Abstract
Fifty-two healthy adult male and female volunteers were enrolled in this double-blind study to determine the maximum tolerated dose, characterize the pharmacokinetics, and obtain serum bactericidal activity (SBA) data for intravenous dalbavancin. Subjects were assigned to single- or multiple-dose groups and randomized to receive dalbavancin or placebo intravenously over 30 min. Doses started at 140 mg in the single-dose group and with a 300-mg loading dose (LD), followed by six daily 30-mg maintenance doses (MDs), in the multiple-dose cohort and escalated to a 1,120-mg single dose and a 1,000-mg LD and 100-mg MD regimen. Plasma, urine, and skin blister fluid aspirate drug concentrations were measured, and pharmacokinetic parameters were determined via noncompartmental methods. SBA against methicillin-resistant Staphylococcus aureus (MRSA) was determined at several time points. Adverse events and changes from the baseline for laboratory data, electrocardiograms, audiologic assessments, physical examinations, and vital signs were assessed. Concentrations increased in proportion to the dose. Steady-state concentrations were achieved by day 3 with the 10:1 LD-MD regimen. The half-life averaged 181 h, and the mean volume of distribution and clearance were 9.75 liters and 0.0473 liters/h, respectively. Mean values were similar in all groups and in males and females. The portion of the dose excreted renally averaged 33.5%. Bactericidal activity was demonstrated in serum at 7 days in all subjects receiving single doses of ≥500 mg. All doses were well tolerated. Dose-limiting toxicity was not encountered. No changes in auditory or vestibular function occurred. The long half-life and maintenance of SBA against MRSA for 1 week suggest that weekly dosing may be feasible.
Nosocomial infections with multiple-drug-resistant organisms such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci, and penicillin-resistant Streptococcus pneumoniae pose a particular challenge to clinicians. An increase in the incidence of serious infections caused by resistant gram-positive organisms (4, 8, 11) has led to the need for new antibiotics with activity against these pathogens.
Dalbavancin (also known as BI 397) is a novel semisynthetic glycopeptide antibiotic obtained by chemical modification of a natural glycopeptide (A40926). Although dalbavancin has the same mechanism of action as vancomycin (7), some vancomycin-resistant enterococci (3) and staphylococci with intermediate sensitivity to glycopeptides (C. J. Hackbarth, S. Lopez, G. Romano, A. Malabarba, J. Trias, and R. White, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1283, 1999) remain sensitive to dalbavancin.
Dalbavancin has demonstrated in vitro activity against more than 6,000 strains of gram-positive bacteria (3, 5, 6, 10a). The minimum concentration that inhibited 90% of the isolates (MIC90) ranged from 0.06 to 0.5 mg/ml for methicillin-sensitive S. aureus, MRSA, and coagulase-negative staphylococci and from 0.03 to 0.13 mg/ml for streptococci. Dalbavancin is also active against several anaerobic gram-positive bacteria, including Clostridium sp., Peptostreptococcus sp., and Actinomyces sp. Dalbavancin is also active against Corynebacterium sp. and Bacillus subtilis. The MICs of dalbavancin against staphylococci are generally lower than those of vancomycin, teicoplanin (3), linezolid (10a), and quinupristin-dalfopristin (6). Several immunocompetent and immunocompromised animal infection models have demonstrated the in vivo efficacy of dalbavancin (3). As expected, dalbavancin is not active against most gram-negative species (6).
An initial phase 1, randomized, double-blind, single- and multiple-dose escalation study was conducted with 23 normal male subjects (R. J. White, G. L. Brown, M. Cavaleri, and G. Romano, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2196, 2000). Levels of dalbavancin in plasma showed dose-related increases following administration of single (70, 140, 220, and 360 mg) and multiple (70-mg daily dose for 7 days) intravenous doses of dalbavancin. The 24-h trough values following multiple-dose administration showed an approximately fivefold increase from day 1 to day 7. The terminal half-life (t1/2) of dalbavancin ranged from 123 to 210 h. Dalbavancin was well tolerated, as evidenced by no clinically significant changes in vital signs, electrocardiogram (ECG), or laboratory assessments during the 28-day follow-up period.
