Abstract
Study Design
A prospective study.
Purpose
To Investigate the prevalence of magnetic resonance imaging (MRI) changes of the lumbar spine in low back pain (LBP) and the associated risk factors in young Arab population.
Overview of Literature
Studies on the prevalence of MRI findings and their relationship with LBP have been conducted; these have occurred in adult populations in developed countries. The prevalence of MRI changes in the young Arab population with LBP is not known.
Methods
Two hundred and fourteen patients of Arab origin in the 16 to 29 year age group with LBP symptoms underwent MRI examinations. The prevalence of MRI changes in the lumbar spine and associated risk factors were determined and compared to age, race, and gender-matched controls.
Results
A majority (64%) of the patients with LBP (138 out of 214) were found to have MRI evidence of degenerative disc disease (DD) compared to 10% (22 out of 214) in the control group. The majority (61%) of patients had multiple level disease, most commonly involving the lowest 2 disc levels. Reduced signal of the disc followed by disc bulge was the most common MRI features seen in the symptomatic subjects. Obesity correlated with MRI prevalence of abnormalities, while activity demonstrated a positive trend.
Conclusions
The MRI prevalence of DD among the young Arab patients with LBP is high when compared to other reports in literature. Obesity correlated with MRI prevalence of abnormalities while activity demonstrated a positive trend.
Keywords: Magnetic resonance imaging, Lumbar spine, Disc, Degeneration, Prevalence, Young, Arabs
Introduction
Low back pain (LBP) is a commonly reported musculoskeletal (MSK) condition, as reflected by a high life-time prevalence among people living in the developed countries [1-4]. The direct and indirect cost of LBP in terms of quality of life, productivity, and employee absenteeism, are enormous making this common condition the single largest contributor to the MSK disability worldwide [5,6]. The life time prevalence of LBP is reported to vary between 32% to 83% in the developed countries [2,7,8]. A survey in Kuwait of 7,670 adults in 2003 has shown that 43% of the MSK pain is due to LBP [8] and among Kuwaiti school children, there was a high prevalence of LBP that was related to age (31% at age 10 years and 74% at age 18 years), gender (more common in females), and physical activity [9].
