Abstract
Background
To determine the frequency of chronic kidney disease (CKD) and its associated risk factors in Chinese type 2 diabetic patients, we conducted a cross-sectional study in Nanjing, China, in the period between January 2008 and December 2009.
Methods
Patients with type 2 diabetes under the care by Jiangsu Province Official Hospital, Nanjing, China were invited for assessment. CKD was defined as the presence of albuminuria or estimated glomerular filtration rate <60 mL/min/1.73 m2. Albuminuria was defined as urinary albumin-to-creatinine ratio ≥30 mg/g.
Results
We recruited 1,521 urban Chinese patients with type 2 diabetes (mean age, 63.9±12.0 years). The frequency of CKD and albuminuria was 31.0% and 28.9%, respectively. After adjusted by age and sex, hypertension, anemia and duration of diabetes were significantly associated with CKD with odds ratio (95% confidence interval) being 1.93 (1.28 to 2.93), 1.70 (1.09 to 2.64), and 1.03 (1.00 to 1.06), respectively.
Conclusion
In conclusion, CKD was common in the urban Nanjing Chinese with type 2 diabetes. Strategies to prevent or delay progression of kidney disease in diabetes should be carried out at the early disease course of type 2 diabetes.
Keywords: Albuminuria; Chronic kidney disease; Diabetes mellitus, type 2; Risk factors
INTRODUCTION
Chronic kidney disease (CKD) is a worldwide public health problem with increasing incidence and prevalence. Recently, diabetes has become the leading cause of end-stage renal disease in many developed countries accounting for about 40% of all new cases who require renal replacement therapy [1,2]. Studies have demonstrated that CKD was an independent risk factor for cardiovascular disease (CVD) and all-cause death in diabetic individuals [3-6]. The early detection of CKD in diabetic patients is therefore of critical importance.
The conventional approach for screening CKD was the determination of albumin excretion rate (AER). As a manifestation of kidney damage, albuminuria is a component of CKD and has been suggested to be an important marker of early-stage CKD and a useful predictor of overt diabetic nephropathy. However, epidemiologic evidence indicated that a substantial fraction of those with CKD in the setting of diabetes have little or no detectable albuminuria [7,8]. In keeping with the National Kidney Foundation guidelines [9], American Diabetes Association (ADA) recommends the screening of CKD in diabetic patients using both AER and glomerular filtration rate (GFR) [10]. Direct measurement of GFR was difficult to perform, not to mention the issues of being time-consuming and expensive. However, GFR can be estimated using formulae such as the Cockroft-Gault equation or a prediction formula based on data from the Modification of Diet and Renal Disease (MDRD) study [10]. The MDRD equation was more accurate [11], more robust when glucose control was poor [12], and not biased by body weight [13].
Recently, Yang et al. [14] reported the age-standardized prevalence of diabetes were 9.7%, accounting for 92.4 million adults with diabetes in China. The rapidly increasing rate of diabetes implied an escalating demand in managing progressive renal failure. Unlike albuminuria, measurement of GFR to screen for kidney disease was not popular among diabetic patients in China. To our knowledge, epidemiological data regarding the prevalence of CKD in China is limited. There were only a few studies reporting the association between CKD and albuminuria among Chinese patients with diabetes.
Against this background, we conducted this study aiming to: 1) determine the frequency of CKD in Chinese type 2 diabetic patients in Nanjing; 2) identify the risk factors associated with CKD; and 3) investigate the inter-relation between estimated GFR (eGFR) and albuminuria.
METHODS
Participants
This study was a cross-sectional study designed to raise the attainment rate of treatment of hyperglycemia, hypertension, and dyslipidemia in type 2 diabetics and was performed by Diabetes Care and Research Center of Jiangsu Province Official Hospital and Jiangsu Province Institute of Geriatrics, Nanjing, China. All enrolled patients are urban resident population of Nanjing City, the capital of Jiangsu Province. Patients were divided into three stages, including initiating stage, regulating stage and maintaining stage, and were managed using target software through multifactoral intervention. A total of 2,256 type 2 diabetics aged over 30 year-old were recruited between January 2008 to December 2009 from Diabetes Care and Research Center of Jiangsu Province Official Hospital and Jiangsu Province Institute of Geriatrics, Nanjing, China. Comprehensive clinical evaluations including history taking, physical examination, and laboratory assessment were performed at baseline for all study patients. Seven hundred thirty-five patients were excluded due to missing urine data, concurrent infection, fever, pyuria and/or hematuria due to any cause, decompensated cardiac failure, severe hepatic dysfunction, known non-diabetic renal disease, eGFR below 15 mL/min/1.73 m2 (CKD stage 5) or receiving renal replacement therapy and pregnant or menstruating women. Eventually, 1,521 Chinese type 2 diabetics were included in this present analysis. This study was approved by Jiangsu Province Health Administrative Department and its Ethics Committee. The study complied with the Declaration of Helsinki and informed written consents were obtained from all participants.