The present study was conducted to further assess the safety and tolerance of intravenous dalbavancin administration following administration of single and multiple doses in healthy volunteers and to establish the maximum tolerated dose. Additionally, the serum bactericidal activity (SBA) against two strains of MRSA was determined.
(These data were presented in part at the 41st Interscience Conference on Antimicrobial Agents Chemotherapy, Chicago, Ill., 16 to 19 December 2001 [Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-951 and A-2192, 2001].)
MATERIALS AND METHODS
This study was conducted from September 2000 to May 2001 at the Clinical Research Center, Robert Wood Johnson Medical School, University of Medicine and Dentistry of New Jersey. The protocol was approved by the Institutional Review Board of the Robert Wood Johnson Medical School, University of Medicine and Dentistry of New Jersey. Each subject gave witnessed, written informed consent prior to undergoing any study procedures.
Selection of subjects
Healthy males or females between 18 and 55 years of age were eligible for enrollment. All subjects had to be surgically sterile, postmenopausal (female), or using effective contraception. Each subject had to have a normal audiologic assessment at baseline. Subjects were excluded for significant prior or current exposure to aminoglycoside antibiotics, chemotherapeutic agents, loop diuretics, and need for over-the-counter, herbal, or prescription medications during the study. Clinically significant laboratory, ECG, or physical assessment abnormalities on pretherapy screening tests also precluded participation in this study.
Study design
This was a phase 1, randomized, double-blind, placebo-controlled, single- and multiple-dose dose escalation study of dalbavancin administered as a 30-min intravenous infusion to healthy subjects. Fasting was not required, and there were no dietary restrictions. Subjects were assigned to either a single- or multiple-dose group and then block randomized to receive either placebo (one per group) or dalbavancin (three per group). The study drug was administered intravenously once in the single-dose cohort and daily for 7 days in the multiple-dose cohort.
The dose of dalbavancin given to the single-dose groups was 140, 350, 500, 630, 840, or 1,120 mg. Pharmacokinetic modeling based on data from an earlier phase 1 study was used to design loading dose (LD) and maintenance dose (MD) regimens for the multiple-dose groups to rapidly achieve steady-state concentrations. It was calculated that an LD/MD ratio of 10:1 would be optimal. Subjects were administered the LD as two equal doses separated by 12 h (e.g., 150 mg at 8 a.m. and at 8 p.m. for the 300-mg LD). The dose regimens given in the multiple-dose groups were 300 and 30, 400 and 40, 600 and 60, 800 and 80, and 1,000 and 100 mg.
Doses were escalated via a modified Fibonacci series (10) starting with 140 mg in the single-dose cohort, and the 300- and 30-mg regimen in the multiple-dose cohort. The investigator could proceed to the next dose level if dose-limiting toxicity was not reported. Dose-limiting toxicity was defined as any drug-related adverse event (AE) occurring in two or more subjects that was scored as grade 2 or greater according to the World Health Organization toxicity grading scale.
Blood sampling and analytical method
In the single-dose cohort, blood samples were collected immediately prior to administration of the dose, at the end of the infusion, and at 1, 2, 4, 6, 12, 18, 24, 48, 72, 96, 120, 144, 312, 480, and 648 h after the start of the infusion. In the multiple-dose cohort, blood samples were collected at baseline, at the end of the first infusion, 1 and 6 h after the start of the first infusion, 30 min and immediately prior to the start of the second infusion, at the end of the second infusion, and 1 and 6 h after the start of the second infusion; on days 2 through 7, blood samples were collected immediately prior to the daily infusion and at the end of the infusion; on day 7, additional blood samples were collected at 1, 6, 12, 24, 72, 120, 144, 312, 480, and 648 h after the final infusion.
Urine was quantitatively collected for the first 24 h in all single- and multiple-dose groups and for 24 h after the last infusion in the multiple-dose cohort. Random urine samples were also collected on days 4, 7, 14, 21, and 28 in single- and multiple-dose groups and on day 35 in the multiple-dose cohort.
Blister fluid was collected at several time points from two subjects, one in the 1,120-mg group and one in the 800- and 80-mg group. To produce a blister, a cantharadin plaster solution (0.7%) was applied to the forearm. The solution was rinsed off within 4 to 6 h or as soon as a blister formed.