Studies on the prevalence of magnetic resonance imaging (MRI) findings and their relationship with LBP have mainly been conducted on the elderly. Studies on younger populations are very few and to our knowledge, the youngest age groups investigated for the prevalence of MRI features in degenerative disease (DD) of the lumbar spine are a Danish study on a cohort of 13-year-old school children [10], a study on juveniles of 13-20 years from China [11], 20 to 22 year-old subjects from Finland [10], and 20 to 30 year-old subjects from the United Kingdom [12]. While the prevalence of LBP in Kuwait is high [8,9], no studies have been performed to determine the imaging features in the young Arab population. This study was conducted to study the latter, as well as to identify modifiable risk factors associated with their symptoms.
Materials and Methods
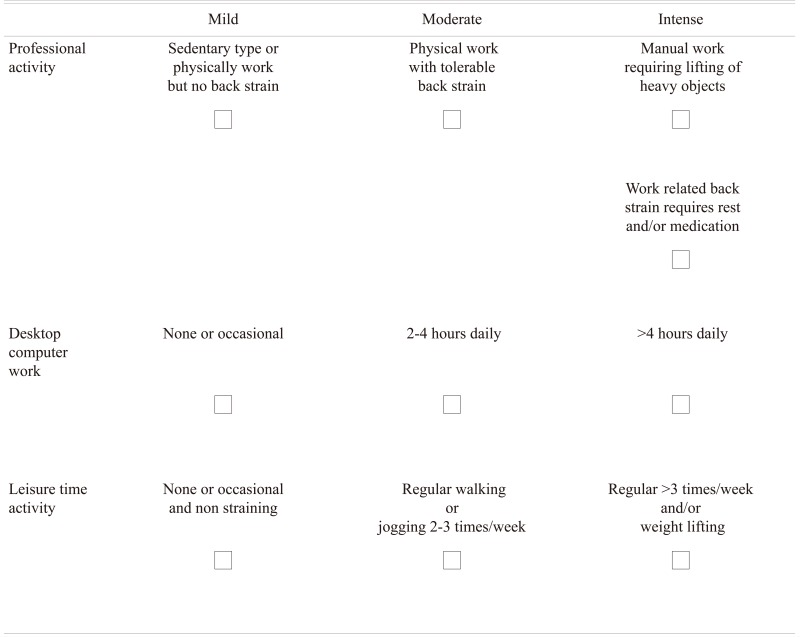
This prospective case-control study was conducted on 214 consecutive patients complaining of low back pain, with or without sciatica, who had been referred to the radiology department of a tertiary care center in Kuwait for an MRI examination of the lumbar spine from January 2010 to September 2011. These patients were of Arab origin in the 16 to 29 years age group. The exclusion criteria included causes of the back pain such as trauma, congenital spine disorders, post-operative, infections, autoimmune disorders, and muscle spasms. The following patient information was obtained and recorded as part of the demographic profile: clinical details, body mass index (BMI), physical activity (and other activity that can influence backache), and family history. Based on age, the subjects were divided into 2 groups (≥16 to <23 years and ≥23 to ≤29 years) to compare the imaging features between these 2 groups. The activity levels of the patient were determined by a self-completed questionnaire and classified as 'routine', 'moderate,' and 'intense' (Appendix 1). Patients, whose profession involved a sedentary type of lifestyle or any such activity that did not strain the back, such as executive type office work and/or working on desk top computer and who did not indulge in any additional physical exercise, were classified as 'routine' activity. Patients who indulged in regular physical exercise like walking or jogging at least 2 to 3 times a week and/or occasional lifting heavy objects as part of their profession were classified as 'moderate' activity, while 'high' activity was classified as those who besides