Anthropometric and clinical assessments
Blood pressure (BP) was measured twice using a standard mercury manometer in the sitting position after the subject rested for at least 15 minutes. The average of the two measurements was recorded. Hypertension was defined by systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg [15] or current use of anti-hypertensive agents. Body weight, height and waist circumference were measured with patients just on light clothing without shoes. Waist circumference was measured with a non-metallic, constant tension tape placed around the body at the midpoint between the highest point of the iliac crest and the lowest part of the costal margin in the mid-axillary line. Body mass index (BMI) was calculated as weight (kg) divided by squared height (m2). Overweight/obesity were defined as BMI ≥25 kg/m2 [16]. Both brachial and ankle systolic BPs were measured in the supine position with an 8-MHz Doppler ultrasound device (ES-1000SPM Smartdop; Hadeco, Kawasaki, Japan). According to the guidelines of American Heart Association [17], ankle-brachial index (ABI) was calculated as the ratio of the higher value of the systolic BP of the two ankle arteries of that limb (either the anterior or the posterior tibial artery) and the higher value of the two brachial systolic BP. For each patient, the lower ABI from both legs was used for further evaluation. Peripheral artery disease was defined as being present if ABI <0.9 in at least one leg. CVD was defined as a self-reported history of coronary arterial disease (including a previous history of myocardial infarction, the presence of coronary interventions) and/or stroke.
Laboratory assay
Venous blood was collected from the antecubital vein after 12 hours overnight fast. Glycated hemoglobin (HbA1c) was measured by a dedicated ion exchange high-performance liquid chromatography instrument (Bio-Rad Variant II; Bio-Rad Laboratories, Hercules, CA, USA). Plasma glucose levels were measured using the glucose oxidase method (Roche Module P800; Roche Diagnostics Ltd., Basel, Switzerland). Diabetes was diagnosed according to the ADA 1997 criteria [18]. Serum urea nitrogen, creatinine, uric acid, total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), and triglyceride (TG) concentrations were determined enzymatically (Roche Module P800). Dyslipidemia was defined by fasting serum TC ≥5.18 mmol/L and/or TG ≥1.70 mmol/L, and/or LDL-C ≥3.37 mmol/L, and/or HDL-C <1.04 mmol/L [19]. Anemia was defined as hemoglobin level less than 130 g/L in male and 120 g/L in female [20]. A morning spot urine sample was obtained from participants for measuring urinary albumin-to-creatinine ratio (ACR). Due to resources limitation, only one urine specimen was collected for each patient. The DCA-2000 microalbumin/creatinine assay system (Bayer Diagnostics, München, Germany) detected urine albumin using an immunoturbidimetric direct antibody-antigen aggregation method and measured creatinine colorimetrically based on the Benedict-Behre reaction [21]. Urinary ACR was computed and reported in milligrams per gram (mg/g). Albuminuria was defined as urinary ACR ≥30 mg/g. Microalbuminuria was defined as ACR of 30 to 299 mg/g, and macroalbuminuria was defined as ACR of 300 mg/g or higher [10].
Kidney function was stratified according to eGFR using the MDRD equation [22] as follows: eGFR=186×[serum creatinine (mg/dL)]-1.154×[age (year)]-0.203×(0.742 if female). According to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) working group [9], CKD was defined as the presence of albuminuria or eGFR <60 mL/min/1.73 m2. The stages of CKD were defined as follows: Stage 1, albuminuria with an eGFR ≥90 mL/min/1.73 m2; Stage 2, albuminuria with an eGFR of 60 to 89 mL/min/1.73 m2; Stage 3, an eGFR of 30 to 59 mL/min/1.73 m2 regardless of urinary ACR; and Stage 4, an eGFR of 15 to 29 mL/min/1.73 m2 regardless of urinary ACR. Renal insufficiency was defined as an eGFR <60 mL/min/1.73 m2 (CKD stage 3 to 4).
Statistical analysis
Data were presented as mean±standard deviation for continuous variables and proportions for categorical variables. Serum TG, urinary ACR and duration of diabetes were logarithmically transformed for analyses and the geometric means were presented. The mean values of duration of diabetes, BP, BMI, waist circumference, HbA1c, lipid profile, uric acid, urea nitrogen, creatinine, hemoglobin, eGFR and urinary ACR were estimated using the general linear model after adjusting for age and sex. Previous studies suggested urinary ACR levels fell into a non-linear distribution pattern [23]. In order to estimate the relationship between decreasing eGFR and the increment of urinary ACR, and to ensure that each sub-group has similar sample size for more appropriate statistical comparison, urinary ACR was stratified into 12 grades [23] and a polynominal regression analysis and curve fitting were performed.
The difference among the CKD stages was tested using ANOVA. The chi-squared test was employed to estimate the difference for categorical variables. The odds ratio between the factors and CKD was calculated using logistic regression analysis with or without adjustment for sex and age. Polynominal regression and curve fitting were performed to estimate the trends of eGFR with the increment of urinary ACR. Statistical analyses were performed using SPSS for Windows version 15.0. (SPSS Inc., Chicago, IL, USA). All statistical tests were two-sided and a P value of less than 0.05 was considered statistically significant.