Plasma, blister fluid, and urine samples were stored at −20°C or below until analysis for dalbavancin concentrations. Concentrations were measured by high-performance liquid chromatography with atmospheric pressure chemical ionization tandem mass spectrometry detection. The plasma and urine assays had a lower limit of quantitation of 0.5 mg/liter. The intraday variability of the plasma assay as measured by the percent relative standard deviation (standard deviation/mean · 100) of quality control samples was 3.77 to 8.82%, and the interday variability was 2.37 to 11.7%. The corresponding values for the urine assay were 4.38 to 9.62% and 4.20 to 13.9%. Blister aspirates were quantitated with the plasma assay.
Pharmacokinetic calculations
Dalbavancin plasma pharmacokinetic data were analyzed by noncompartmental methods with WinNonlin (standard version 1.1; Pharsight Corporation, Mountain View, Calif.) and the model for a constant intravenous infusion dose. The pharmacokinetic parameters calculated for the single-dose groups were Cmax (maximum concentration observed), Tmax (time at which Cmax occurred), AUC (total area under the plasma concentration-time curve), t1/2, CL (plasma clearance), and Vss (volume of distribution at steady state). For the multiple-dose groups, Cmax, Tmax, Cmin (trough plasma concentration at 24 h following administration of the last dose), and Tmin were recorded from the observed data on day 1. On day 7, the steady-state Cmax, Tmax, and Cmin were also obtained by visual inspection of the concentration-versus-time curve; AUC0-24 (24-h AUC), t1/2, and CL (MD/AUC0-24) were calculated.
Renal clearance (CLR) was calculated as the amount of dalbavancin excreted into urine through the 24-h period divided by the corresponding day 1 or day 7 AUC0-24 for the single- and multiple-dose groups, respectively. Quantitative excretion of dalbavancin into urine was calculated as the ratio of CLR/CL × 100. All urine pharmacokinetic calculations were carried out with Excel (version 7.0; Microsoft Corporation, Redmond, Wash.).
Statistical analysis of the pharmacokinetic data included descriptive statistics calculated with WinNonlin and Excel and linear regression analysis performed with Sigma Plot (SPSS, Inc., Chicago, Ill.). Linear regression analysis was carried out for Cmax and AUC versus dose (single doses) and steady-state Cmin and AUC0-24 versus dose (multiple doses) to test for dose proportionality. Because of the small sample size, inferential statistical comparisons were not performed.
SBA
In all groups, blood samples were collected at 24 (day 2), 48 (day 3), and 144 (day 7) h relative to the start of the single infusion (single-dose cohort) or final infusion (multiple-dose cohorts) for SBA testing.
S. aureus 3886 and 3897, both methicillin resistant (methicillin MIC, 128 mg/liter), were used as test organisms. The dalbavancin MICs for these strains were 0.13 and 0.25 mg/liter, respectively. The dalbavancin minimum bactericidal concentration (MBC) was equal to the MIC. Tests were performed in accordance with National Committee for Clinical Laboratory Standards recommendations (9). Heat-inactivated pooled human serum (CELLect, lot. no. R10738) was used as a diluent for the serum samples. The absence of antimicrobial activity in human serum against the test strains was previously verified.
Twofold serial dilutions of the test samples in human serum were prepared in microtiter plates. For each strain, exponentially growing cultures were diluted to 106 CFU/ml in Mueller-Hinton broth (Difco, Detroit, Mich.) supplemented with Mg2+ and Ca2+, and 50 μl of the bacterial suspension was distributed into each well to give a final bacterial concentration of 5 × 105 CFU/ml. All experiments were conducted in triplicate on three different days. Plates were incubated at 35°C for 24 h. Two aliquots, 10 μl each, were withdrawn from the wells that did not show visible growth and subcultured on Todd-Hewitt agar (Difco, Detroit, Mich.). A possible antibiotic carryover effect was reduced by plating three 10-fold dilutions of each sample. A colony count was performed after 48 h of incubation at 35°C. SBA was defined as the highest dilution of each sample that reduced the initial inoculum by ≥99.9%.