their routine work performed daily regular exercise and/or lifting heavy weight as part of profession or as an exercise. From the questionnaire the highest recorded activity was taken into consideration. We recruited 214 subjects of matched age and gender, but without any symptoms of LBP to serve as controls. Verbal consent was obtained from these volunteers after explaining the purpose and the procedure of performing the MRI of the lumbar spine.
1. Imaging technique
Each MRI scan of the lumbar spine was performed on a 1.5T MRI machine (GE Signa 1.5 T Echo speed, GE healthcare, Milwaukee, WI, USA) using a dedicated receive-only, spine coil. The imaging protocol included sagittal T1-Weighted Spin Echo (repetition time [TR] 700 msec/echo time [TE] 12 msec) and T2 weighted fast spin-echo (FSE) (TR 5,000 msec/TE 130 msec) images with the following parameters: matrix = 320 × 250; field of view = 24; slice thickness 4 mm; inter-slice gap = 0.8 mm; number of excitations = 4; echo train length (ETL) = 15 and axial T2 weighted, FSE scans (TR 5000 msec/TE 72 msec; matrix 320 × 250; field view = 24 mm; inter-slice gap = 0.8 mm; number of excitations = 2; echo train length = 6). All sequences where acquired as routine with no fat saturation.
All the MRI examinations were read independently by 2 experienced radiologists (OA and MI) that had substantial experience in MSK imaging. Any differences in opinion were settled by consensus. The following standardized terms were used to classify the intervertebral discs in the images: normal, bulge (circumferential symmetric extension of the disc beyond the interspace), protrusion (focal or asymmetric extension of the disc beyond the interspace), and extrusion (more extreme extension of the disc beyond the interspace). Nerve compression, thecal sac compression, and/or canal stenosis, were identified. Other findings unrelated to disk abnormalities, such as facet arthropathy were reported, as well.
2 .Statistical analysis
A bivariate and multivariate logistic regression test was performed to determine the significance of the risk factors.
Results
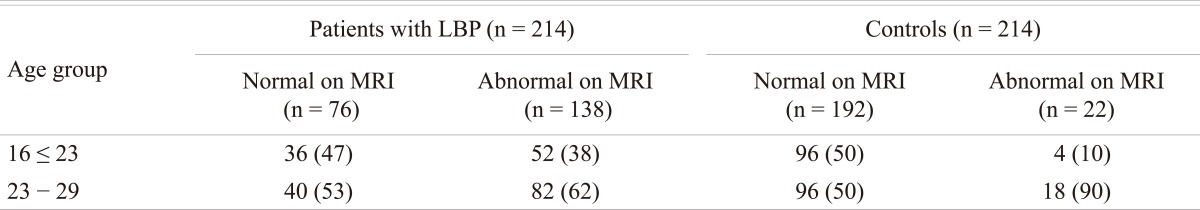
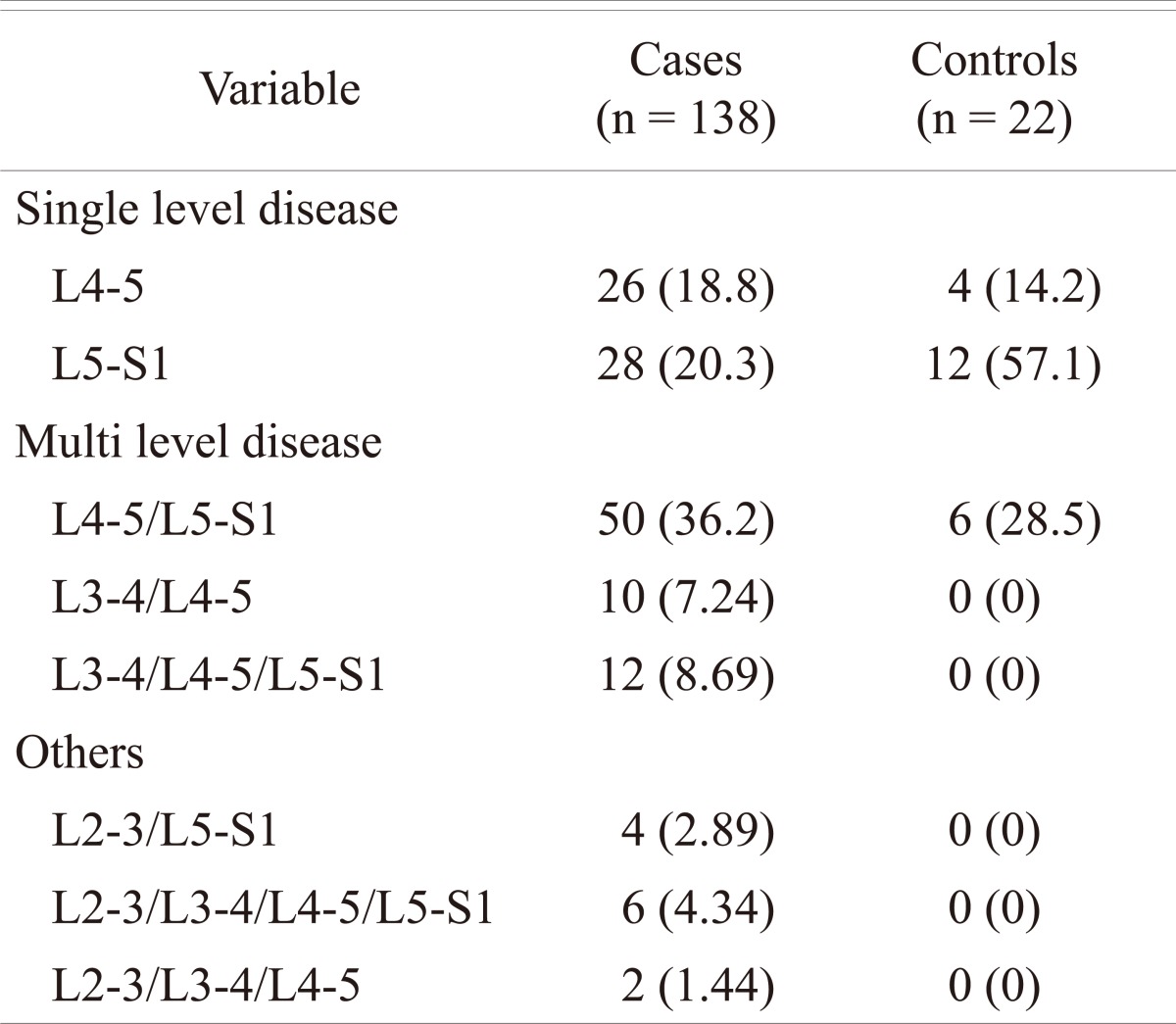
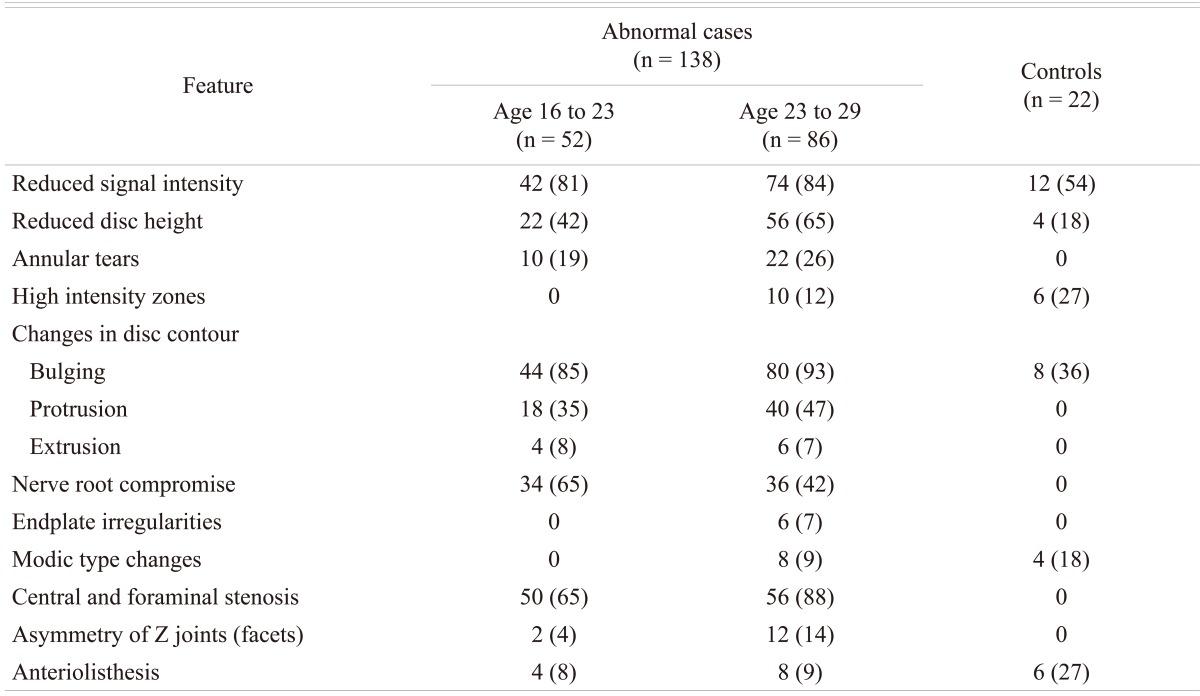
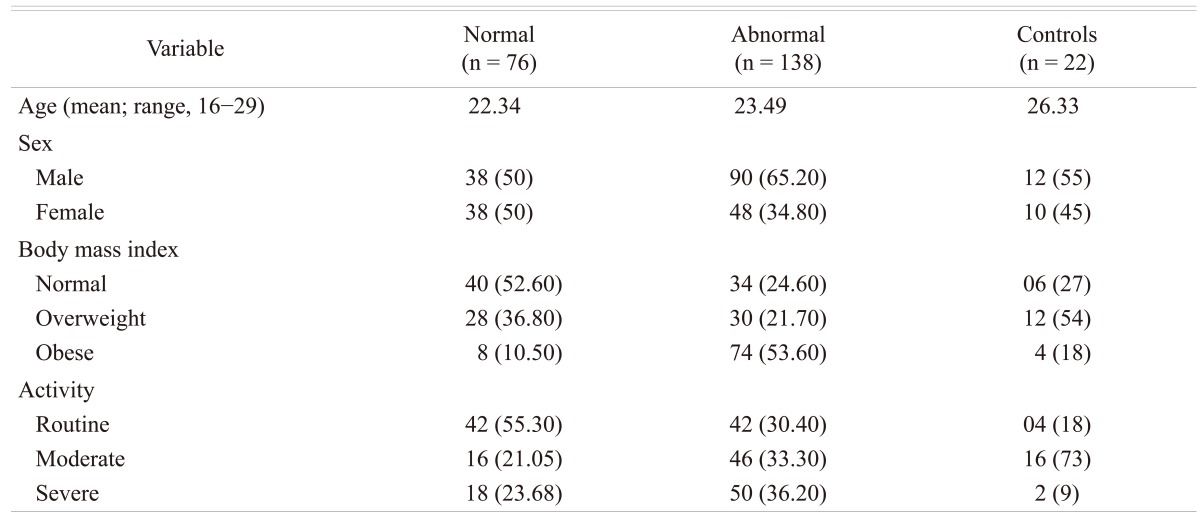
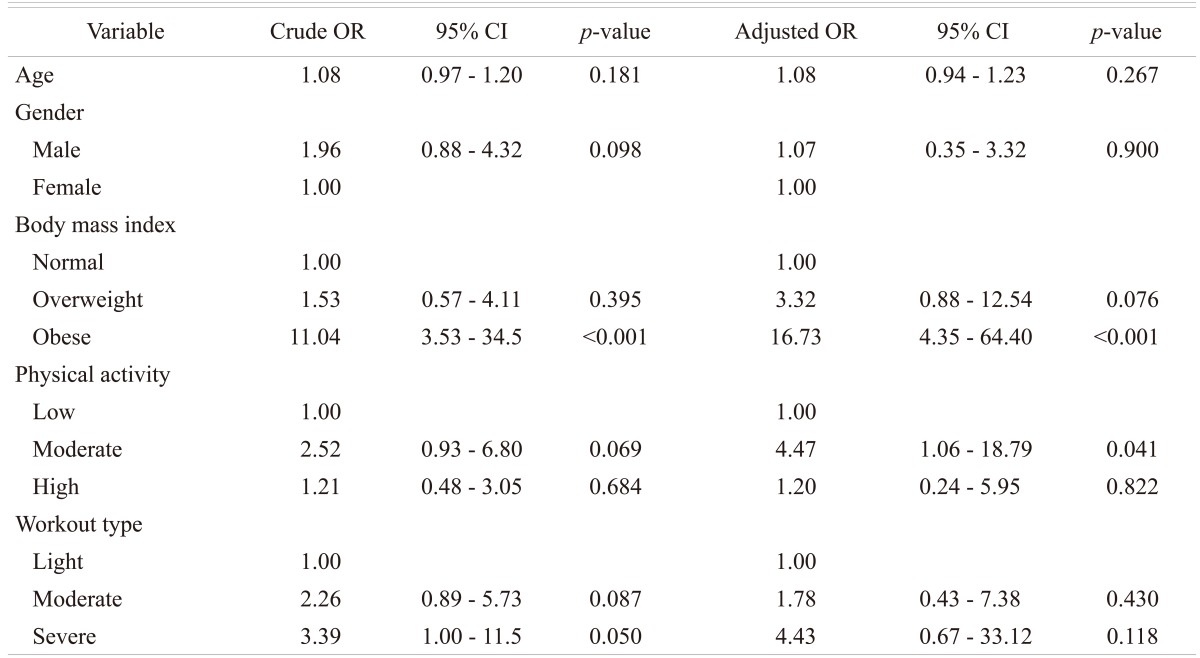