RESULTS
Population characteristics and the frequency of CKD
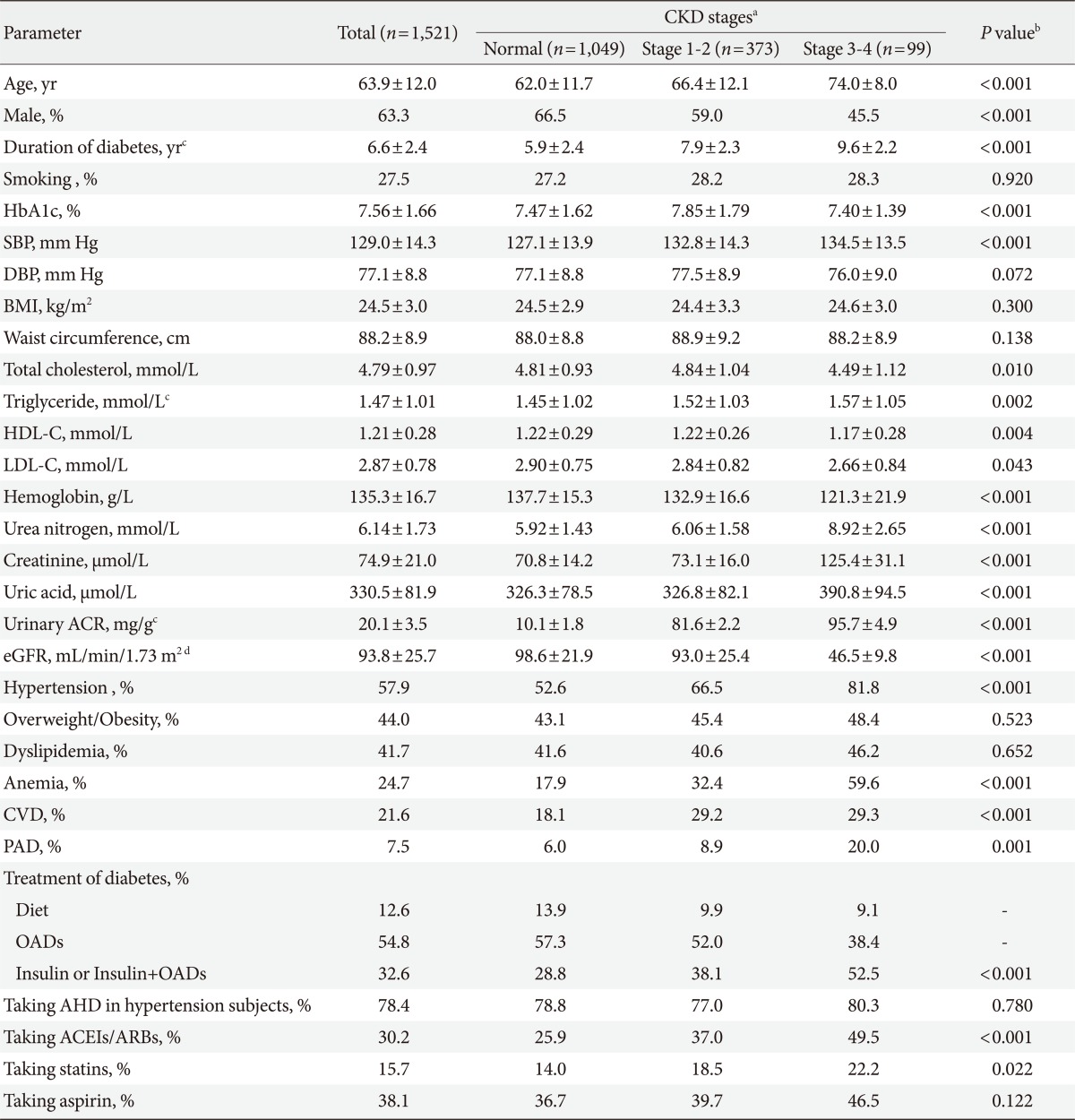
The mean age of the study cohort was 63.9±12.0 years (median, 65 years; range, 30 to 88 years), duration of diabetes was 6.6±2.4 years (median, 8 years; range, 0 to 30 years) and HbA1c was 7.56±1.66%. The rate of hypertension, dyslipidemia, overweight/obesity, CVD, PAD, and anemia was 57.9%, 41.7%, 44.0%, 21.6%, 7.5%, and 24.7%, respectively. The clinical and metabolic parameters of patients according to the stages of CKD were shown in Table 1. As compared to those who had no CKD, the patients with CKD stage 1 to 2 or stage 3 to 4 were older, had higher rate of hypertension, CVD, PAD and anemia, and had longer duration of diabetes. The CKD stage 1 to 2 patients had higher HbA1c level than those without CKD and those with stage 3 to 4, and there was no difference of HbA1c between patients without CKD and those with CKD stage 3 to 4.
Table 1.
Clinical and metabolic parameters across the stages of chronic kidney disease
CKD, chronic kidney disease; HbA1c, glycated hemoglobin; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; HDL-D, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; ACR, albumin-to-creatinine ratio; eGFR, estimated glomerular filtration rate; CVD, cardiovascular disease; PAD, peripheral arterial disease; OADs, oral antidiabetic drugs; AHD, antihypertensive drugs; ACEIs, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers.
aCKD stages 1-2 were defined based on the level of kidney function and the presence of albuminuria; stages 3-4 were defined solely based on kidney function; subjects with eGFR below 15 mL/min/1.73 m2 (stage 5) receiving maintenance dialysis were excluded in this study, bAge and sex adjusted P value among the CKD stages except for age and categorical variables compared, cNon-normally distributed variables were expressed as Geometric mean, dGFR estimated by Modification of Diet in Renal Disease (MDRD) study equation.