Safety assessments
Assessments for AEs were performed throughout the duration of the study and were graded by the investigator as mild, moderate, severe, or life threatening and in accordance with the World Health Organization toxicity grading system (e.g., any body temperature of >37.1°C was reported as pyrexia). The investigator was also asked to categorize the events in regard to their relationship to the study drug. Laboratory data (chemistry panel, blood count with differential, and urinalysis) were collected on days 4 and 7 for all groups and additionally on days 2, 10, and 14 in the multiple-dose groups. Laboratory values were assessed for changes from the baseline; out-of-range values were also identified. ECGs were performed on day 3 in the single-dose groups and on days 3 and 7 in the multiple-dose groups, and changes from the baseline were assessed. All postbaseline ECGs were reviewed in a blinded fashion against the corresponding baseline ECG by an independent reviewer.
Audiologic assessments were conducted at the baseline and on days 2, 7, and 14 in the single-dose groups and on days 2, 7, 14, and 21 in the multiple-dose groups. The audiologic methods used in this study were in accordance with the guidelines of the American Speech-Language Hearing Association (1) and are detailed in a separate publication (2).
RESULTS
Patients
Fifty-two subjects were enrolled; 39 received dalbavancin, and 13 received placebo. The majority were Caucasian (n = 25) or Black (n = 19). The subjects were between 19 and 52 years of age (mean of 28.1 years), and roughly half (n = 25) were male. Their heights ranged from 152.4 to 190.5 cm (mean, 169 cm), and their weights ranged from 48.1 to 104.3 kg (mean, 71.3 kg). Fifty-one subjects completed the study; one subject in the dalbavancin 800- and 80-mg group voluntarily withdrew on day 1. Pharmacokinetic parameters from this subject were included in the day 1 mean values.
Pharmacokinetics
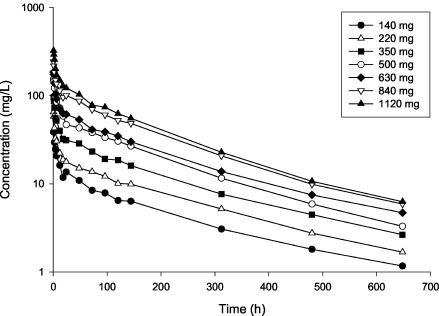
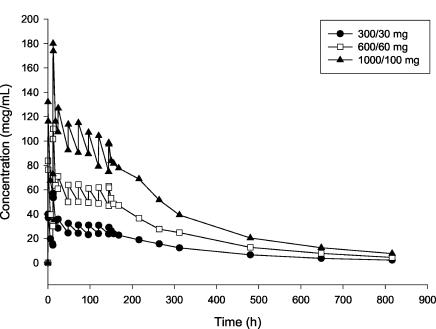
Mean dalbavancin plasma concentration-time profiles for the single- and multiple-dose cohorts are shown in Fig. 1 and 2, respectively. Mean dalbavancin concentrations in plasma increased proportionally with dose and declined in a log-linear manner in the terminal portion of the profile.
FIG. 1.
Mean dalbavancin concentrations in plasma following administration of single 30-min intravenous infusion doses (n = 3 per group).
FIG. 2.
Mean dalbavancin concentrations in plasma following administration of multiple 30-min intravenous infusion doses (n = 3 per group).
Mean dalbavancin pharmacokinetic parameters are shown in Table 1 for the single-dose groups and in Table 2 for the multiple-dose groups. In all groups, Cmax was observed following completion of the intravenous infusion. The mean t1/2 of dalbavancin ranged from 149 to 198 h, was similar in the single- and multiple-dose cohorts, and showed no tendency to change with the dose. In the single-dose cohort, the mean CL and Vss were also similar across all dose levels. The dalbavancin CLR was lower than the CL and ranged from 0.0115 to 0.0228 liters/h. The proportion of dalbavancin excreted unchanged in the urine ranged from 25.0 to 45.5% of the administered dose. Pharmacokinetic parameters showed low intersubject variability on the basis of the percent relative standard deviation calculated across all subjects in the single-dose cohort: t1/2, 11.0%; CL, 12.7%; Vss, 20.1%. With the multiple-dose regimens, steady-state concentrations were generally achieved on day 2 or 3 following administration of the initial dose. Dalbavancin blister fluid concentrations correlated well with simultaneously obtained drug levels in plasma. The ratio of the concentration in blister fluid to the concentration in plasma ranged from 0.83 to 1.11. Urine concentrations were above the limit of quantitation of the assay (0.5 mg/liter) at all times measured through 28 days postdose.