Of the 214 (128 male and 86 females; age range 16 to 29 years) patients with LBP that were subjected to MRI examination, 138 (64%) patients (90 males and 48 females; mean age 23 years) were found to have disc disease, while the remaining 76 patients (38 males and 38 females; mean age 22 years) were considered normal. In the control group, 22 subjects (10%) were found to have MRI evidence of early degenerative changes of the lumbar spine. The distribution of the disease in the different age groups is shown in Table 1. The distribution of the disease in the lumbar spine is shown in Table 2. The majority (61%; 84 out of 138) of the symptomatic patients had multiple level disease, most commonly involving the lowest 2 spine levels (L4-5 and L5-S1). Single level disease was seen in 54 out of 138 (39%), with close to equal involvement of the L4-5 and the L5-S1 discs. Overall the L4-5 disc, whether by itself or in combination with other discs, was diseased in the majority of the patients (80%). The various MRI features of the DD in symptomatic patients, as well as in the controls, are shown in Table 3. The most common feature observed was disc bulge followed by reduced signal intensity of the disc in DD of both the symptomatic group, as well as in the controls. The MRI features of the DD were more frequently seen in the higher (22 to 29 years) age group (Table 4). Analysis of the risk factors for the DD revealed that the majority of patients (75%) were either obese or overweight and the majority (69%) indulged in moderate or severe physical activity, but only obesity was retained as a significant risk factor in our analysis (Table 5).
Table 1.
Showing age distribution
Values are presented as number (%).
LBP: Low back pain, MRI: Magnetic resonance imaging.
Table 2.
Distribution of disc disease in symptomatic patients and controls
Values are presented as number (%).
Table 3.
Magnetic resonance imaging features of disc disease
Values are presented as number (%).
Table 4.
Risk factors
Values are presented as number (%).
Table 5.
Statistical analysis of risk factors
OR: Odds ratio, CI: Confidence intervals.
Discussion
In the present study, we reported on a young Arab population, the frequency of MRI changes in the spine in the symptomatic patients appears to be higher (65%) when compared to other reports in the literature [10-14] and these changes were more frequent (79%) in the higher (23 to 29 years) age group.
