, CA) were also evaluated. Epstein-Barr Virus Probe ISH Kit (Leica Microsystems, Wetzlar, Germany) was used for in situ hybridization for Epstein-Barr virus (EBV) RNA (EBER). Patients were positive when > 20% neoplastic cells were immune reactive, except: BCL-2 positivity was > 50% of cells with moderate to strong positivity, and FOXP1 positivity required moderate to bright staining in > 80% tumor cell nuclei. Nuclear Ki-67 expression was semiquantitative, as percentage of positive tumor cells. Hybridization signal in ≥ 50% neoplastic cells defined EBV EBER positivity. Patients with adequate tissue were categorized as germinal center B cell–like (GCB) versus non-GCB according to Hans algorithm.27 Full immunohistochemical characterization was compromised because of exhaustion of diagnostic tissue.
Study Design and Statistical Considerations
This was a nonrandomized, single-arm, phase II study. Forty patients were felt sufficient to test the null hypothesis that the CR rate was 0.40 against the alternative of 0.60, at the one-sided .10 significance level, with power of 0.87. Forty-four enrolled patients would allow for 10% dropout.
Binomial proportions and 95% CIs were used to describe CR and overall response rates.28 Kaplan-Meier methods were used for duration of response, EFS, and overall survival times. Binomial proportions were used to estimate proportion of patients with infection.28,29
Cox proportional hazards model evaluated the relationship between response and survival. Wilcoxon rank sum test compared patients who developed infection with those who did not, with respect to baseline CD4 lymphocyte count and HIV-1 viral load.29
RESULTS
Demographic Characteristics
Forty-three patients were accrued. One was never treated, one was erroneously removed after one cycle, and one was ineligible. Forty patients are reported. Median age was 44 years (range, 21 to 68 years). Thirty were men; 15 (38%) were African American, and 10 (25%) were Hispanic.
HIV-Related Characteristics
Entry median CD4 cell count (n = 38) was 114/μL (range, 5 to 1,026/μL). Median HIV-1 viral load (n = 39) was 25,000 copies/mL (range nondetectable to 9,276,210 copies/mL).
Lymphoma Characteristics
Lymphoma characteristics are listed in Table 1. DLBCL was diagnosed by the site pathologist in 39 patients, and lymphoma, not otherwise specified, was diagnosed in one. Central pathologic review was retrospectively accomplished in 33 patients; attempts to collect the remaining seven patient cases were unsuccessful. DLBCL was present in 23 patients; aggressive/high-grade lymphoma, not otherwise specified, in 1; B-cell lymphoma intermediate between DLBCL and Burkitt's in one; and Burkitt's lymphoma in one. Follicular hyperplasia with a clonal B-cell population was diagnosed in one patient, from a nondiagnostic specimen submitted. Diagnostic material available for the remaining six was insufficient. Evaluation of GC cell origin was feasible in 16 patients; GC was found in 11. EBV status was positive in six of 28 patients. BCL-2 was positive in 13 of 28 patients. Stage III or IV disease was confirmed in 82% of patients, with ≥ two extranodal sites in 15 (37.5%). Eleven patients (28%) had high or intermediate/high age-adjusted International Prognostic Index scores.30
Table 1.
Baseline Disease Characteristics
| Characteristic | No. | % |
|---|---|---|
| Ann Arbor stage | ||
| IE | 2 | 5 |
| II | 4 | 10 |
| IIE | 2 | 5 |
| III | 6 | 15 |
| IIIE | 4 | 10 |
| IV | 22 | 55 |
| Pathologic diagnosis | ||
| DLBCL | 39 | 98 |
| Other | 1 | 2 |
| Age-adjusted IPI risk category | ||
| 0 | 15 | 37.5 |
| 1 | 14 | 35 |
| 2 | 10 | 25 |
| 3 | 1 | 2 |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; IPI, International Prognostic Index.
Therapy Administered and Reasons for Early Protocol Withdrawal
A median of six cycles was administered (range, one to eight). Seventeen patients completed all therapy, whereas 11 (27.5%) withdrew early because of PD, and nine (22.5%) because of adverse events (Table 2).
Table 2.