TABLE 1.
Dalbavancin pharmacokinetic parameters following administration of a single 30-min intravenous infusion to healthy male and female subjectsa
| Doseb (mg) | Tmax | Cmax (mg/liter) | AUC (mg · h/liter) | CL (liters/h) | Vss (liters) | t1/2 (h) |
|---|---|---|---|---|---|---|
| 140 | 0.5 (0.5-1.0) | 40.1 (37.9-41.1) | 3,234 (3,174-3,344) | 0.0433 (0.0419-0.0441) | 10.9 (10.7-11.1) | 188 (186-193) |
| 220 | 0.5 (0.5-0.5) | 71.4 (48.4-76.1) | 5,004 (4,370-5,490) | 0.044 (0.0401-0.0503) | 11.0 (10.1-12.7) | 186 (172-207) |
| 350 | 0.5 (0.5-0.5) | 96.3 (89.1-103) | 8,104 (6,904-9,276) | 0.0432 (0.0377-0.0507) | 11.0 (8.2-12.8) | 188 (162-192) |
| 500 | 0.5 (0.5-0.5) | 133 (131-195) | 11,393 (11,200-14,758) | 0.0439 (0.0339-0.0446) | 9.01 (7.19-9.54) | 162 (154-163) |
| 630 | 1.0 (0.5-1.0) | 170 (143-256) | 12,616 (12,257-19,400) | 0.0499 (0.0325-0.0514) | 11.3 (6.96-13.2) | 168 (158-190) |
| 840 | 0.5 (0.5-0.5) | 239 (235-256) | 21,949 (21,253-23,474) | 0.0383 (0.0358-0.0395) | 7.85 (7.15-8.24) | 149 (148-158) |
| 1,120 | 0.5 (0.5-0.5) | 312 (292-371) | 27,103 (22,967-27,299) | 0.0413 (0.041-0.0488) | 7.93 (7.84-9.70) | 149 (145-153) |
The values shown are medians (ranges are in parentheses).
There were three subjects in each group.
TABLE 2.
Dalbavancin pharmacokinetic parameters following administration of multiple daily 30-min intravenous infusions to healthy male and female subjectsa
| Loading dose/ maintenance dose (mg)b |
Cmax (mg/liter)
|
Cmin (mg/liter)
|
AUC0-24 (mg · h/liter), day 7 | CL (liters/h), day 7 | t1/2 (h), day 7 | ||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 1 | Day 7 | ||||
| 300/30 | 57.8 (49.0-65.2) | 29.5 (24.5-34.9) | 14.9 (10.1-18.1) | 22.1 (19.6-26.5) | 607 (517-667) | 0.0494 (0.045-0.0581) | 189 (179-207) |
| 400/40 | 77.4 (65.9-89.4) | 38.8 (37.7-51.0) | 21.4 (21.1-22.2) | 32.3 (27.3-33.2) | 849 (729-896) | 0.0471 (0.0446-0.0549) | 185 (166-200) |
| 600/60 | 111 (110-124) | 63.5 (60.6-66.9) | 29.9 (27.0-33.8) | 46.0 (45.3-50.1) | 1,248 (1,134-1,282) | 0.0481 (0.0468-0.0529) | 204 (177-213) |
| 800/80c | 127 (102-164) | NAd (66.2-69.2) | 33.3 (31.4-44.8) | NA (53.5, 54.5) | NA (1,345, 1,397) | NA (0.0573, 0.0595) | NA (189, 208) |
| 1,000/100 | 180 (155-205) | 92.6 (84.1-120) | 54.4 (51.3-66.0) | 71.0 (70.3-91.9) | 1,871 (1,770-2,349) | 0.0535 (0.0426-0.0565) | 188 (177-201) |
The values shown are medians (ranges are in parentheses).