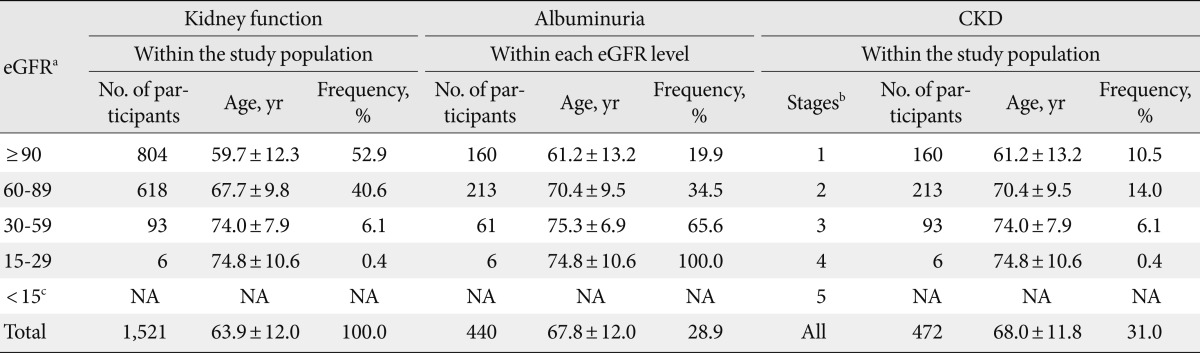
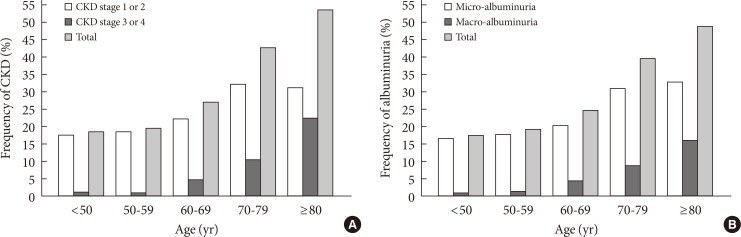
Table 2 showed the frequency and age of the subjects according to their levels of kidney function, albuminuria, and stages of CKD. As earlier stages of CKD (stage 1 to 2) were classified based on a combination of eGFR and albuminuria, the frequency of albuminuria were categorized within each level of eGFR. There were 717 (47.1%) patients whose eGFR levels were less than 90 mL/min/1.73 m2. The total frequencies of CKD and albuminuria in diabetes subjects were 31.0% and 28.9%. The frequency of renal insufficiency (defined as eGFR <60 mL/min/1.73 m2) was 6.5%, and the corresponding frequencies were 1.0%, 0.9%, 4.6%, 10.4%, and 22.4% in those aged <50, 50 to 59, 60 to 69, 70 to 79 and ≥80 years, respectively. In patients with renal insufficiency, the frequency of albuminuria and normoalbuminuria was 67.7% and 32.3%, respectively. Fig. 1 showed the frequency of CKD stage 1 to 2, stage 3 to 4, and micro-/macro-albuminuria which increased gradually with the increase of age (P<0.001).
Table 2.
Kidney functions, albuminuria and stages of CKD of the 1,521 Chinese patients with type 2 diabetes
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; NA, not applicable.
aGFR estimated by Modification of Diet in Renal Disease (MDRD) study equation, in the unit of mL/min/1.73 m2, bCKD stages 1-2 were defined based on the level of kidney function and the presence of albuminuria; stages 3-4 were defined solely based on kidney function, cSubjects with eGFR below 15 mL/min/1.73 m2 (CKD stage 5) receiving maintenance dialysis were excluded in this study.
Fig. 1.
The frequency of chronic kidney disease (CKD) (A) and albuminuria (B) in 1,521 Nanjing Chinese type 2 diabetic patients stratified by age.
Risk factors associated with CKD
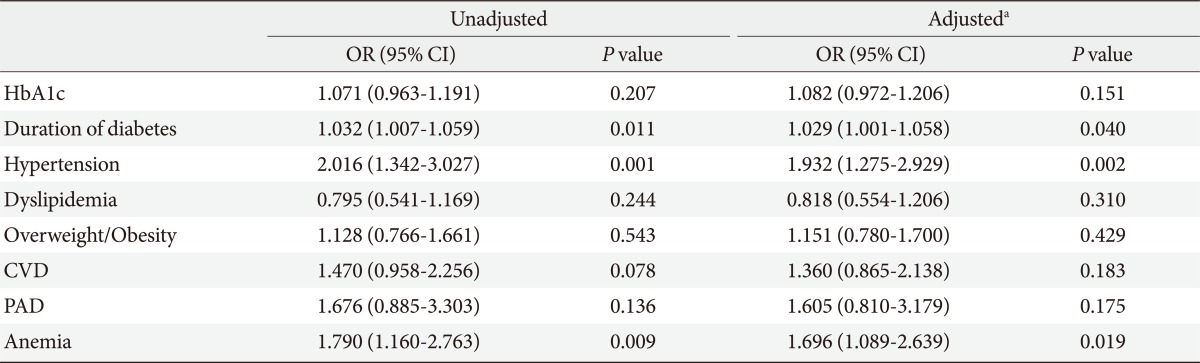
We analyzed the association between the presence of CKD and HbA1c, duration of diabetes, hypertension, dyslipidemia, overweight/obesity, CVD, PAD and anemia. After adjusting for age and gender, hypertension, anemia, and duration of diabetes were independently associated with CKD with an odds ratio (95% confidence interval) of 1.93 (1.28 to 2.93, P=0.002), 1.70 (1.09 to 2.64, P=0.019), and 1.03 (1.00 to 1.06, P=0.040), respectively (Table 3).