Treatment Summary and Reasons for Protocol Termination
| No. of Cycles Completed | Reason for Termination |
Total |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Completed Per Protocol |
Disease Progression |
Adverse Event |
Patient Withdrawal |
Other |
||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| 1 | 0 | 1 | 1 | 0 | 1* | 3 | 7.5 | |||||
| 2 | 0 | 3 | 0 | 1 | 0 | 4 | 10 | |||||
| 3 | 0 | 3 | 2 | 0 | 0 | 5 | 12.5 | |||||
| 4 | 0 | 3 | 0 | 0 | 0 | 3 | 7.5 | |||||
| 5 | 0 | 0 | 4 | 0 | 0 | 4 | 10 | |||||
| 6 | 15 | 1 | 2 | 0 | 0 | 18 | 45 | |||||
| 7 | 0 | 0 | 0 | 0 | 0 | 1 | 2.5 | |||||
| 8 | 2 | 0 | 0 | 0 | 1† | 2 | 5 | |||||
| Total | 17 | 42.5 | 11 | 27.5 | 9 | 22.5 | 1 | 2.5 | 2 | 5.0 | 40 | 100 |
Noncompliance.
Delayed > 6 weeks.
Infections and Adverse Events
Twenty-two infections were reported in 16 patients during therapy (Table 3), including opportunistic infection (Mycobacterium avium intracellulare [MAI]) in one and grade 4 infection in two (abdominal and catheter-related infections). No patient died as a result of infection while receiving therapy. Mucositis was diagnosed in eight patients (all grade 1 or 2). Eight developed hand-foot syndrome (grade 1 or 2 in six; grade 3 in two, causing protocol withdrawal in these two; Table 4).
Table 3.
Summary of Infections During Protocol Therapy
| Infection | CTCAE Grade (No. of patients) |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | All | |
| Abdominal | — | — | — | 1 | 1 |
| Catheter related | — | — | 2 | 1 | 3 |
| Infections and infestations—other, specify | 1 | 3 | — | — | 4* |
| Lung | — | — | 1 | — | 1 |
| Mucosal | 3 | 2 | — | — | 5 |
| Nail | 1 | — | — | — | 1 |
| Rhinitis | — | 1 | — | — | 1 |
| Skin | — | — | 1 | — | 1 |
| Small intestine | — | — | 1 | — | 1 |
| Upper respiratory | — | 2 | — | — | 2 |
| Urinary tract | — | 1 | 1 | — | 2 |
| All | 5 | 9 | 6 | 2 | 22 |
Abbreviation: CTCAE, Common Terminology Criteria for Adverse Events.
Toe inflammation (grade 2), clostridium difficile infection (grade 1), Mycobacterium avium intracellulare = (grade 2), infection with grade 3 or 4 neutropenia (grade 2).
Table 4.
Specific Adverse Events Leading to Termination of Protocol Therapy
| Cycle | Patient | Adverse Event | Grade | Relationship to Study Drugs |
|---|---|---|---|---|
| 1 | 15 | MAI infection | 2 | Unlikely |
| Dyspnea | 4 | Possible | ||
| 3 | 26 | Confusion | 4 | Unrelated |
| 9 | Pneumonitis | 3 | Unlikely | |
| 5 | 16 | Anemia | 2 | Probable |
| Neutropenia | 3 | Probable | ||
| Thrombocytopenia | 3 | Probable | ||
| 20 | Catheter-related infection | 4 | Possible | |
| 10 | Palmar-plantar erythrodysesthesia syndrome | 3 | Definite | |
| 18 | Neutropenia | 3 | Probable | |
| 6 | 12 | Fatigue | 3 | Probable |
| 24 | Anorexia | 3 | Probable | |
| Optic Nerve Disorder | 4 | Unrelated | ||
| Gait disturbance | 2 | Unlikely | ||
| Cognitive disturbance | 2 | Unrelated | ||
| Dizziness | 2 | Unrelated | ||
| Leukoencephalopathy | 1 | Possible | ||
| Peripheral motor neuropathy | 3 | Unlikely | ||
| Palmar-plantar erythrodysesthesia syndrome | 3 | Probable |
Abbreviation: MAI, Mycobacterium avium intracellulare.
