Abstract
Treatment of nosocomial infections is becoming difficult due to the increasing trend of antibiotics resistance. Current knowledge on antibiotic resistance pattern is essential for appropriate therapy. We aimed to evaluate antibiotic resistance profiles in nosocomial bloodstream and urinary tract pathogens. A total of 129 blood stream and 300 urinary tract positive samples were obtained from patients referring to Besat hospital over a two-year period (2009 and 2010). Antibiotic sensitivity was ascertained using the Kirby-Bauer disk diffusion technique according to CLSI guidelines. Patient's data such as gender and age were recorded. The ratio of gram-negative to gram-positive bacteria in BSIs was 1.6 : 1. The most prevalent BSI pathogen was Coagulase-Negative Staphylococci (CoNS). The highest resistance rate of CoNS was against penicillin (91.1%) followed by ampicillin (75.6%), and the lowest rate was against vancomycin (4.4%). Escherichia coli was the most prevalent pathogen isolated from urinary tract infections (UTIs). Ratio of gram-negative to gram-positive bacteria was 3.2 : 1. The highest resistance rate of E. coli isolates was against nalidixic acid (57.7%). The present study showed that CoNS and E. coli are the most common causative agents of nosocomial BSIs and UTIs, and control of infection needs to be addressed in both antibiotic prescription and general hygiene.
1. Introduction
Nosocomial or hospital-acquired infections are defined as infections which are acquired during the hospital stay. Nosocomial infections are usually defined as infections that are identified at least 48–72 hours following admission to health institutions [1]. Nosocomial infections are also important public health problems in developing countries as well as in developed countries [2]. The most frequent types of nosocomial infections are urinary tract infection (UTI), surgical-wound infection, pneumonia, and bloodstream infection (BSI) [3]. BSIs are responsible for approximately 10–30% of the cases [4]. UTI is the presence of bacteria in the urine (bacteriuria) and defined as the growth of a single pathogen of >105 colony-forming units/mL from properly collected mid-stream urine specimens [5]. The common bacterial pathogens present in the BSIs and UTIs are Staphylococcus aureus, Coagulase-Negative Staphylococci (CoNS), Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, Enterobacter spp., Enterococcus spp., and Acinetobacter spp. [6]. As the result of extensive uses of antimicrobial agents, nosocomial pathogens have shifted away from easily treatable bacteria towards more resistant bacteria. This change is important problem for nosocomial infection control and prevention [7]. Area-specific monitoring studies aimed to gain knowledge about type of pathogens and antimicrobial resistance patterns can optimize treatment and decrease mortality rates [8, 9]. In Iran, broad-spectrum antibiotics are commonly used in the hospitals, and there is limited data on antibiotic resistance. The present study was aimed to ascertain the resistance patterns of the most common bacterial isolates from hospital-acquired bloodstream and urinary tract infections over a two-year period.
2. Materials and Methods
2.1. Subjects
This study was conducted between January 2009 and December 2010 at Besat Hospital affiliated to Artesh University of Medical Sciences, Tehran, Iran. During the study, all patients admitted to different hospital wards with suspected nosocomial BSI or UTI were included. Patients who had been admitted for more than 48 h, with no sign of bacterial colonization at the time of admission were included. Clinical examination was conducted by physician to exclude community-acquired infections. Nosocomial UTI patients included in this study were classified in four age groups. This study was approved by the Ethics Committee of the Besat Hospital in Tehran.
2.2. Sample Collection and Bacterial Identification
For BSI, an appropriate volume of blood sample was collected with aseptic technique. Two independent blood samples for a patient were recruited. Obtained samples were inoculated into Castaneda (biphasic blood culture bottles) medium, immediately and incubated in aerobic conditions with and without CO2. Primary check was done after 24 hours. Constitutive check was performed until 21 days. Subculture on ship blood, chocolate, and Mac Conkey agar plates was performed for suspected Castaneda inoculated specimens [10, 11]. Multimicrobial growth on culture media was excluded. Nonrepetitive positive cultures were recruited for study. In UTI suspected patients, the midstream specimens of urine were taken following the recommendations of Kass [12]. The plates were incubated in aerobic atmosphere at 37°C for 24–48 hrs. Presence of more than 105 (cfu/mL) bacteria signified as UTI. Isolated bacteria were microbiologically identified with standard biochemical identification methods [13, 14].