MRI is the first line investigation performed in the examination for DD of the spine. The imaging features of the DD of the spine are well established and include changes in the intervertebral disc, as well as other parts of the spine. The findings described in the disc on MRI include reduced signal intensity, irregular shape of the nucleus, reduced disc height, annular tears, high intensity zone (HIZ) within the annulus, and changes in disc contour (bulge, protrusion, extrusion and sequestration). The non-disc changes can be comprised of nerve root compression, end plate irregularities, Modic type changes, central and foraminal stenosis, degeneration and asymmetry of the Z-joints (Facets Joints) and spondylolisthesis. Salminen et al. [14] studied the prevalence of DD disease in adolescence and reported that 31% and 42% of subjects had at least one level of DD at the age of 15 and 18, respectively. In a Danish study, 21% of 13-year-old school children were reported to have at least one disc with decreased disc signal intensity in the lumbar spine [10]. In a study from Finland on an group aged 20-22 years, Takatalo et al. [12] found a 47% prevalence of DD. The study did not mention any difference in prevalence between the symptomatic and the asymptomatic subjects, although 45% of the male subjects and 41% of the female subjects in their study had LBP symptoms. Savage et al. [13] from UK compared MRI features between 2 age groups (20 to 30 years versus 31 to 59 years). In the 20 to 30 years age group, they found that 34% of subjects DD on MRI prevalence of compared to 59% in the older age group. In the 20 to 30 year age group, 47% were asymptomatic. A study on Chinese juveniles (13 to 20 years) reported that 35% of spinal DD, 72% had LBP [11]. In the current study, the frequency of MRI changes in the spine in the symptomatic patients appears to be higher (65%) when compared to other reports in literature [10-14] and these changes were more frequent (62%) in the higher (23 to 29 years) age group. The prevalence of degenerative changes is shown to increase with age [12,13] which can explain the age-related higher frequency in our study given that, when compared to other studies of young adults [10-14], had patients in a relatively higher (16 to 29 years) age group. Our observation is consistent with observations made in other studies on LBP conducted in this region and in similar age groups [9]. Sixty one percent of the patients in this study had multiple level disease with the highest prevalence (36%) at the 2 lowest lumbar levels (L4-5 and L5-S1), which is consistent with the observations made by others [10,12,15].