Response and Relapses After Protocol Therapy
The overall response rate was 67.5%, with 19 CRs (47.5%; 95% CI, 31.5% to 63.9%) and eight PRs (20%). Thirteen patients experienced no response (32.5%). With median follow-up of 25.5 months, one CR patient relapsed.
Twenty-two patients met criteria for CNS prophylaxis, and 12 (54.4%) received it, consisting of intrathecal (IT) cytarabine in four, methotrexate in three, cytarabine and methotrexate in three, liposomal cytarabine in one, and IT hydrocortisone in one. Four patients, none of whom attained CR, experienced CNS progression. One had stage II disease, attained PR, and, as per protocol, received no prophylaxis, whereas the other three had multiple sites of extranodal lymphoma and had received IT methotrexate and/or cytarabine, three times in two and once in the third.
Biologic Correlates of Disease
Of 11 patients with GC subtype of DLBCL, all were alive at 1 year, versus 50% of those with non-GC sub-=type (P = .074; Table 5).
Table 5.
Prognostic Significance of Biologic Markers
| Marker | No. of Patients | CR* |
CR + PR* |
1-Year OS† |
|||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | P | No. | % | P | % | P | ||
| BCL-2 | 1.000 | .153 | .384 | ||||||
| Negative | 15 | 9 | 60 | 14 | 93.3 | 73.3 | |||
| Positive | 13 | 7 | 53.9 | 9 | 69.2 | 72.7 | |||
| EBER | .354 | .286 | .282 | ||||||
| Negative | 22 | 14 | 63.6 | 19 | 86.4 | 76.2 | |||
| Positive | 6 | 2 | 33.3 | 4 | 66.7 | 60.0 | |||
| GC/non-GC | .300 | 1.000 | .074 | ||||||
| GC | 11 | 8 | 72.7 | 9 | 81.8 | 100 | |||
| Non-GC | 5 | 2 | 40.0 | 4 | 80.0 | 50 | |||
Abbreviations: CR, complete remission; EBER, Epstein-Barr virus RNA; GC, germinal center B cell–like; OS, overall survival; PR, partial response.
Fisher's exact test.
Log-rank test.
Characteristics of Complete Responders
Sixteen (84%) of 19 complete responders had extranodal disease, and seven (37%) had multiple extranodal sites. The median CD4 cell count was 180/μL (range, 39 to 824/μL), and nine (47%) had CD4 counts < 100/μL. Median HIV-1 viral load was 38,693 copies/mL (range < 75 to 9,276,210 copies/mL). Nine CR patients (47%) had viral loads ≥ 50,000 copies/mL; three, > 100,000 copies/mL; and two, > 1,000,000/mL at entry. The relationship between viral load and response to therapy was not significant (P = .207). Of the 11 patients with GC subtype, eight (73%) achieved CR, versus two (40%) of five with non-GC subtype. GC subtype, EBER, and BCL-2 expression were not statistically associated with CR. Central pathologic review was accomplished in 18 of 19 complete responders. DLBCL was confirmed in 12, whereas one each had Burkitt's lymphoma, aggressive B-cell lymphoma, and B-cell lymphoma between DLBCL and Burkitt's. Three complete responders (including one reviewed as follicular hyperplasia with clonal B-cell proliferation) had submission of nondiagnostic material.
Survival and Causes of Death
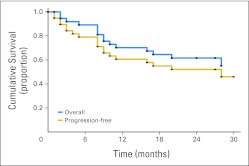
With 25.5-month median follow-up, overall survival (OS) at 1 year was 70.3% (95% CI, 52.8 to 82.3), and at 2 years, it was 61.6% (95% CI, 43.9 to 75.2; Fig 1). Progression-free survival at 1 year was 60.6% (95% CI, 43.4 to 74.1), and at 2 years, it was 52.1% (95% CI, 35.1 to 66.6). Median survival for CR has not been reached, whereas that for PR was 9.5 months (range, 3 to ≥ 27 months). OS of patients with pathologically confirmed DLBCL was 71.4% (95% CI, 47.2 to 86) at 1 year and 56.4% (95% CI, 32.8 to 74.5) at 2 years, with 2-year progression-free survival of 49.3% (95% CI, 27.3 to 68.1).