2.3. Antibiotic Susceptibility Testing
Antibiotic susceptibility testing was performed by Kirby-Bauer's disk diffusion method on Muller-Hinton agar (Hi Media, Mumbai, India) in accordance with the standards of the Clinical Laboratory Standards Institute (CLSI, formerly National Committee for Clinical Laboratory Standards [NCCLS]) guidelines [15]. The antibiotic concentration per disk was as follows: penicillin 10 units, ampicillin 10 μg, erythromycin 5 μg, ceftazidime 30 μg, ciprofloxacin 5 μg, cotrimoxazole 25 μg, gentamycin 10 μg, tetracycline 10 μg, amikacin 30 μg, imipenem 10 μg, nitrofurantoin 30 μg, nalidixic acid 30 μg, clindamycin 2 μg, oxacillin 1 μg, cefoxitin 10 μg, cephalothin 30 μg, and vancomycin 30 μg. S. aureus ATCC 25923 and E. coli ATCC 25922 were used as control strains.
2.4. Statistical Analysis
Data entry and statistical analysis were performed using SPSS v.13 software. Comparisons were made using Pearson Chi-square and Fisher exact tests. A P value of <0.05 was considered indicative of a statistically significant difference.
3. Results
3.1. Nosocomial BSI
A total of 1200 blood samples which were obtained from nosocomial BSI of suspected patients were analyzed. Among them, 129 (10.7%) were documented BSI with monomicrobial origin. Of the 129 patients, 61 (47.3%) were females and 68 (52.7%) were males (P > 0.05).
CoNS (34.8%) and E. coli (29.4%) were the most prevalent microorganisms isolated from nosocomial BSI patients followed by K. pneumoniae (11%), Acinetobacter spp. (8.5%), Enterobacter spp. (7%), S. aureus (3.9%), P. aeruginosa (3.9%), and Proteus vulgaris (1.5%). Figure 1 represents the gender wise prevalence of bacteria isolated from blood cultures. No significant correlation was observed between gender and BSIs (P > 0.05). The highest resistance rate of the CoNS was against penicillin (91.1%) followed by ampicillin (75.6%), and the lowest rate was against vancomycin (4.4%). Proteus vulgaris was the least prevalent bacteria isolated from blood cultures and had a high sensitivity to different antibiotics (data not shown). E. coli had the highest resistance rates to tetracycline (63.2%) and ampicillin (63.2%), while imipenem and gentamycin showed the lowest level of resistance (15.8%). The resistance rates of bacteria isolated from nosocomial BSI against different antibiotics are presented in Table 1.
Figure 1.
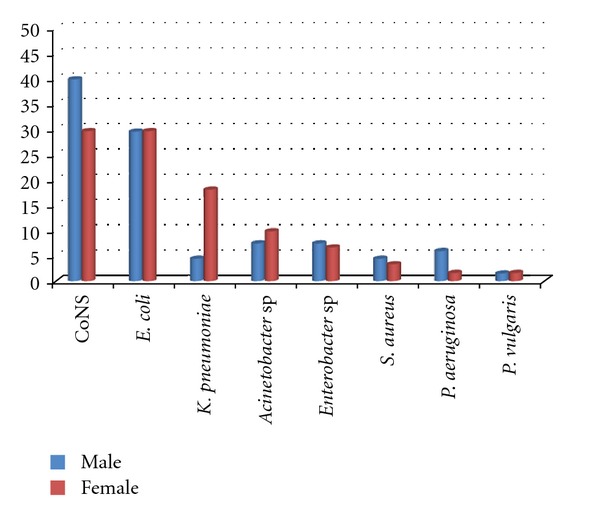
Frequency and distribution of bacterial isolates from blood cultures. CoNS*: Coagulase-Negative Staphylococci.