Table 3.
Risk factors associated with CKD in Nanjing Chinese type 2 diabetic patients
CKD, chronic kidney disease; OR, odds ratio; CI, confidence interval; HbA1c, glycated hemoglobin; CVD, cardiovascular disease; PAD, peripheral arterial disease.
aOdds ratio and P value after adjustment for age and gender.
Association between eGFR and albuminuria
The frequency of micro- and macro-albuminuria increased significantly with decreasing eGFR levels (P<0.001). When the patients were stratified according to their urinary ACR status, the frequency of renal insufficiency was 3.0%, 9.3%, and 40.5%, and the frequency of eGFR <90 mL/min/1.73 m2 was 40.4%, 57.6%, and 89.3% for patients with normo-, micro-, and macroalbuminuria, respectively.
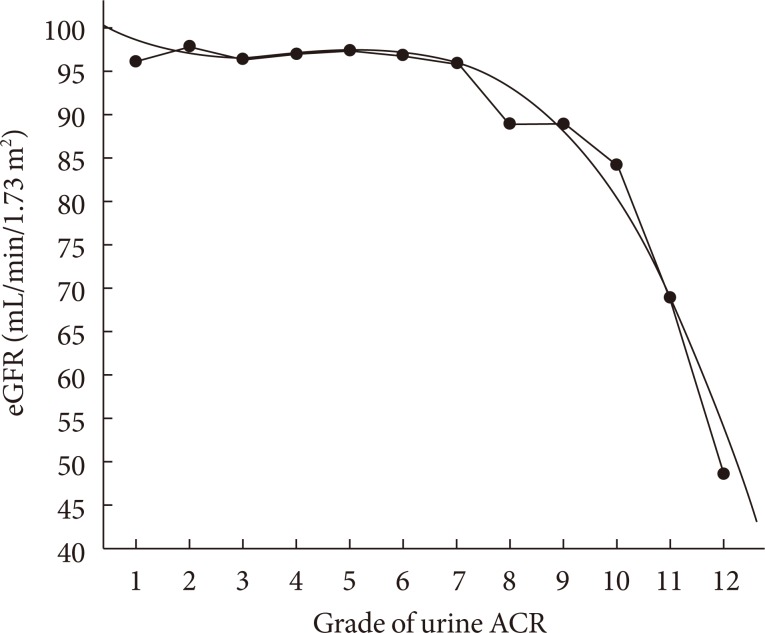
With urinary ACR being stratified into 12 grades and a polynominal regression analysis and curve fitting were performed, as shown in Fig. 2, a cubic parabola regression equation of eGFR and the increment of urinary ACR was established (r2=0.978, P<0.001). In subjects with the ACR ranging from grade 1 to grade 7, the levels of eGFR decreased slowly and steadily and maintained more than 90 mL/min/1.73 m2. However, from grade 9 of ACR (approximately equivalent to urinary ACR more than 90 mg/g), the levels of eGFR decreased rapidly and were all lower than 90 mL/min/1.73 m2.
Fig. 2.
Polynominal regression and curve fitting of estimated glomerular filtration rate (eGFR) across the grade of urinary albumin-to-creatinine ratio (ACR). ACR was stratified into 12 grades: grade 1, <5 mg/g; grade 2, 5 to 9 mg/g; grade 3, 10 to 14 mg/g; grade 4, 15 to 19 mg/g; grade 5, 20 to 24 mg/g; grade 6, 25 to 29 mg/g; grade 7, 30 to 59 mg/g; grade 8, 60 to 89 mg/g; grade 9, 90 to 149 mg/g; grade 10, 150 to 299 mg/g; grade 11, 300 to 499 mg/g; and grade 12, ≥500 mg/g.
DISCUSSION
In this study involving 1,521 Chinese type 2 diabetic patients, 47.1% patients had an eGFR less than 90 mL/min/1.73 m2 and 28.9% had albuminuria. The frequency of CKD was 31.0%, of which 6.5% had renal insufficiency. Our result is in keeping with the result of Shanghai Diabetic Complications Study in which 29.6% Shanghai Chinese diabetic patients had CKD and 26.2% had albuminuria [23]. Kong et al. [24] also reported that 15.6% Hong Kong Chinese type 2 diabetic patients had CKD. A community-based study of CKD among type 2 diabetic Chinese patients in Taiwan showed the prevalence of CKD was 38.0% [25]. However, Lu et al. [26] reported the prevalence of CKD was as high as 63.9% in downtown Shanghai, China. The marked variation in the prevalence of CKD might be due to the difference in study cohort characteristics and difference in GFR evaluation methods [25,26], while some studies did not include albuminuria in the evaluation [24]. Nevertheless, all the above studies suggested that CKD was common in Chinese population with type 2 diabetes.