There were three subjects per group, unless noted otherwise.
There were two subjects on day 7.
NA, not applicable.
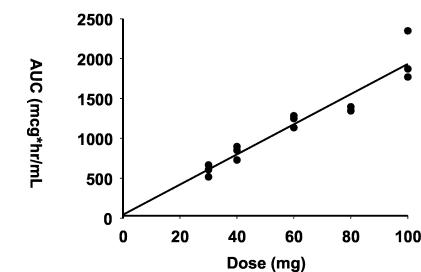
The dalbavancin Cmax increased in proportion to the increase in the dose in subjects receiving single doses. AUCs showed a similar linear relationship to the dose increment (Fig. 3). Linear regression analyses of Cmax and AUC versus the dose administered demonstrated a linear relationship, with correlation coefficients of 0.930 and 0.944, respectively.
FIG. 3.
Linear regression analysis of dalbavancin AUC versus dose following administration of single 30-min intravenous infusion doses of 140 to 1,120 mg.
Although this study was not designed to compare dalbavancin pharmacokinetics between male and female subjects, an evaluation of t1/2, Vss, and CL values from the single-dose cohort was performed. The mean t1/2s were similar for the two genders (172 h for males and 169 h for females). Mean Vss and CL values were 13% higher in males, but after correction for body weight, these parameters were found to be similar in males and females.
SBA
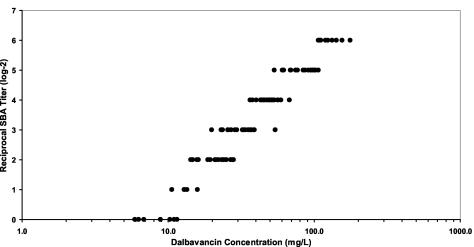
The MIC and MBC of dalbavancin against the two MRSA test strains with 50% human serum were determined as the internal control for each experiment. The MIC and MBC in the presence of 50% human serum were 1.71 and 2.04 mg/liter, respectively, for the 3886 strain and 1.22 and 1.61 mg/liter, respectively, for the 3897 strain. Samples from all subjects treated with single doses of ≥500 mg, and from all subjects receiving multiple doses, showed bactericidal activity against both MRSA strains through 7 days after dalbavancin administration. The median reciprocal SBA titer for the 3886 strain and the corresponding plasma concentration data for all of the time points studied are shown in Fig. 4. Reciprocal SBA titers increased with drug concentrations in plasma. All samples in which the corresponding drug concentrations in plasma exceeded 20 mg/liter had a detectable bactericidal titer.
FIG. 4.
Reciprocal SBA titer against a strain of MRSA versus corresponding dalbavancin concentration.
Safety and tolerability
There were no serious AEs or deaths reported in this study. No dose effects were seen for AEs or laboratory values. Sixty-seven percent of the subjects reported at least one treatment-emergent AE. Most AEs were mild in severity, and none met the criteria for dose-limiting toxicity. The most common AEs in both the single- and multiple-dose groups were pyrexia (50%), headache (25%), and nausea (6%). Subjects receiving placebo also reported pyrexia (38%) and headache (31%). Pyrexia was defined, in accordance with World Health Organization toxicity grading criteria, as any oral temperature of >37.1°C; no subject had a temperature exceeding 37.5°C at any time during the study. There were no clinically significant changes from the baseline regarding laboratory findings, vital signs, physical examinations, or ECGs. Mild (less than five times the upper limit of normal), transient, asymptomatic elevations in alanine aminotransferase and aspartate aminotransferase were observed in one subject who received 350 mg of dalbavancin and in no subjects who received higher doses. One dalbavancin-treated subject experienced a transient hyperglycemia (blood glucose level of 160 mg/dl) that was considered mild, and one subject in the placebo group also experienced mild hyperglycemia (135 mg of glucose/dl).
Results of audiologic assessments were published in detail elsewhere (2). Overall, these assessments showed no audiologic changes in any subject. Analysis of variance results indicated that there was no significant change over time related to treatment condition or stimulus frequency. The power to detect a change, if it had occurred, was >0.99.