Table 1.
Antimicrobial resistance profiles of hospital-acquired bacterial isolates from clinical blood specimens.
| Antimicrobial agents | Microorganisms | Total n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| CoNS* n (%) |
E. coli
n (%) |
K. pneumonia
n (%) |
Aceinetobacter spp.n (%) |
En
te
ro
ba
c
te
r
spp. n (%) |
S. aureus
n (%) |
P. aeruginosa
n (%) |
||
| Ciprofloxacin | 22 (48.9) | 18 (47.4) | 6 (42.9) | 5 (45.5) | 1 (11.1) | 2 (40) | 2 (40) | 56 (43.4) |
| Ceftazidime | 18 (40) | 15 (39.5) | 6 (42.9) | 6 (54.5) | 3 (33.3) | 3 (60) | 3 (60) | 54 (41.9) |
| Amikacin | 20 (44.4) | 6 (15.8) | 6 (42.9) | 6 (54.5) | 0 (0) | 3 (60) | 3 (60) | 44 (34.1) |
| Imipenem | 16 (35.6) | 6 (15.8) | 6 (42.9) | 6 (54.5) | 2 (22.2) | 4 (80) | 4 (80) | 44 (34.1) |
| Clindamycin | 28 (62.2) | 19 (50) | 6 (42.9) | 6 (54.5) | 2 (22.2) | 3 (60) | 4 (80) | 68 (52.7) |
| Vancomycin | 2 (4.4) | NA* | NA | NA | NA | 0 (0) | NA | 2 (1.6) |
| Cephalothin | 22 (48.9) | 16 (42.1) | 9 (64.3) | 9 (81.8) | 3 (33.3) | 2 (40) | 3 (60) | 64 (49.6) |
| Ampicillin | 34 (75.6) | 24 (63.2) | 7 (50) | 7 (63.6) | 4 (44.4) | 5 (100) | 5 (100) | 86 (66.7) |
| Cotrimoxazole | 26 (57.8) | 19 (50) | 12 (85.7) | 7 (63.6) | 1 (11.1) | 5 (100) | 5 (100) | 75 (58.1) |
| Cefoxitin | 26 (57.8) | 17 (44.7) | 8 (47.1) | 6 (54.5) | 2 (22.2) | 4 (80) | 4 (80) | 67 (51.9) |
| Oxacillin | 28 (62.2) | NA | NA | NA | NA | 2 (40) | NA | 30 (23.3) |
| Erythromycin | 17 (37.8) | 13 (34.2) | 6 (42.9) | 4 (36.4) | 1 (11.1) | 3 (60) | 4 (80) | 48 (37.2) |
| Tetracycline | 23 (51.1) | 24 (63.2) | 9 (64.3) | 5 (45.5) | 6 (66.6) | 4 (80) | 5 (100) | 76 (58.9) |
| Penicillin | 41 (91.1) | 22 (58) | 11 (78.6) | 7 (63.6) | 4 (44.4) | 5 (100) | 5 (100) | 95 (73.6) |
| Gentamicin | 16 (35.6) | 6 (15.8) | 4 (28.6) | 7 (63.6) | 2 (22.2) | 4 (80) | 2 (40) | 41 (31.8) |
|
| ||||||||
| Total | 45 (34.8) | 38 (29.4) | 14 (11) | 11 (8.5) | 9 (7) | 5 (3.9) | 5 (3.9) | 127 (98.5) |
CoNS*: Coagulase-negative Staphylococci.
NA*: Not applicable.
3.2. Nosocomial UTI
A total of 1000 urine samples which were obtained from patients suspected of nosocomial UTI were analyzed. Among them, 300 samples (30%) were distinguished as UTIs, which were isolated from 204 (68%) females and 96 (32%) males (P < 0.05), respectively (mean age: 46.8 years, range: 15–91 years). The most frequency of UTIs (22.7%) was found in females with mean age of >60 yrs (Table 3). E. coli (66.7%) was the most common gram-negative bacilli isolated from nosocomial UTI patients followed by CoNS (11.7%), S. aureus (6.7%), Proteus vulgaris (6.7%), Enterococcus spp. (5%), P. aeruginosa (1.7%), and Enterobacter spp. (1.7%). The highest resistance rate of E. coli was against nalidixic acid (57.7%). Results of the resistance rates to different antibiotics were showed in Table 2.