Fig 1.
Overall and progression-free survival in 40 evaluable patients.
Causes of death included progressive lymphoma in 14 patients (35%), progressive multifocal leukoencephalopathy (PML) in one CR patient, nonmalignancy complications of HIV in one CR patient, and cardiac arrest in one. No patient experienced death resulting from infection while receiving treatment, although three were removed early because of MAI after cycle one, pneumonitis after cycle three, and catheter-related infection after cycle five (Tables 2 and 3).
DISCUSSION
By substituting long-acting pegylated liposomal doxorubicin in a regimen similar to R-CHOP,7,8 we hoped to improve outcome in newly diagnosed ARL, while obviating the need for multiple-day continuous infusions.5,6 Although well tolerated and associated with 62% 2-year survival, DR-COP was associated with a CR rate of only 48%, inferior to the 74% CR and 5-year survival of 73% first reported with infusional EPOCH5 and the 80% 5-year survival with R-EPOCH.6 In the AMC (AIDS Malignancy Consortium) 034 trial, in a multi-institutional setting, EPOCH was associated with 70% 2-year survival when rituximab was administered concurrently, and 67% when rituximab was administered at the completion of all EPOCH infusions.18 Thus, the 2-year survival rate of 62% with DR-COP seems similar to that of either concomitant or sequential R-EPOCH, as reported by the AMC,18 although inferior to EPOCH or R-EPOCH, as reported by the National Cancer Institute.5,6
Characteristics of complete responders were informative, in that neither low CD4 cells nor high viral load precluded attainment of CR. The median CD4 cell count at entry among complete responders was 180/μL, and 47% had CD4 cells < 100/μL, with 47% demonstrating viral loads > 50,000 copies/mL; there was no statistically significant relationship between viral load and response. The findings of EPOCH and R-EPOCH from the National Cancer Institute were consistent, because HAART was started only after completion of all cycles of therapy; despite falling CD4 cells and rising HIV viral load during chemotherapy, outcome was not compromised.5,6
Although most biomarkers were not associated with response, we found a higher proportion of complete responses in GC versus non-GC subtypes. The prognostic significance of DLBCL subclassification in HIV-infected patients is controversial.26,31 Existing discrepancies could be attributed to the various therapeutic modalities employed or to the accuracy of GC subclassification by immunohistochemistry versus gene expression profiling. It remains to be seen whether use of new algorithms or molecular technologies will reliably predict outcome in ARL.32,33
Median survival in the current study has not been reached, and 62% of patients remain alive at 2 years. The rather high rate of OS may reflect the relatively short follow-up, or may indicate some improvement when compared with CHOP/R-CHOP.9 Only three CR patients have died (as a result of relapse, complications of PML, and complications of nonmalignancy HIV), and the median survival of PR patients is 9.5 months (range, 3 to > 27 months), indicating that some of these PR patients may have had a better biologic response than indicated by CT evaluations. PET scan results were not required for determination of response.