DISCUSSION
Tolerability, pharmacokinetic, and pharmacodynamic data from 51 subjects were analyzed in this phase 1, double-blind, randomized, placebo-controlled, single- and multiple-dose dose escalation study.
Dalbavancin was well tolerated at all of the doses studied, including a single dose of 1,120 mg and multiple doses of 1,000 mg on the first day, followed by 100 mg daily for an additional 6 days (1,600-mg total exposure). The most frequently reported treatment-emergent AEs in both dalbavancin-treated and placebo-treated patients were pyrexia (temperature range, 37.1 to 37.5°C) and headache. There was no relationship between the incidence or severity of AEs and the dose of dalbavancin administered and no difference in the incidence of AEs between the dalbavancin and placebo groups. No AEs met the criteria for dose-limiting toxicity.
In the absence of protocol-defined dose-limiting toxicity, additional dose escalation of dalbavancin could have proceeded in the present study. The study concluded with dose levels of 1,120 mg as a single dose and 1,000 and 100 mg as a multiple-dose regimen because the dalbavancin concentrations in plasma achieved with these doses exceeded the concentrations expected to be required for clinical use.
This study used well-designed, carefully standardized, and tightly controlled audiologic assessments with statistical power exceeding 0.99. No significant changes occurred with any audiologic test for any individual subject or when analyzed by group.
Mean pharmacokinetic parameters found in the present study were similar to those obtained in a previous study (White et al., 40th ICAAC). In single- and multiple-dose cohorts, the dalbavancin Cmax and AUC increased in proportion to the dose given while the t1/2, CL, and Vss remained essentially unchanged. Accordingly, dalbavancin demonstrated linear, dose-proportional pharmacokinetics. The 10:1 LD-to-MD ratio achieved the desired objective, as a steady state was achieved as early as 2 or 3 days following administration of the initial dose. The dalbavancin t1/2 is approximately 1 week, and the estimated CL (overall mean = 0.0472 liters/h) and CLR (overall mean = 0.0157 liters/h) are slower than the average glomerular filtration rate of 7.8 liters/h in normal subjects. The mean Vss is approximately 10 liters, a value larger than the average blood volume (5.4 liters) but smaller than the average extracellular water volume (16 liters for normal subjects).
Approximately one-third of the dose of dalbavancin was excreted into urine unchanged (overall mean quantitative excretion of dalbavancin into urine = 33.5%), suggesting that nonrenal routes of elimination play an important role in the elimination of dalbavancin. In contrast, vancomycin and teicoplanin, currently available glycopeptide antibiotics, are excreted almost entirely via the kidneys (12).
In these preliminary assessments, dalbavancin concentrations in blister fluid were roughly equivalent to the concentrations in plasma. These data are consistent with preclinical data (5a).
We have previously shown that dalbavancin is highly bound to serum proteins (M. Cavaleri, A. Cooper, M. A. Nutley, and M. Stogniew, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1385, 2002) and that MICs in the presence of serum are higher (four- to eightfold) than in broth (3). This difference is less pronounced when looking at the effect of serum on MBCs (one- to twofold higher in the presence of serum). In this study, SBAs were determined under test conditions (50% serum) identical to those used to measure MICs and MBCs. The physical-chemical properties of dalbavancin have, to date, precluded development of an assay for free drug concentrations in plasma samples. With an indirect method by which to measure protein binding, Cavaleri et al. have shown that the free fraction is fairly constant across a large range of concentrations, including those observed in this study. Therefore, total concentrations of dalbavancin in plasma have been used as a surrogate for the free concentrations. Serum samples from subjects who received single or multiple doses of dalbavancin demonstrated bactericidal activity when tested against two MRSA strains. The bactericidal titer and persistence of activity correlated with the dose of dalbavancin administered and with the dalbavancin concentrations determined in the same plasma samples. The data from the present study suggest that bactericidal activity is present when concentrations of dalbavancin in plasma are 20 mg/liter or greater. Given the long t1/2 of dalbavancin, single doses of 500 mg or higher will maintain concentrations above the minimum bactericidal level for at least 1 week. More extensive pharmacodynamic evaluations are needed to determine the importance of this observation.