Table 3.
Age and gender distribution in hospital-acquired UTI patients.
| Gender | Age groups | Total n (%) | |||
|---|---|---|---|---|---|
| <20 n (%) |
20–40 n (%) |
40–60 n (%) |
>60 n (%) |
||
| Male | 15 (5) | 24 (8) | 26 (8.6) | 31 (10.3) | 96 (32) |
| Female | 30 (10) | 51 (17) | 55 (18.3) | 68 (22.7) | 204 (68) |
|
| |||||
| Total | 45 (15) | 75 (25) | 81 (26.9) | 99 (33) | 300 (100) |
Table 2.
Antimicrobial resistance profiles of hospital-acquired bacterial isolates from clinical urine specimens.
| Antimicrobial agents | Microorganisms | Total n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| CoNS* n (%) |
E. coli
n (%) |
P. vulgaris
n (%) |
Enterococcus sp. n (%) |
En
te
ro
ba
c
te
r sp. n (%) |
S. aureus
n (%) |
P. aeruginosa
n (%) |
||
| Ciprofloxacin | 20 (57.1) | 80 (40) | 10 (50) | 7 (46.6) | 2 (40) | 3 (15) | 3 (60) | 125 (41.6) |
| Ceftazidime | 15 (42.8) | 18 (9) | 8 (40) | 4 (26.6) | 1 (20) | 4 (20) | 3 (60) | 53 (17.7) |
| Amikacin | 22 (62.8) | 5 (2.5) | 7 (35) | 6 (40) | 1 (20) | 5 (25) | 3 (60) | 49 (16.3) |
| Imipenem | 10 (28.5) | 5 (2.5) | 4 (20) | 2 (13.3) | 0 (0) | 5 (25) | 2 (40) | 28 (9.3) |
| Clindamycin | 20 (57.1) | 19 (9.5) | 4 (20) | 8 (53.3) | 1 (20) | 6 (30) | 3 (60) | 61 (20.3) |
| Nalidixic acid | 7 (20) | 115 (57.7) | 7 (35) | 7 (46.6) | 1 (20) | 6 (30) | 4 (80) | 147 (49) |
| Cephalothin | 18 (51.4) | 20 (10) | 8 (40) | 10 (66.6) | 1 (20) | 6 (30) | 4 (80) | 67 (22.3) |
| Ampicillin | 34 (97.1) | 80 (40) | 14 (70) | 12 (80) | 2 (40) | 18 (90) | 5 (100) | 165 (55) |
| Cotrimoxazole | 20 (57.1) | 100 (50) | 10 (50) | 10 (66.6) | 1 (20) | 10 (50) | 3 (60) | 154 (51.3) |
| Nitrofurantoin | 28 (80) | 80 (40) | 13 (65) | 7 (46.6) | 2 (40) | 8 (40) | 3 (60) | 141 (47) |
| Erythromycin | 15 (42.8) | 43 (21.5) | 7 (35) | 4 (26.6) | 1 (20) | 12 (60) | 2 (40) | 84 (28) |
| Tetracycline | 25 (71.4) | 24 (12) | 3 (15) | 5 (33.3) | 1 (20) | 10 (50) | 3 (60) | 71 (23.7) |
| Penicillin | 35 (100) | 55 (27.5) | 12 (60) | 13 (86.6) | 3 (60) | 18 (90) | 5 (100) | 141 (47) |
| Gentamicin | 10 (28.5) | 10 (5) | 6 (30) | 9 (60) | 2 (40) | 9 (45) | 2 (40) | 48 (16) |
|
| ||||||||
| Total | 35 (11.7) | 200 (66.7) | 20 (6.7) | 15 (5) | 5 (1.7) | 20 (6.7) | 5 (1.7) | 300 (100) |
CoNS*: Coagulase-negative Staphylococci.