The phase III R-CHOP versus CHOP trial from the AMC reported that rituximab-treated patients had a statistically increased rate of death resulting from infection, even though R-CHOP was not associated with higher rates of neutropenia, neutropenic infections, or hypogammaglobulinemia.9 Rather, these deaths occurred among patients with severe immunocompromise. In the subsequent AMC study of R-EPOCH,18 no increase in death resulting from infection was apparent among rituximab-treated patients, similar to results from others.33 Our study confirms the relative safety of rituximab when administered with chemotherapy in ARL, because no patient died as a result of infection during treatment, grade 4 infection occurred in only two patients, and opportunistic infection (MAI) occurred in only one patient (during cycle one), whereas another developed PML 2 months after protocol completion. Prophylactic antibiotics were mandated for patients with < 100/m3 CD4 cells, and HAART was required, perhaps explaining the relative paucity of infections, despite low entry median CD4s (114/μL). Death resulting from bacterial infection has been reported in HIV-infected patients with < 50/μL CD4 cells, without malignancy.35 Furthermore, PML and mycobacterium tuberculosis reactivation have been reported with rituximab in the absence of HIV.36
Twenty-two patients were eligible for CNS prophylaxis per protocol, yet only 12 received it, and three of these 12 experienced CNS along with extracranial progression. The only other CNS progressor had localized disease, achieved PR, did not receive IT prophylaxis (per protocol), and had no extracranial site of progression. Although ARL remains a risk factor for CNS relapse, the efficacy of IT prophylaxis remains unproven in this setting.37,38
A weakness of this study is that centralized pathologic review confirmed the original impression of DLBLC in only 70% (23 of 33 reviewed). Nonetheless, inadequate tissue was received in six of these 33 patients, and CR occurred in three of the discrepant patient cases, centrally diagnosed as Burkitt's, aggressive B cell, and lymphoma intermediate between DLBC and Burkitt's, whereas in a fourth, reviewed as high-grade lymphoma, PR was attained. Furthermore, when analyzing only those patients with DLBCL by central review, data were similar, with 25-month survival of 56% versus 62% in the 40-patient protocol-eligible group. The protocol required eligibility based on diagnosis made at the treating hospital, because rapid institution of therapy is often required. We found it difficult to obtain tissue for central review because of small biopsies performed in an attempt to avoid surgical procedures and exhaustion of diagnostic tissue. Inconsistency of lymphoma diagnosis among pathologists is well described,39,40 and use of more objective measures of diagnosis will be important for future studies.
In summary, DR-COP is associated with 62% overall survival at 25 months, with almost half of complete responders initiating chemotherapy at CD4 counts < 100/μL and HIV viral loads > 50,000 copies/mL. Despite concurrent use of rituximab, no patient died as a result of infection. This approach may be useful in patients for whom more intensive infusional regimens are impractical.
Footnotes
Listen to the podcast by Dr Smith at www.jco.org/podcasts
Written on behalf of the AIDS Malignancy Consortium.
Supported by Grant No. U01 CA 121947 from the Department of Health and Human Services (R.M.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00389818.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Lee Ratner, Galen (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Alexandra M. Levine, Jeannette Y. Lee, Bruce J. Dezube, Anil Tulpule
Financial support: Alexandra M. Levine, Ronald Mitsuyasu
Administrative support: Alexandra M. Levine
Provision of study materials or patients: Alexandra M. Levine, Ronald Mitsuyasu, Bruce J. Dezube, Lee Ratner
Collection and assembly of data: Alexandra M. Levine, Juan Carlos Ramos, Samir Parekh, Ronald Mitsuyasu, Timothy Cooley, Bruce J. Dezube, Lee Ratner, Ethel Cesarman, Anil Tulpule
Data analysis and interpretation: Alexandra M. Levine, Ariela Noy, Jeannette Y. Lee, Wayne Tam, Juan Carlos Ramos, David H. Henry, Erin G. Reid, Ronald Mitsuyasu, Ethel Cesarman, Anil Tulpule