The prevalence of the various MRI features in the DD of the spine, known to occur in subjects with or without symptoms, have again mainly been described in the adult or the elderly population [16,17]. In the asymptomatic subjects the prevalence of reduced disc signal (56% to 72%), disc bulging (20% to 81%), protrusions (27% to 33%), extrusions (0% to 18%), HIZ lesions (6% to 33%), and annular tears (56%) is reported to range widely [15,17]. On the other hand, in symptomatic subjects, Kjaer et al. [10] observed that reduced disc signal, disc bulging, protrusions, extrusions, HIZ lesions and annular tears occurred in 86%, 27%, 23% and, 1.2%, 40%, and 39%. respectively and found these figures largely comparable with other reports in the literature [13,15,18-22]. A study from the UK [13] reported a higher prevalence of reduced disc signal, disc herniation/protrusion, and facet hypertrophy in the older age groups (30 to 59 year-old vs. 20 to 30 year-old), while the Chinese study [11] reported a higher prevalence of disc bulge/extrusion and HIZ; neither study mentions the difference between the symptomatic and the asymptomatic subjects, although in general, studies have shown higher prevalence rates of DD findings in symptomatic compared to non symptomatic populations [16,17,20,21,23]. In our study, the prevalence of MRI features for reduced disc signal, disc bulging, and protrusions, were highest followed by the prevalence of annular tears, extrusions and HIZ lesions. Our figures appear higher than that reported in the literature and for a relatively younger age group. The prevalence of protrusion in a study from Finland [12] on persons aged 20 to 22 years and in a study on a cohort of 13-year-old school children [10] there was a lesser prevalence of extrusion in these studies and the condition was rare.
Annular tears and HIZ in this study was present in 23% and 7% respectively, with preponderance at the 2 lowest levels in the lumbar spine (L4-5 and L5-S1) and was more common in men. Our findings on the annular tears regarding location and gender distribution are similar to the studies on 13-year-old Danish children [10] and on young adults 20 to 22 year-old from Finland [12], but unlike that of the study on the 40 year old Danes which reported prevalence of annular tears higher among women [13]. The HIZ lesions in the Danish study were more likely at L5-S1 and were more likely in boys [10]; however, there was no significant difference between the 2 lowest levels, or in gender observed in a study performed in a 40-year-old Danish population [14]. The Finnish study [10] showed that the lesions occurred more often in women.
Modic changes are reported to range from 12% to 58% and appear related to age and symptoms [24]. In the Danish population it was reported to occur in 0.5% and 6.6% in the 13-year-old [10] and the 40-year-old subjects [14], respectively, and in 1.4% in the 20 to 22 year age group from Finland [12]. In the present study (16 to 29 years age group), modic change was seen in 6%. Although our figures are high, comparison between these studies is difficult because of the disparity in age.
The causal relationship between lumbar disc degeneration, LBP, physical activity, and obesity is not firmly established. It appears that lack of physical loading on one hand and the high activity in sports on the other hand could both be harmful on the spine and relate to back troubles in the young. While there are reports that low physical activity (e.g., being sedentary) is associated with increased occurrence of DD in adults [25] and with higher prevalence of LBP in the young [26], there are studies in which a high activity in competitive sports correlates with increased prevalence of LBP [27] or increased occurrence of MRI findings in the young [28]. In adults, injuries or heavy work have been found to be a risk factor for DD [25,28]. In the present study with MRI features of DD, 69.5% of the subjects reported moderate to intense activity and the majority of these were men. On the other hand, in our study obesity proved to be a significant risk factor for DD of the spine, as nearly 75% of the patients with this condition were either overweight or obese.