4. Discussion
Nosocomial infections occur worldwide and affect both developed and developing countries [16]. Many of these infections are associated with microorganisms that are resistant to antibiotics and can easily spread by hospital personnel [17]. Guidelines for antibiotic therapy can be helpful for clinicians to select more appropriate antibiotics for effective treatment and prevent the development of drug resistance. This study shows the distribution of antibiotic resistance pattern of bacterial species isolated from patients with nosocomial BSI or UTI at a hospital in Tehran, Iran.
This study revealed that 129 (10.7%) out of 1200 bloodstream samples which were obtained from nosocomial BSI suspected patients were positive. Of the 129 patients, 61 (47.3%) were females and 68 (52.7%) were males (P > 0.05). The CoNS (34.8%) and E. coli (29.4%) were the most prevalent microorganisms that have been isolated. Similar findings have been observed in Turkey and in a Children's Medical Center, Iran [18, 19]. A study in Brazil revealed the predominance of S. aureus (14%) followed by CoNS (12.6%) and Klebsiella (12%) [20]. In several studies, CoNS followed by S. aureus comprised the most prevalent bacteria isolated from BSIs [21–23]. In our study, gram-negative bacteria were more regularly involved in nosocomial BSI than gram-positive bacteria (P < 0.05). This finding is in accordance with the results of recent studies [24]. Based on our data, the highest resistance rate of the CoNS was against penicillin followed by ampicillin and oxacillin (Table 1). Oxacillin-resistant staphylococcus spp. are an increasing global problem in nosocomial infections [25–27]. Oxacillin-resistant strains show the high level of resistance to penicillin, cephalosporins, and other beta-lactams like imipenem. In the present study, 62% of the isolated CoNS were oxacillin resistant with the high rates of resistance to penicillin, cephalothin, and imipenem (100%, 100%, and 62%, resp.). E. coli strains which were isolated from BSI patients had the highest resistance rates to tetracycline and ampicillin (63.2%), which is similar to the recent study from Ireland [28]. In the present study, the E. coli strains which were isolated from BSI patients showed a resistance rate of 47.4% to ciprofloxacin which is consistent with the other studies from Iran [29, 30]. In our study, the 8.5% of microorganisms which were isolated from nosocomial BSI patients were Acinetobacter spp. The Acinetobacter spp. isolates showed the highest resistance rate to cephalothin (81.8%) followed by cotrimoxazole and gentamicin (63.6%) (Table 1). Reports of Acinetobacter spp. bacteremia are increasing, especially from Asian countries and neighboring countries of Iran such as Iraq, Kuwait, Turkey, and Afghanistan [31–33]. A recent surveillance study from Iran reported that Acinetobacter spp. were the most frequently isolated bacteria in the hospital and community-acquired BSIs (32%) followed by E. coli (13.7%) and Klebsiella sp. (12%), respectively [28]. In the present study, vancomycin was the most effective antibiotic against CoNS (95.6% susceptible) and S. aureus (100% susceptible). This is in agreement with another study performed in Iran [34]. In our study, statistical analysis showed a significant correlation between nosocomial UTI prevalence and gender (P < 0.05). It has been extensively reported that adult women have a higher prevalence of UTI than men because of anatomic and physical situations [35, 36].
The present study indicates that E. coli is still the most common cause of nosocomial UTI. This finding is consistent with the other studies from Iran and other countries [35, 37, 38]. The highest resistance rate of E. coli isolate which was obtained from urine samples was against nalidixic acid followed by cotrimoxazole, ciprofloxacin, and ampicillin, respectively (Table 2). These results were predictable because these antibiotics have been used as a long time in our hospital. In this study, amikacin and imipenem had the widest coverage against E. coli isolates (97.5%). In a recent surveillance study in Iran authors reported that the highest resistance rate of E. coli isolates which were obtained from various clinical specimens at 11 hospitals was against tetracycline followed by amoxicillin and penicillin, respectively [39].