Hypertension is a well recognized risk factor for diabetic patients to develop CKD. From the result of our study, hypertension is a prevalent condition in diabetic patients, particularly those with CKD. Up to 66.5% and 81.8% diabetic patients with CKD stage 1 to 2 and stage 3 to 4, respectively, have hypertension. Moreover, hypertension imposes the strongest risk, when compared to other risk factors identified, associated with CKD. United Kingdom Prospective Diabetes Study (UKPDS) has demonstrated that every 10 mm Hg reduction in systolic BP was associated with a 13% reduction in the risk of microvascular complications [27]. Our result supports that BP control is of crucial importance in diabetes management.
Duration of diabetes was the other risk factor associated with CKD. On the other hand, as an indicator of the average level of glucose, HbA1c was not associated with CKD in the present study. UKPDS study indicated a reduction of 1% in HbA1c was associated with a 37% decrease in microvascular endpoints [28]. However, in the glucose control and vascular complications in veterans with type 2 diabetes (VADT) study, patients in the intensive arm after a mean follow-up of 5.6 years did not show any benefit regarding changing serum creatinine or GFR values and only a minor effect on albuminuria levels was observed [29]. The same was observed in the intensive blood glucose control and vascular outcomes in patients with type 2 diabetes (ADVANCE) trial [30]. The group in the intensive arm after an average follow-up of 5 years only showed a minimal reduction in the number of cases with new-onset microalbuminuria compared to the standard therapy group (23.7% vs. 25.7%) and no effect was observed in the serum creatinine values [30]. The patients in VADT and ADVANCE study had an longer duration of diabetes (11.5 years and 8.0 years, respectively) compared to those in UKPDS study, in which all patients were newly diagnosed diabetes. This may be one of the major reasons causing the different results among these three studies and also demonstrated the importance of duration of diabetes in the incidence of CKD. In addition, the 10-year follow-up of the UKPDS study has shown that the effects of intensive glucose therapy on diabetes complications could be seen many years later [31]. Therefore, in order to prevent or delay progression of kidney disease in diabetes, earlier intervention in the disease course seemed to be far more important.
Anemia is common among those with diabetes. Ahmed et al. [32] reported in a large multiethnic cohort study with 65,696 diabetic patients that the prevalence of anemia was 28.0% in Asians and 33.6% in Whites. In our study, 24.7% subjects with type 2 diabetes had anemia. The patients with renal insufficiency had the lowest level of hemoglobin, and almost 60% of these subjects had anemia. It has been reported that anemia may contribute to the progression of kidney disease in diabetes [33]. A more recent study indicated that anemia was the risk factor for both renal prognosis and survival in patients with diabetes [34]. Our study also showed anemia being an independent associated factor with CKD in type 2 diabetic patients. Anemia in diabetic patients with CKD may mainly result from iron and erythropoietin deficiencies and hypo-responsiveness to the actions of erythropoietin [35]. In addition, renin-angiotensin system (RAS) inhibitors could cause a reversible decrease in hemoglobin concentration in patients with diabetes and CKD. Mohanram et al. [36] reported that long-term administration of losartan in a dose of 50 to 100 mg once daily in patients with type 2 diabetes was expected to have lower hemoglobin by about 10 g/L. In the present study, although more angiotensin converting enzyme inhibitors/angiotensin receptor blockers were prescribed in patients with anemia as compared those without anemia (data not shown), whether it is one of the reasons of anemia is worth exploring in the future.
Assessment of the association between GFR and urinary albumin excretion was scarcely reported in Chinese type 2 diabetic patients. In this study, we found that in subjects with a urinary ACR less than 90 mg/g, the average level of eGFR maintained relatively steadily but decreased rapidly in those with an urinary ACR more than 90 mg/g. This finding suggested that when patients presented with urinary ACR more than 90 mg/g, their kidney function might deteriorate very rapidly in the future. This finding was similar to that reported by Jia et al. [23] about patients with urinary ACR of around 100 mg/g, and that study used the same GFR estimating formula as ours. However, increases in AER and decreased GFR do not always occur in parallel. A substantial proportion of normoalbuminuria may present with a reduced GFR in diabetic patients [7,8]. Using eGFR alone as a screening test for CKD in diabetes is inadequate. Many patients with diabetes and CKD may have elevated or high normal GFRs, particularly in the early years after diagnosis. Therefore markers of kidney damage are required to detect early stages of CKD while eGFR alone may be able to detect CKD only at a later stage such as stage 3 or above [37]. Our study also showed that even in patients with renal insufficiency, 32.3% subjects presented with normoalbuminuria. These results suggested albuminuria could imply, but not necessarily confirm, the onset of a decline in GFR. Therefore, both parameters (GFR and urinary albumin) should be measured in assessing the severity of CKD in diabetes patients.