Statistical analysis confirms that BMI is the independent factor associated with abnormal findings in our study. A patient who is obese was 16.7 times more likely to have abnormal MRI compared to a patient with a normal BMI. A large population based study from Norway [29] as well as a studies from the Middle East [30] and from China [1] have revealed a high prevalence of LBP among obese persons.
Conclusions
The MRI prevalence of DD among the young Arab patients with LBP is high when compared to other reports in literature. Obesity correlated with MRI prevalence of abnormalities while activity demonstrated a positive trend. In view of the high prevalence among a young Arab population, a larger cross sectional study is warranted before any further recommendations can be developed regarding a public health intervention.
Appendix 1
How do you rate your physical activity?
References
- 1.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 2.Clays E, De Bacquer D, Leynen F, Kornitzer M, Kittel F, De Backer G. The impact of psychosocial factors on low back pain: longitudinal results from the Belstress study. Spine (Phila Pa 1976) 2007;32:262–268. doi: 10.1097/01.brs.0000251884.94821.c0. [DOI] [PubMed] [Google Scholar]
- 3.Oksuz E. Prevalence, risk factors, and preference-based health states of low back pain in a Turkish population. Spine (Phila Pa 1976) 2006;31:E968–E972. doi: 10.1097/01.brs.0000247787.25382.3c. [DOI] [PubMed] [Google Scholar]
- 4.Gatchel RJ, Polatin PB, Noe C, Gardea M, Pulliam C, Thompson J. Treatment- and cost-effectiveness of early intervention for acute low-back pain patients: a one-year prospective study. J Occup Rehabil. 2003;13:1–9. doi: 10.1023/a:1021823505774. [DOI] [PubMed] [Google Scholar]
- 5.Ricci JA, Stewart WF, Chee E, Leotta C, Foley K, Hochberg MC. Back pain exacerbations and lost productive time costs in United States workers. Spine (Phila Pa 1976) 2006;31:3052–3060. doi: 10.1097/01.brs.0000249521.61813.aa. [DOI] [PubMed] [Google Scholar]
- 6.Ihlebaek C, Hansson TH, Laerum E, et al. Prevalence of low back pain and sickness absence: a "borderline" study in Norway and Sweden. Scand J Public Health. 2006;34:555–558. doi: 10.1080/14034940600552051. [DOI] [PubMed] [Google Scholar]
- 7.Schneider S, Randoll D, Buchner M. Why do women have back pain more than men? A representative prevalence study in the federal republic of Germany. Clin J Pain. 2006;22:738–747. doi: 10.1097/01.ajp.0000210920.03289.93. [DOI] [PubMed] [Google Scholar]
- 8.Al-Awadhi AM, Olusi SO, Moussa M, et al. Musculoskeletal pain, disability and health-seeking behavior in adult Kuwaitis using a validated Arabic version of the WHO-ILAR COPCORD Core Questionnaire. Clin Exp Rheumatol. 2004;22:177–183. [PubMed] [Google Scholar]
- 9.Shehab DK, Al-Jarallah KF. Nonspecific low-back pain in Kuwaiti children and adolescents: associated factors. J Adolesc Health. 2005;36:32–35. doi: 10.1016/j.jadohealth.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Kjaer P, Leboeuf-Yde C, Sorensen JS, Bendix T. An epidemiologic study of MRI and low back pain in 13-year-old children. Spine (Phila Pa 1976) 2005;30:798–806. doi: 10.1097/01.brs.0000157424.72598.ec. [DOI] [PubMed] [Google Scholar]
- 11.Samartzis D, Karppinen J, Mok F, Fong DY, Luk KD, Cheung KM. A population-based study of juvenile disc degeneration and its association with overweight and obesity, low back pain, and diminished functional status. J Bone Joint Surg Am. 2011;93:662–670. doi: 10.2106/JBJS.I.01568. [DOI] [PubMed] [Google Scholar]
- 12.Takatalo J, Karppinen J, Niinimäki J, et al. Prevalence of degenerative imaging findings in lumbar magnetic resonance imaging among young adults. Spine (Phila Pa 1976) 2009;34:1716–1721. doi: 10.1097/BRS.0b013e3181ac5fec. [DOI] [PubMed] [Google Scholar]