The rate of nosocomial UTI is determined by the interactions of several factors such as primary disease and its severity, duration of hospitalization and treatment, and invasive interventions like use of urinary catheters; Moreover, the incidence of nosocomial UTI has been increasing and its treatment has become more complicated because of the pathogens with increasing resistance to antibiotics [40, 41].
5. Conclusion
It is concluded that in our hospital gram-negative bacteria were more frequently involved in nosocomial BSI than gram-positive bacteria. CoNS and E. coli were the most common isolated bacteria from blood cultures and included the 64% of total isolates. Vancomycin was the most effective antibiotic against gram-positive bacteria, and gentamicin, amikacin, and imipenem are proposed for treatment of nosocomial UTI caused by Gram-negative bacteria. E. coli was the most common cause of nosocomial UTI in our hospital and imipenem and aminoglycosides (gentamicin, amikacin) were the most effective antibiotics against this infection. Finally, to reduce the incidence of nosocomial infections, the appropriate use of antibiotics according to the standard antimicrobial susceptibility tests is proposed.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgments
The authors would like to thank Dr. Aghighi, Dr. Keyghobad, and the laboratory personnel of Besat Hospital for their excellent assistance.
References
- 1.Saraçli MA, Baysallar M, Gun H. Nosocomial uropathogens and their antibiotic susceptibilities in a Turkish Military Hospital: a prospective and microbiological study. Turkish Journal of Medical Sciences. 1999;29(2):165–168. [Google Scholar]
- 2.Celik I, Inci N, Denk A, Sevim E, Yasar D, Yasar M. Prevalence of Hospital acquired infections in Anesthesiology intensive care unit. Fırat Tıp Dergisi. 2005;10(3):132–135. [Google Scholar]
- 3.Nguyen Q. Hospital-Acquired Infections. New York, NY, USA: Medicine from WEBMD eMedicine; 2004. [Google Scholar]
- 4.Arnoni MV, Berezin EN, Martino MDV. Risk factors for nosocomial bloodstream infection caused by multidrug resistant gram-negative bacilli in pediatrics. Brazilian Journal of Infectious Diseases. 2007;11(2):267–271. doi: 10.1590/s1413-86702007000200020. [DOI] [PubMed] [Google Scholar]
- 5.Prais D, Straussberg R, Avitzur Y, Nussinovitch M, Harel L, Amir J. Bacterial susceptibility to oral antibiotics in community acquired urinary tract infection. Archives of Disease in Childhood. 2003;88(3):215–218. doi: 10.1136/adc.88.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleven BEE, Palka-Santini M, Gielen J, Meembor S, Krönke M, Krut O. Identification and characterization of bacterial pathogens causing bloodstream infections by DNA microarray. Journal of Clinical Microbiology. 2006;44(7):2389–2397. doi: 10.1128/JCM.02291-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain A, Awasthi A, Kumar M. Etiological and antimicrobial susceptibility profile of nosocomial blood stream infections in neonatal intensive care unit. Indian Journal of Medical Microbiology. 2007;25(3):299–300. doi: 10.4103/0255-0857.34783. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118(1):146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 9.Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically III patients. Chest. 1999;115(2):462–474. doi: 10.1378/chest.115.2.462. [DOI] [PubMed] [Google Scholar]