In summary, the results of this study provide evidence that dalbavancin is well tolerated and has pharmacokinetic and pharmacodynamic properties that support its use in the treatment of serious gram-positive infections. Because of the long t1/2 of dalbavancin and its persistent bactericidal activity, weekly dosing regimens are expected to be a clinical option. Further studies are under way.
Acknowledgments
This study was funded by Vicuron Pharmaceuticals, Inc., King of Prussia, Pa.
REFERENCES
- 1.American Speech-Language Hearing Association. 1994. Guidelines for the audiologic management of individuals receiving cochleotoxic drug therapy. Am. Speech-Lang. Hear. Assoc. 36:11-19. [Google Scholar]
- 2.Campbell, K. C. M., E. Kelly, and N. Targovnik, L. Hughes, C. Van Saders, A. B. Gottlieb, M. B. Dorr, and A. Leighton. 2003. Audiologic monitoring for potential ototoxicity in a phase 1 clinical trial of a new glycopeptide antibiotic. J. Am. Acad. Audiol. 14:157-168. [PubMed] [Google Scholar]
- 3.Candiani, G., M. Abbondi, M. Borgonovi, G. Romanò, and F. Parenti. 1999. In vitro and in vivo antibacterial activity of BI 397, a new semi-synthetic glycopeptide antibiotic. J. Antimicrob. Chemother. 44:179-192. [DOI] [PubMed] [Google Scholar]
- 4.Diekema, D. J., M. A. Pfaller, R. N. Jones, G. V. Doern, K. C. Kuegler, M. L. Beach, H. S. Sader, and The SENTRY Participants Group. 2002. Trends in antimicrobial susceptibility of bacterial pathogens isolated from patients with bloodstream infections in the USA, Canada and Latin America: report from the SENTRY Antimicrobial Surveillance Programme, 1998. Int. J. Antimicrob. Agents 13:257-271. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein, E. J. C., D. M. Citron, C. V. Merriam, U. Warren, K. Tyrell, and H. T. Fernandez. 2003. In vitro activities of dalbavancin and nine comparator agents against anaerobic gram-positive species and corynebacteria. Antimicrob. Agents Chemother. 47:1968-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Jabes, D., G. Candiani, G. Romanò, C. Brunati, S. Riva, and M. Cavaleri. Antimicrob. Agents Chemother., in press. [DOI] [PMC free article] [PubMed]
- 6.Jones, R. N., D. J. Biedenbach, D. M. Johnson, and M. A. Pfaller. 2001. In vitro evaluation of BI 397, a novel glycopeptide antimicrobial agent. J. Chemother. 13:244-254. [DOI] [PubMed] [Google Scholar]
- 7.Malabarba, A., and S. Donadio. 1999. BI 397, glycopeptide antibiotics. Drugs Future 24:839-846. [Google Scholar]
- 8.Maranan, M. C., B. Moreira, S. Boyle-Vavra, and R. S. Daum. 1997. Antimicrobial resistance in Staphylococci: epidemiology, molecular mechanisms, and clinical relevance. Infect. Dis. Clin. N. Am. 11:813-849. [DOI] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. 1999. Methodology for the serum bactericidal test. Approved standard M21-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 10.Ratain, M. J., R. Mick, R. L. Schilsky, R. L. Siegler, and M. Siegler. 1993. Statistical and ethical issues in the design and conduct of phase I and II clinical trials of new anticancer agents. J. Natl. Cancer Inst. 85:1637-1643. [DOI] [PubMed] [Google Scholar]
- 10a.Streit, J. M., T. R. Fritsche, H. S. Sader, and R. N. Jones. Worldwide assessment of dalbavancin activity and spectrum against over 6,000 clinical isolates. Diagn. Microbiol. Infect. Dis., in press. [DOI] [PubMed]
- 11.Voss, A., D. Milatovic, and C. Wallrauch-Schwarz, V. T. Rosdahl, and I. Braveny. 1994. Methicillin-resistant Staphylococcus aureus in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 13:50-55. [DOI] [PubMed] [Google Scholar]
- 12.Wilson, A. P. 2000. Clinical pharmacokinetics of teicoplanin. Clin. Pharmacokinet. 39:167-183. [DOI] [PubMed] [Google Scholar]