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:653–660. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Mocroft A, Ledergerber B, Katlama B, et al. Decline in the AIDS and death rates in the EuroSIDA study: An observational study. Lancet. 2003;362:22–29. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 3.International Collaboration on HIV and Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus infected adults. J Natl Cancer Inst. 2000;92:1823–1830. doi: 10.1093/jnci/92.22.1823. [DOI] [PubMed] [Google Scholar]
- 4.Engels EA, Pfeiffer RM, Landgen O, et al. Immunologic and virologic predictors of AIDS-related non-Hodgkin lymphoma in the highly active antiretroviral therapy era. J Acquir Immune Defic Syndr. 2010;54:78–84. doi: 10.1097/01.qai.0000371677.48743.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Little RF, Pittaluga S, Grant N, et al. Highly effective treatment of acquired immunodeficiency syndrome-related lymphoma with dose adjusted EPOCH: Impact of antiretroviral therapy suspension and tumor biology. Blood. 2003;101:4653–4659. doi: 10.1182/blood-2002-11-3589. [DOI] [PubMed] [Google Scholar]
- 6.Dunleavy K, Little RF, Pittaluga S, et al. The role of tumor histogenesis, FDG-PET and short course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV associated diffuse large B cell lymphoma. Blood. 2010;115:3017–3024. doi: 10.1182/blood-2009-11-253039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large B cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 8.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan LD, Lee JY, Ambinder RF, et al. Rituximab does not improve clinical outcome in a randomized phase 3 trial of CHOP with or without rituximab in patients with HIV associated non-Hodgkin's lymphoma: AIDS Malignancies Consortium Trial 010. Blood. 2005;106:1538–1543. doi: 10.1182/blood-2005-04-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pastan I, Gottesman MM. Multidrug resistance. Annu Rev Med. 1991;42:277–286. doi: 10.1146/annurev.me.42.020191.001425. [DOI] [PubMed] [Google Scholar]
- 11.Ueda K, Coswell MM, Gottesman MM, et al. The MDR1 gene, responsible for multidrug-resistance, codes for p-glycoprotein. Biochem Biophys Res Commun. 1986;141:956–962. doi: 10.1016/s0006-291x(86)80136-x. [DOI] [PubMed] [Google Scholar]
- 12.Hamada H, Tsuruo T. Purification of the 170 to 180 kilodalton membrane glycoprotein associated with multidrug resistance. J Biol Chem. 1997;263:1454–1458. [PubMed] [Google Scholar]
- 13.Yuen AR, Sikic BI. Multidrug resistance in lymphoma. J Clin Oncol. 1994;12:2453–2459. doi: 10.1200/JCO.1994.12.11.2453. [DOI] [PubMed] [Google Scholar]
- 14.Miller TP, Grogan TM, Dalton WS, et al. P-glycoprotein expression in malignant lymphoma and reversal of clinical drug resistance with chemotherapy plus high dose verapamil. J Clin Oncol. 1991;9:17–24. doi: 10.1200/JCO.1991.9.1.17. [DOI] [PubMed] [Google Scholar]
- 15.Tulpule A, Sherrod A, Dharmapala D, et al. Multidrug resistance (MDR-1) expression in AIDS related lymphomas. Leuk Res. 2002;26:121–127. doi: 10.1016/s0145-2126(01)00113-8. [DOI] [PubMed] [Google Scholar]
- 16.Lee CG, Gottesman MM, Cardarelli CO, et al. HIV-1 protease inhibitors are substrates for the MDR-1 multidrug transporter. Biochemistry. 1998;37:3596–3601. doi: 10.1021/bi972709x. [DOI] [PubMed] [Google Scholar]
- 17.Kim RB, Fromm MF, Wandel C, et al. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;1091:289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sparano JA, Lee JY, Kaplan LD, et al. Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV associated B cell non Hodgkin lymphoma. Blood. 2010;115:3008–3016. doi: 10.1182/blood-2009-08-231613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spina M, Jaeger U, Sparano JA, et al. Rituximab plus infusional cyclophosphamide, doxorubicin and etoposide in HIV associated non-Hodgkin lymphoma: Pooled results from 3 phase 2 trials. Blood. 2005;105:1891–1897. doi: 10.1182/blood-2004-08-3300. [DOI] [PubMed] [Google Scholar]
- 20.McKelvey EM, Gottlieb JA, Wilson HE, et al. Hydroxyldaunomycin (Adriamycin) combination chemotherapy in malignant lymphoma. Cancer. 1976;38:1484–1493. doi: 10.1002/1097-0142(197610)38:4<1484::aid-cncr2820380407>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Rahman A, Husain SR, Siddiqui J, et al. Liposome mediated modulation of multi-drug resistance in human HL-60 leukemia cells. J Natl Cancer Inst. 1992;84:1909–1915. doi: 10.1093/jnci/84.24.1909. [DOI] [PubMed] [Google Scholar]