There were several limitations in our study. Firstly, our study is a cross-sectional study that precluded the exploration of a causal relationship between risk factors and the development of CKD. Secondly, we used MDRD to estimate GFR rather than the measurement GFR using iothalamate clearance to determine kidney function. However, a number of studies have demonstrated that eGFR is a reliable and commonly used method to assess kidney function [11-13]. Thirdly, the urinary ACR was checked only once. Because of potential variability in the UAE, ideally 3 specimens being collected within a 3 to 6 month period was recommended. Fourthly, when analyzing the association between eGFR and albuminuria, we had not taken into account the potential influence on the use of anti-hypertensive and/or lipid modifying agents, such as RAS inhibitors and statins [38,39]. Finally, since all our subjects were recruited from urban area, the study data may or may not be applicable to other diabetic patients in mainland China, not to mention the generalizability of our results to other populations that need to be interpreted with caution. Interestingly, Xu et al. [40] recently reported that among Chinese type 2 diabetic patients living in rural area, the rate of kidney diseases (including albuminuria) was 41.3%, which is similar if not even more prevalent than our figure being derived from urban area.
In conclusion, our study demonstrated that CKD and albuminuria were highly prevalent in the Chinese population with type 2 diabetes. Hypertension, duration of diabetes and anemia were the main risk factors associated with CKD. Screening programs using a combination of eGFR and the urinary ACR are necessary, especially among those diabetic patients at the middle or late phase of CKD, while urinary ACR only can be accepted for early cases with short history of disease. Strategies to prevent or delay progression of kidney disease in diabetes should be carried out at the early stages of CKD.
ACKNOWLEDGMENTS
We thank all medical and nursing staffs of Diabetes Care and Research Center of Jiangsu Province Institute of Geriatrics for the design and conduct of the study, and team members of Department of Medicine and Therapeutics, the Chinese University of Hong Kong for the guidance and comments. This study was supported by a grant from Jiangsu Province Science and Technology Department (BS2006073) and a grant from Jiangsu Province Health Department (H200931), China.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Burrows NR, Li Y, Williams DE. Racial and ethnic differences in trends of end-stage renal disease: United States, 1995 to 2005. Adv Chronic Kidney Dis. 2008;15:147–152. doi: 10.1053/j.ackd.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Iseki K. Chronic kidney disease in Japan. Intern Med. 2008;47:681–689. doi: 10.2169/internalmedicine.47.0906. [DOI] [PubMed] [Google Scholar]
- 3.So WY, Kong AP, Ma RC, Ozaki R, Szeto CC, Chan NN, Ng V, Ho CS, Lam CW, Chow CC, Cockram CS, Chan JC, Tong PC. Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes Care. 2006;29:2046–2052. doi: 10.2337/dc06-0248. [DOI] [PubMed] [Google Scholar]
- 4.Bruno G, Merletti F, Bargero G, Novelli G, Melis D, Soddu A, Perotto M, Pagano G, Cavallo-Perin P. Estimated glomerular filtration rate, albuminuria and mortality in type 2 diabetes: the Casale Monferrato study. Diabetologia. 2007;50:941–948. doi: 10.1007/s00125-007-0616-1. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh MC, Hsiao JY, Tien KJ, Chang SJ, Hsu SC, Liang HT, Chen HC, Lin SR, Tu ST. Chronic kidney disease as a risk factor for coronary artery disease in Chinese with type 2 diabetes. Am J Nephrol. 2008;28:317–323. doi: 10.1159/000111388. [DOI] [PubMed] [Google Scholar]
- 6.Targher G, Zoppini G, Chonchol M, Negri C, Stoico V, Perrone F, Muggeo M, Bonora E. Glomerular filtration rate, albuminuria and risk of cardiovascular and all-cause mortality in type 2 diabetic individuals. Nutr Metab Cardiovasc Dis. 2011;21:294–301. doi: 10.1016/j.numecd.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18:1353–1361. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 8.Premaratne E, MacIsaac RJ, Finch S, Panagiotopoulos S, Ekinci E, Jerums G. Serial measurements of cystatin C are more accurate than creatinine-based methods in detecting declining renal function in type 1 diabetes. Diabetes Care. 2008;31:971–973. doi: 10.2337/dc07-1588. [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Standards of medical care in diabetes: 2009. Diabetes Care. 2009;32(Suppl 1):S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rigalleau V, Lasseur C, Perlemoine C, Barthe N, Raffaitin C, Liu C, Chauveau P, Baillet-Blanco L, Beauvieux MC, Combe C, Gin H. Estimation of glomerular filtration rate in diabetic subjects: Cockcroft formula or modification of Diet in Renal Disease study equation? Diabetes Care. 2005;28:838–843. doi: 10.2337/diacare.28.4.838. [DOI] [PubMed] [Google Scholar]
- 12.Rigalleau V, Lasseur C, Raffaitin C, Perlemoine C, Barthe N, Chauveau P, Combe C, Gin H. Glucose control influences glomerular filtration rate and its prediction in diabetic subjects. Diabetes Care. 2006;29:1491–1495. doi: 10.2337/dc06-0407. [DOI] [PubMed] [Google Scholar]
