Abstract
This study investigated the effects of chronic in vivo AMP-kinase activation with 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) on lipolysis in subcutaneous inguinal, epididymal, and retroperitoneal fat pads. Male Wistar rats received daily single intraperitoneal injections of either saline or AICAR (0.7 g/kg body wt) for a period of 8 wk. The fat pads were used either to isolate adipocytes and measure basal and catecholamine-stimulated lipolysis or to assess signaling steps of lipolysis after 4 and 8 wk of AICAR treatment. Blood was sampled weekly to measure nonesterified fatty acids (NEFAs). AICAR treatment reduced basal and catecholamine-stimulated lipolysis at week 4 in adipocytes from all fat depots. However, at week 8, catecholamine-induced lipolysis significantly increased in inguinal and retroperitoneal adipocytes. Interestingly, plasma levels of NEFAs were also decreased and subsequently increased at 4 and 8 wk, respectively. The lipolytic cascade of the inguinal fat pad was the most drastically affected by the treatment, since the phosphorylation and content of most proteins involved in lipolysis were consistently undetected in this tissue after 4 and 8 wk of AICAR treatment. The enhancement of catecholamine-induced lipolysis in inguinal and retroperitoneal adipocytes after 8 wk of AICAR treatment was accompanied by increased contents of adipose triglyceride lipase (ATGL) and perilipin A in these fat depots. In summary, despite depot-specific regulation of the lipolytic cascade, catecholamine-induced lipolysis in isolated adipocytes correlated well with plasma NEFA concentrations in the course of chronic AICAR-induced AMPK activation. The mechanisms underlying these effects also involved time-dependent and depot-specific regulation of hormone-sensitive lipase, ATGL, and perilipin.
Keywords: AMPK, lipolysis, visceral fat, ATGL, perilipin
one of the main functions of the white adipose tissue (WAT) is to release fatty acids (FAs) into the bloodstream under conditions of increased metabolic demand (e.g., exercise) or energy deficit (e.g., fasting). To accomplish this, adipocytes are equipped with the machinery required to break down the high-energy triacylglycerol (TAG) molecules and release nonesterified fatty acids (NEFAs) to provide substrate for energy production in peripheral tissues (15). In humans and rodents WAT lipolysis involves a cascade of intracellular events triggered by the activation of G protein-coupled receptors leading to activation of protein kinase A (PKA), hormone-sensitive lipase (HSL), and adipose tissue TAG lipase (ATGL; Refs. 4, 15). In rodents, ATGL and HSL seem to be the main TAG and diacylglycerol (DAG) lipases, respectively, accounting together for ∼95% of lipase activity in murine WAT (19). Besides phosphorylating and activating HSL, PKA also phosphorylates the lipid droplet-coating protein perilipin A, which causes the latter to dissociate from the comparative gene identification-58 (CGI-58) protein (14). Once dissociated, CGI-58 interacts with ATGL to enhance the activity of this TAG lipase by ∼20-fold in mouse adipocytes (14, 16), leading to the release of one fatty acid (FA) and the generation of DAG. Phosphorylated and activated HSL acts on DAG and releases another FA, which is then followed by the cleavage of a third FA by monoacylglycerol lipase (MAGL; Ref. 15). Therefore, regulation of ATGL, HSL, and MAGL activity can profoundly affect the breakdown of TAG and the release of NEFAs by adipocytes. In this context; it was originally demonstrated that the cellular energy sensor AMP-activated protein kinase (AMPK) could phosphorylate HSL on Ser565 in rat adipocytes, and this could prevent the subsequent phosphorylation by PKA of other HSL serine residues involved in activation of this lipase (13). In fact, studies using acute AICAR-induced AMPK activation indeed increased HSL Ser565 phosphorylation and reduced catecholamine-stimulated adipocyte lipolysis (3, 22). Furthermore, pharmacological inhibition of AMPK prevented AICAR-induced HSL Ser565 phosphorylation, increased epinephrine-stimulated HSL Ser660 phosphorylation, and promoted lipolysis in isolated visceral (VC) and subcutaneous (SC) rat adipocytes (3). These findings were supported by observations that adipocytes from AMPKα1 knockout mice elicited increased lipolysis (8) and also by in vivo human (6) and rat (5) studies demonstrating that acute intravenous AICAR administration reduced whole body lipolysis. Besides regulating HSL activity, AMPK also seems to alter ATGL content in adipocytes. In fact, prolonged exposure (15 h) of isolated rat adipocytes to AICAR led to a reduction in HSL phosphorylation and a time-dependent increase in ATGL content and TAG lipase activity (10). Interestingly, these antagonistic regulatory effects on HSL and ATGL were accompanied by reductions and increases in the release of glycerol and NEFAs, respectively, in AICAR-treated cells (10). The physiological implications of these findings are still unclear; but recent in vivo studies have demonstrated that rats treated for 8 wk with AICAR show increased energy expenditure and reduced fat mass (12), indicating that WAT lipolysis is indeed affected by chronic AICAR-induced AMPK activation. Based on previous in vitro studies (3, 10), one would expect that upregulation of ATGL content/activity would be an important adaptive response to chronic AMPK activation, since it would promote FA release from adipocytes even though HSL activity could be suppressed. However, to the best of our knowledge, no studies have assessed the regulation of major steps of the lipolytic cascade in the WAT in the course of chronic in vivo pharmacological AMPK activation. Besides HSL and ATGL, other molecular steps of lipolysis such as CGI-58 and perilipin could also be affected by AICAR-induced AMPK activation and exert important regulatory effects in different fat depots. Therefore, the goal of this study was to assess basal and catecholamine-stimulated lipolysis, as well as the responses of major molecular steps involved in TAG breakdown under chronic AMPK activation in VC and SC WAT. Here we provide novel evidence that catecholamine-stimulated lipolysis is initially reduced and then increased in adipocytes isolated from VC and SC fat depots of rats exposed to chronic systemic pharmacological AMPK activation. The plasma concentration of NEFAs in AICAR-treated rats coincided with the in vitro lipolytic profile of VC and SC adipocytes, which could be explained by time-dependent and tissue-specific alterations in major regulators of lipolysis such as ATGL and perilipin.