- 13.Savage RA, Whitehouse GH, Roberts N. The relationship between the magnetic resonance imaging appearance of the lumbar spine and low back pain, age and occupation in males. Eur Spine J. 1997;6:106–114. doi: 10.1007/BF01358742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salminen JJ, Erkintalo MO, Pentti J, Oksanen A, Kormano MJ. Recurrent low back pain and early disc degeneration in the young. Spine (Phila Pa 1976) 1999;24:1316–1321. doi: 10.1097/00007632-199907010-00008. [DOI] [PubMed] [Google Scholar]
- 15.Weishaupt D, Zanetti M, Hodler J, Boos N. MR imaging of the lumbar spine: prevalence of intervertebral disk extrusion and sequestration, nerve root compression, end plate abnormalities, and osteoarthritis of the facet joints in asymptomatic volunteers. Radiology. 1998;209:661–666. doi: 10.1148/radiology.209.3.9844656. [DOI] [PubMed] [Google Scholar]
- 16.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–408. [PubMed] [Google Scholar]
- 17.Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- 18.Boos N, Rieder R, Schade V, Spratt KF, Semmer N, Aebi M. 1995 Volvo Award in clinical sciences. The diagnostic accuracy of magnetic resonance imaging, work perception, and psychosocial factors in identifying symptomatic disc herniations. Spine (Phila Pa 1976) 1995;20:2613–2625. doi: 10.1097/00007632-199512150-00002. [DOI] [PubMed] [Google Scholar]
- 19.Stadnik TW, Lee RR, Coen HL, Neirynck EC, Buisseret TS, Osteaux MJ. Annular tears and disk herniation: prevalence and contrast enhancement on MR images in the absence of low back pain or sciatica. Radiology. 1998;206:49–55. doi: 10.1148/radiology.206.1.9423651. [DOI] [PubMed] [Google Scholar]
- 20.Videman T, Battié MC, Gibbons LE, Maravilla K, Manninen H, Kaprio J. Associations between back pain history and lumbar MRI findings. Spine (Phila Pa 1976) 2003;28:582–588. doi: 10.1097/01.BRS.0000049905.44466.73. [DOI] [PubMed] [Google Scholar]
- 21.Borenstein DG, O'Mara JW, Jr, Boden SD, et al. The value of magnetic resonance imaging of the lumbar spine to predict low-back pain in asymptomatic subjects: a seven-year follow-up study. J Bone Joint Surg Am. 2001;83:1306–1311. doi: 10.2106/00004623-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Jarvik JJ, Hollingworth W, Heagerty P, Haynor DR, Deyo RA. The Longitudinal Assessment of Imaging and Disability of the Back (LAIDBack) Study: baseline data. Spine (Phila Pa 1976) 2001;26:1158–1166. doi: 10.1097/00007632-200105150-00014. [DOI] [PubMed] [Google Scholar]
- 23.Carragee EJ, Paragioudakis SJ, Khurana S. 2000 Volvo Award winner in clinical studies: Lumbar high-intensity zone and discography in subjects without low back problems. Spine (Phila Pa 1976) 2000;25:2987–2992. doi: 10.1097/00007632-200012010-00005. [DOI] [PubMed] [Google Scholar]
- 24.Jensen TS, Karppinen J, Sorensen JS, Niinimäki J, Leboeuf-Yde C. Vertebral endplate signal changes (Modic change): a systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J. 2008;17:1407–1422. doi: 10.1007/s00586-008-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Videman T, Nurminen M, Troup JD. 1990 Volvo Award in clinical sciences. Lumbar spinal pathology in cadaveric material in relation to history of back pain, occupation, and physical loading. Spine (Phila Pa 1976) 1990;15:728–740. [PubMed] [Google Scholar]
- 26.Salminen JJ. The adolescent back. A field survey of 370 Finnish schoolchildren. Acta Paediatr Scand Suppl. 1984;315:1–122. [PubMed] [Google Scholar]
- 27.Kujala UM, Salminen JJ, Taimela S, Oksanen A, Jaakkola L. Subject characteristics and low back pain in young athletes and nonathletes. Med Sci Sports Exerc. 1992;24:627–632. [PubMed] [Google Scholar]
- 28.Goldstein JD, Berger PE, Windler GE, Jackson DW. Spine injuries in gymnasts and swimmers. An epidemiologic investigation. Am J Sports Med. 1991;19:463–468. doi: 10.1177/036354659101900507. [DOI] [PubMed] [Google Scholar]
- 29.Heuch I, Hagen K, Heuch I, Nygaard Ø, Zwart JA. The impact of body mass index on the prevalence of low back pain: the HUNT study. Spine (Phila Pa 1976) 2010;35:764–768. doi: 10.1097/BRS.0b013e3181ba1531. [DOI] [PubMed] [Google Scholar]
- 30.Bener A, Alwash R, Gaber T, Lovasz G. Obesity and low back pain. Coll Antropol. 2003;27:95–104. [PubMed] [Google Scholar]