- 13.Rigalleau V, Lasseur C, Perlemoine C, Barthe N, Raffaitin C, Chauveau P, Combe C, Gin H. Cockcroft-Gault formula is biased by body weight in diabetic patients with renal impairment. Metabolism. 2006;55:108–112. doi: 10.1016/j.metabol.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Shan G, He J China National Diabetes and Metabolic Disorders Study Group. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. 1999 World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension: guidelines subcommittee. J Hypertens. 1999;17:151–183. [PubMed] [Google Scholar]
- 16.Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Am J Clin Nutr. 1998;68:899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Assoication. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 19.Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults. Chinese guidelines on prevention and treatment of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:390–419. [PubMed] [Google Scholar]
- 20.Dirren H, Decarli B, Lesourd B, Schlienger JL, Deslypere JP, Kiepurski A. Nutritional status: haematology and albumin. Euronut SENECA investigators. Eur J Clin Nutr. 1991;45(Suppl 3):43–52. [PubMed] [Google Scholar]
- 21.Poulsen PL, Mogensen CE. Clinical evaluation of a test for immediate and quantitative determination of urinary albumin-to-creatinine ratio. A brief report. Diabetes Care. 1998;21:97–98. doi: 10.2337/diacare.21.1.97. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 23.Jia W, Gao X, Pang C, Hou X, Bao Y, Liu W, Wang W, Zuo Y, Gu H, Xiang K. Prevalence and risk factors of albuminuria and chronic kidney disease in Chinese population with type 2 diabetes and impaired glucose regulation: Shanghai diabetic complications study (SHDCS) Nephrol Dial Transplant. 2009;24:3724–3731. doi: 10.1093/ndt/gfp349. [DOI] [PubMed] [Google Scholar]
- 24.Kong AP, So WY, Szeto CC, Chan NN, Luk A, Ma RC, Ozaki R, Ng VW, Ho CS, Lam CW, Chow CC, Cockram CS, Chan JC, Tong PC. Assessment of glomerular filtration rate in addition to albuminuria is important in managing type II diabetes. Kidney Int. 2006;69:383–387. doi: 10.1038/sj.ki.5000061. [DOI] [PubMed] [Google Scholar]
- 25.Lin CH, Yang WC, Tsai ST, Tung TH, Chou P. A community-based study of chronic kidney disease among type 2 diabetics in Kinmen, Taiwan. Diabetes Res Clin Pract. 2007;75:306–312. doi: 10.1016/j.diabres.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Lu B, Song X, Dong X, Yang Y, Zhang Z, Wen J, Li Y, Zhou L, Zhao N, Zhu X, Hu R. High prevalence of chronic kidney disease in population-based patients diagnosed with type 2 diabetes in downtown Shanghai. J Diabetes Complications. 2008;22:96–103. doi: 10.1016/j.jdiacomp.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, Holman RR. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–419. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 30.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 31.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed AT, Go AS, Warton EM, Parker MM, Karter AJ. Ethnic differences in anemia among patients with diabetes mellitus: the Diabetes Study of Northern California (DISTANCE) Am J Hematol. 2010;85:57–61. doi: 10.1002/ajh.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babazono T, Hanai K, Suzuki K, Kiuchi Y, Inoue A, Tanaka M, Tanaka N, Hase M, Ishii A, Iwamoto Y. Lower haemoglobin level and subsequent decline in kidney function in type 2 diabetic adults without clinical albuminuria. Diabetologia. 2006;49:1387–1393. doi: 10.1007/s00125-006-0247-y. [DOI] [PubMed] [Google Scholar]
- 34.Sasatomi Y, Kaneoka H, Abe Y, Ishimura A, Ogahara S, Murata T, Uesugi N, Takebayashi S, Iwasaki H, Saito T. Anemia and hypertension are risk factors for both renal prognosis and survival in patients with diabetes mellitus. Clin Exp Nephrol. 2009;13:473–479. doi: 10.1007/s10157-009-0191-5. [DOI] [PubMed] [Google Scholar]
- 35.Mehdi U, Toto RD. Anemia, diabetes, and chronic kidney disease. Diabetes Care. 2009;32:1320–1326. doi: 10.2337/dc08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohanram A, Zhang Z, Shahinfar S, Lyle PA, Toto RD. The effect of losartan on hemoglobin concentration and renal outcome in diabetic nephropathy of type 2 diabetes. Kidney Int. 2008;73:630–636. doi: 10.1038/sj.ki.5002746. [DOI] [PubMed] [Google Scholar]
- 37.KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 suppl 2):S12–154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsarinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 39.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Charlton-Menys V, DeMicco DA, Fuller JH CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS) Am J Kidney Dis. 2009;54:810–819. doi: 10.1053/j.ajkd.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 40.Xu R, Zhong YH, Chen B, Yuan M, Fang Y, Lin J, Jiang SH, Xu XL, Gong SM, Heng YY, Ding XQ, Jin TY. The prevalence and risk factors of kidney disease in type 2 diabetic patients in rural Shanghai. Zhonghua Nei Ke Za Zhi. 2012;51:18–23. [PubMed] [Google Scholar]