- 10.Kassa-Kelembho E, Mbolidi CD, Service YB, Morvan J, Minssart P. Bacteremia in adults admitted to the Department of Medicine of Bangui Community Hospital (Central African Republic) Acta Tropica. 2003;89(1):67–72. doi: 10.1016/j.actatropica.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Mantur BG, Mangalgi SS. Evaluation of conventional Castaneda and lysis centrifugation blood culture techniques for diagnosis of human brucellosis. Journal of Clinical Microbiology. 2004;42(9):4327–4328. doi: 10.1128/JCM.42.9.4327-4328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girou E, Rioux C, Brun-Buisson C, Lobel B. The postoperative bacteriuria score: a new way to predict nosocomial infection after prostate surgery. Infection Control and Hospital Epidemiology. 2006;27(8):847–854. doi: 10.1086/506398. [DOI] [PubMed] [Google Scholar]
- 13.Forbes B, Sahm D, Weissfeld A. Bailey & Scott’s DiagnoStic Microbiology-Text and Study Guide Package. 12th edition. New York, NY, USA: Elsevier; 2007. [Google Scholar]
- 14.Mahon C, Lehman D, Manuselis G. Text Book of Diagnostic Microbiology. 4th edition. New York, NY, USA: Elsevier; 2011. [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Approved Standards. M7- A5. Wayne, Pa, USA: National Committee for Clinical Laboratory Standards; 2000. Prefomance standards for antimicrobial disk susceptibility tests, 5th ed. [Google Scholar]
- 16.World Health Organization. Prevention of Hospital-Acquired Infections. A Practical Guide. 2nd edition. Geneva, Switzerland: WHO Press; 2002. [Google Scholar]
- 17.Durmaz B, Durmaz R, Otlu B, Sonmez E. Nosocomial infections in a new medical center, Turkey. Infection Control and Hospital Epidemiology. 2000;21(8):534–536. doi: 10.1086/501803. [DOI] [PubMed] [Google Scholar]
- 18.Dogru A, Sargin F, Çelik M, Sagiroglu AE, Goksel MM, Sayhan H. The rate of device-associated nosocomial infections in a medical surgical intensive care unit of a training and research hospital in Turkey: one-year outcomes. Japanese Journal of Infectious Diseases. 2010;63(2):95–98. [PubMed] [Google Scholar]
- 19.Pourakbari B, Sadr A, Ashtiani MT, et al. Five-year evaluation of the antimicrobial susceptibility patterns of bacteria causing bloodstream infections in Iran. Journal of Infection in Developing Countries. 2012;6(2):120–125. doi: 10.3855/jidc.1517. [DOI] [PubMed] [Google Scholar]
- 20.Marra AR, Camargo LFA, Pignatari ACC, et al. Nosocomial bloodstream infections in Brazilian hospitals: analysis of 2,563 cases from a prospective nationwide surveillance study. Journal of Clinical Microbiology. 2011;49(5):1866–1871. doi: 10.1128/JCM.00376-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitt P, Adamson V, Lõivukene K, et al. Epidemiology of nosocomial bloodstream infections in Estonia. Journal of Hospital Infection. 2009;71(4):365–370. doi: 10.1016/j.jhin.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Moyo S, Aboud S, Kasubi M, Maselle SY. Bacteria isolated from bloodstream infections at a tertiary hospital in Dar es Salaam, Tanzania-antimicrobial resistance of isolates. South African Medical Journal. 2010;100(12):835–838. doi: 10.7196/samj.4186. [DOI] [PubMed] [Google Scholar]
- 23.Richards M, Thursky K, Buising K. Epidemiology, prevalence, and sites of infections in intensive care units. Seminars in Respiratory and Critical Care Medicine. 2003;24(1):3–22. doi: 10.1055/s-2003-37913. [DOI] [PubMed] [Google Scholar]
- 24.Diekema DJ, Pfaller MA, Jones RN, et al. Trends in antimicrobial susceptibility of bacterial pathogens isolated from patients with bloodstream infections in the USA, Canada and Latin America. International Journal of Antimicrobial Agents. 2000;13(4):257–271. doi: 10.1016/s0924-8579(99)00131-4. [DOI] [PubMed] [Google Scholar]