MATERIALS AND METHODS
Reagents.
Epinephrine, FA-free BSA, free glycerol determination kit, glucose oxidase kit, and isoproterenol were obtained from Sigma. NEFAs were measured using a kit from Wako Chemicals (Richmond, VA). AICAR was purchased from Toronto Research Chemicals (Toronto, ON, Canada). The AMPK, phospho-AMPK, HSL, phospho-HSL Ser563, 565, 660, ATGL, and β-actin antibodies were purchased from Cell Signaling Technology (Beverly, MA). The phospho-acetyl-CoA carboxylase Ser79 (P-ACC) and the perilipin antibodies were obtained from Upstate (Charlottesville, VA) and from American Research Products (Belmont, MA), respectively. The antibody for the comparative gene identification 58 (CGI-58) was a kind gift from Dr. Dawn L. Brasaemle from Rutgers University (New Brunswick, NJ).
In vivo AICAR treatment and plasma analyses.
Male albino rats (Wistar strain), weighing 150–200 g, were maintained on a 12:12-h light/dark cycle at 22°C and fed standard laboratory chow ad libitum. Rats were given a single daily intraperitoneal injection of either saline or AICAR (0.7 g/kg body wt) for 4 and 8 wk. The dosage was chosen based on previous in vivo rat studies that used between 0.5 and 1.0 g/kg body wt for chronic AICAR injections (10, 18, 23). Saline-injected rats were pair-fed to the AICAR-treated group to control for effects of altered food intake induced by the treatment. Weekly blood samples were collected before daily injections from the saphenous vein, and the plasma was frozen (−80°C) for later analyses of glucose and NEFAs using commercially available kits. One group of animals had SC [inguinal (ING)] and VC [epididymal (EPI) and retroperitoneal (RP)] fat pads extracted after 4 wk and another after 8 wk of treatment. The fat tissues were used for adipocyte isolation and for Western blot analyses. The experimental protocol was approved by the York University Animal Care Ethics Committee.
Isolation of adipocytes and measurement of lipolysis.
After the 4- and 8-wk treatment periods, fat depots from ING, EPI, and RP regions were quickly removed and adipocytes were isolated from each tissue as previously described (11). To distribute equal number of adipocytes in each treatment condition, cell diameters and numbers were measured as described by DiGirolamo and Fine (9). For lipolysis, 2.5 × 105 cells were incubated with constant agitation (80 orbital strokes/min) in the absence or presence of either epinephrine (100 nM) or isoproterenol (100 nM) for 75 min (11). Subsequently, aliquots of the media were removed and assayed for glycerol content using a commercially available kit. The concentrations of epinephrine and isoproterenol used in this study were chosen based on our previous studies demonstrating a robust lipolytic response (10, 11), which would allow us to assess potential effects of AICAR-induced AMPK activation on lipolysis in VC and SC adipocytes.
Determination of content and phosphorylation of proteins by Western blot.
Fat depots were extracted and immediately snap frozen in liquid nitrogen and then stored at −80°C for subsequent use. The tissues were homogenized in a buffer containing 25 mM Tris·HCl and 25 mM NaCl (pH 7.4), 1 mM MgCl2, 2.7 mM KCl, 1% Triton-X, and protease and phosphatase inhibitors (0.5 mM Na3VO4, 1 mM NaF, 1 μM leupeptin, 1 μM pepstatin, and 20 mM PMSF). Homogenates were centrifuged, the infranatant was collected, and an aliquot was used to measure protein by the Bradford method. Samples were diluted 1:1 (vol/vol) with 2× Laemmli sample buffer, heated to 95°C for 5 min, and subjected to SDS-PAGE. All primary antibodies were used in a dilution of 1:1, 000 except for p-AMPK (1:500), perilipin (1:2, 500), and CGI-58 (1:5,000). Equal loading was confirmed by Coomassie blue staining of gels and by β-actin detection.
RESULTS
Effects of AICAR on AMPK and ACC phosphorylation.