- 22.Thierry AR, Vigé D, Coughlin SS, et al. Modulation of doxorubicin resistance in multi-drug resistant cells by liposomes. FASEB J. 1993;7:572–579. doi: 10.1096/fasebj.7.6.8097173. [DOI] [PubMed] [Google Scholar]
- 23.Levine AM, Tulpule A, Espina B, et al. Liposome-encapsulated doxorubicin in combination with standard agents (cyclophosphamide, vincristine, prednisone) in patients with newly diagnosed AIDS related non-Hodgkin's lymphoma: Results of therapy and correlates of response. J Clin Oncol. 2004;22:2662–2670. doi: 10.1200/JCO.2004.10.093. [DOI] [PubMed] [Google Scholar]
- 24.Gill PS, Rarick MU, Brynes RK, et al. Azidothymidine (AZT) and bone marrow failure in AIDS. Ann Intern Med. 1987;107:502–505. doi: 10.7326/0003-4819-107-4-502. [DOI] [PubMed] [Google Scholar]
- 25.Hellerstedt B, Ahmed A. Delayed-type hypersensitivity reaction or serum sickness after rituximab treatment. Ann Oncol. 2003;14:1792. doi: 10.1093/annonc/mdg488. [DOI] [PubMed] [Google Scholar]
- 26.Chadburn A, Chiu A, Lee JY, et al. Immunophenotypic analysis of AIDS-related diffuse large B-cell lymphoma and clinical implications in patients from AIDS Malignancies Consortium clinical trials 010 and 034. J Clin Oncol. 2009;27:5039–5048. doi: 10.1200/JCO.2008.20.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 28.Chernick MR, Liu CY, et al. The saw-toothed behavior of power versus sample size and software solutions: Single binomial proportion using exact methods. Am Stat. 2002;56:149–155. [Google Scholar]
- 29.Lawless J. Hoboken, NJ: John Wiley and Sons; 1982. Statistical Models and Methods for Lifetime Data. [Google Scholar]
- 30.International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann C, Tiemann M, Schrader C, et al. AIDS-related B cell lymphoma (ARL): Correlation of prognosis with differentiation profiles assessed by immunophenotyping. Blood. 2005;106:1762–1769. doi: 10.1182/blood-2004-12-4631. [DOI] [PubMed] [Google Scholar]
- 32.Visco C, Li Y, Xu-Monette ZY, et al. Comprehensive gene expression profiling and immunohistochemical studies support application of immunophenotypic algorithm for molecular subtype classification in diffuse large B cell lym-phoma: A report from the International DLBCL Rituximab-CHOP Consortium Program Study. Leukemia. 2012;26:2103–2113. doi: 10.1038/leu.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linton K, Howarth C, Wappett M, et al. Microarray gene expression analysis of fixed archival tissue permits molecular classification and identification of potential therapeutic agents in diffuse large B cell lymphoma. J Mol Diagn. 2012;14:223–232. doi: 10.1016/j.jmoldx.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 34. Reference deleted.
- 35.Borg C, Ray-Coquard I, Philip I, et al. CD4 lymphopenia as a risk factor for febrile neutropenia and early death after cytotoxic chemotherapy in adult patients with cancer. Cancer. 2004;101:2675–2680. doi: 10.1002/cncr.20688. [DOI] [PubMed] [Google Scholar]
- 36.Koo S, Baden LR. Infectious complications associated with immunomodulating monoclonal antibodies used in the treatment of hematologic malignancy. J Natl Compr Canc Netw. 2008;6:202–213. doi: 10.6004/jnccn.2008.0017. [DOI] [PubMed] [Google Scholar]
- 37.Kridel R, Dietrich PY. Prevention of CNS relapse in diffuse large B cell lymphoma. Lancet Oncol. 2011;12:1258–1266. doi: 10.1016/S1470-2045(11)70140-1. [DOI] [PubMed] [Google Scholar]
- 38.Haioun C, Besson C, Lepage E, et al. Incidence and risk factors of central nervous system relapse in histologically aggressive non-Hodgkin's lymphoma uniformly treated and receiving intrathecal central nervous system prophylaxis: A GELA study on 974 patients. Ann Oncol. 2000;11:685–690. doi: 10.1023/a:1008394827806. [DOI] [PubMed] [Google Scholar]
- 39.Kim H, Zelman RJ, Fox MA, et al. Pathology panel for lymphoma clinical studies: A comprehensive analysis of cases accumulated since its inception. J Natl Cancer Inst. 1982;68:43–67. [PubMed] [Google Scholar]
- 40.LaCasce AS, Kho ME, Friedberg JW, et al. Comparison of referring and final pathology for patients with non-Hodgkin's lymphoma in the National Comprehensive Cancer Network. J Clin Oncol. 2008;26:5107–2112. doi: 10.1200/JCO.2008.16.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]