- 25.Diekema DJ, Pfaller MA, Schmitz FJ, et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clinical Infectious Diseases. 2001;32(supplement 2):S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 26.Kang CI, Kim SH, Wan BP, et al. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrobial Agents and Chemotherapy. 2005;49(2):760–766. doi: 10.1128/AAC.49.2.760-766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar S, Rizvi M, Vidhani S, Sharma VK. Changing face of septicaemia and increasing drug resistance in blood isolates. Indian Journal of Pathology and Microbiology. 2004;47(3):441–446. [PubMed] [Google Scholar]
- 28.Barati M, Taher MT, Abasi R, Zadeh MM, Barati M, Shamshiri AR. Bacteriological profile and antimicrobial resistance of blood culture isolates. Iranian Journal of Clinical Infectious Diseases. 2009;4(2):87–95. [Google Scholar]
- 29.Nakhjavani FA, Mirsalehian A, Hamidian M, Kazemi B, Mirafshar M, Jabalameli F. Antimicrobial susceptibility testing for Escherichia coli strains to fluoroquinolones, in urinary tract infections. Iranian Journal of Public Health. 2007;36(1):89–92. [Google Scholar]
- 30.Cheng B, Xie G, Yao S, et al. Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Critical Care Medicine. 2007;35(11):2538–2546. doi: 10.1097/01.CCM.0000284492.30800.00. [DOI] [PubMed] [Google Scholar]
- 31.Houang ETS, Chu YW, Leung CM, et al. Epidemiology and infection control implications of Acinetobacter spp. in Hong Kong. Journal of Clinical Microbiology. 2001;39(1):228–234. doi: 10.1128/JCM.39.1.228-234.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munoz-Price LS, Weinstein RA. Acinetobacter infection. New England Journal of Medicine. 2008;358(12):1214–1281. doi: 10.1056/NEJMra070741. [DOI] [PubMed] [Google Scholar]
- 33.Tien HC, Battad A, Bryce EA, et al. Multi-drug resistant Acinetobacter infections in critically injured Canadian forces soldiers. BMC Infectious Diseases. 2007;7:p. 95. doi: 10.1186/1471-2334-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdollahi A, Moradi Tabrizi H, Mahfoozi S. Frequency of pathogens and antimicrobial susceptibility of bacteria isolated from bloodstream infections. Iran Journal of Pathology. 2010;5(3):143–149. [Google Scholar]
- 35.Kang CI, Chung DR, Son JS, Ko KS, Peck KR, Song JH. Clinical significance of nosocomial acquisition in urinary tract-related bacteremia caused by gram-negative bacilli. American Journal of Infection Control. 2011;39(2):135–140. doi: 10.1016/j.ajic.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 36.Kumar M, Lakshmi V, Rajagopalan R. Occurrence of extended spectrum β-lactamases among Enterobacteriaceae spp. isolated at a tertiary care Institute. Indian Journal of Medical Microbiology. 2006;24(3):208–211. [PubMed] [Google Scholar]
- 37.Mohammadtaheri Z, Pourpaki M, Mohammadi F, Namdar R, Masjedi MR. Surveillance of antimicrobial susceptibility among bacterial isolates from Intensive Care Unit Patients of a Tertiary-Care University Hospital in Iran: 2006–2009. Chemotherapy. 2010;56(6):478–484. doi: 10.1159/000321032. [DOI] [PubMed] [Google Scholar]
- 38.Foxman B. The epidemiology of urinary tract infection. Nature Reviews Urology. 2010;7(12):653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 39.Ahsan B, Beiranvand S, Abdulmaleki N, Mohamadi H, Kalantar E. A surveillance study of antimicrobial susceptibility in 11 hospitals in Kurdistan Province. African Journal of Microbiology Research. 2011;5(20):3157–3161. [Google Scholar]
- 40.Savas L, Guvel S, Onlen Y, Savas N, Duran ND. Nosocomial urinary tract infections: micro-organisms, antibiotic sensitivities and risk factors. West Indian Medical Journal. 2006;55(3):188–193. doi: 10.1590/s0043-31442006000300011. [DOI] [PubMed] [Google Scholar]
- 41.Bean DC, Krahe D, Wareham DW. Antimicrobial resistance in community and nosocomial Escherichia coli urinary tract isolates, London 2005-2006. Annals of Clinical Microbiology and Antimicrobials. 2008;7:p. 13. doi: 10.1186/1476-0711-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