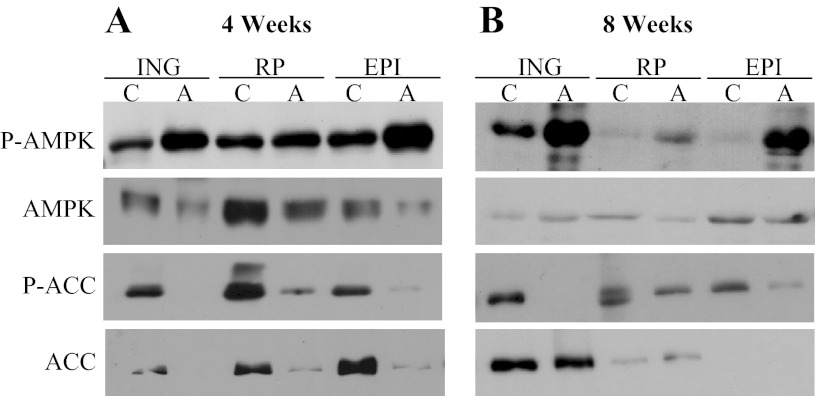
As we have recently described (12), AMPK phosphorylation (Thr172) increased in ING, RP, and EPI fat depots after 4 and 8 wk of AICAR treatment. The most pronounced increase in AMPK phosphorylation was in the ING and EPI fat pads (Fig. 1, A and B). AMPK content in all fat depots was reduced in the AICAR group at week 4, an effect no longer present at week 8. ACC phosphorylation (Ser79) was markedly reduced at week 4 and 8 in ING, RP, and EPI fat pads. ACC content also reduced in all fat depots after 4 wk of AICAR treatment (Fig. 1A). Interestingly, at week 8, the ACC content profile differed among the three fat depots. In fact, while ACC content was unaltered and slightly elevated in the ING and RP fat pads, respectively, in the EPI fat pad ACC was undetectable after 8 wk of AICAR treatment (Fig. 1B).
Fig. 1.
Phosphorylation of AMPK and acetyl-CoA carboxylase (ACC) is increased in inguinal (ING), retroperitoneal (RP), and epididymal (EPI) fat pads extracted from rats after 4 (A) and 8 wk (B) of AICAR (A) treatment. C, control; n = 3–4 per group.
Effects of chronic AICAR treatment on basal and catecholamine-induced lipolysis in isolated VC and SC adipocytes.
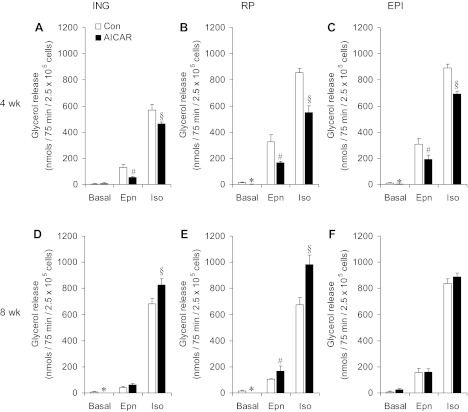
While basal lipolysis was potently suppressed to almost no detectable values in RP and EPI adipocytes after 4 wk of AICAR treatment, in ING adipocytes this variable was similar to control (Fig. 2, A–C). Epinephrine-stimulated glycerol release was significantly reduced after 4 wk by 60, 50, and 38% in ING, RP, and EPI adipocytes, respectively, in AICAR-treated animals (Fig. 2, A–C). The isoproterenol effect on this variable was also blunted by 19, 36, and 23% in adipocytes from ING, RP, and EPI fat pads, respectively (Fig. 2, A–C). After 8 wk of treatment, basal lipolysis in ING and RP adipocytes remained potently suppressed, while in EPI adipocytes from AICAR-treated rats this variable was elevated by approximately threefold (Fig. 2, D–F). The initial inhibitory effect of AICAR on catecholamine-induced lipolysis disappeared after 8 wk of treatment with this AMPK activator. In fact, at the end of the treatment period, the epinephrine-stimulated release of glycerol was restored to the same level as controls in ING and EPI fat depots and increased by 62% in RP adipocytes from AICAR-treated rats. Furthermore, the isoproterenol effect on glycerol release was increased by 22 and 42% in ING and RP from AICAR-treated rats, respectively (Fig. 2, D–F). Even though isoproterenol-induced glycerol release did not differ between control and AICAR-treated EPI adipocytes at week 8, a clear reversal of the inhibitory effect of AICAR detected after 4 wk occurred (Fig. 2, C and F).
Fig. 2.
Lipolysis in ING, RP, and EPI adipocytes reduces after 4weeks and increases after 8 wk of 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) treatment. Glycerol release under basal and epinephrine (Epn)- and isoproterenol (Iso)-stimulated conditions in adipocytes isolated from ING (A and D), RP (B and E), and EPI (C and F) fat pads of rats either injected with saline (control, Con) or AICAR for 4 (A–C) and 8 wk (D–F). Data are compiled from 4 independent experiments with triplicates in each experiment. Two-way ANOVA with Bonferroni post hoc tests. *,#,§P < 0.05 vs. all other conditions.
Effects of chronic AICAR treatment on NEFAs.
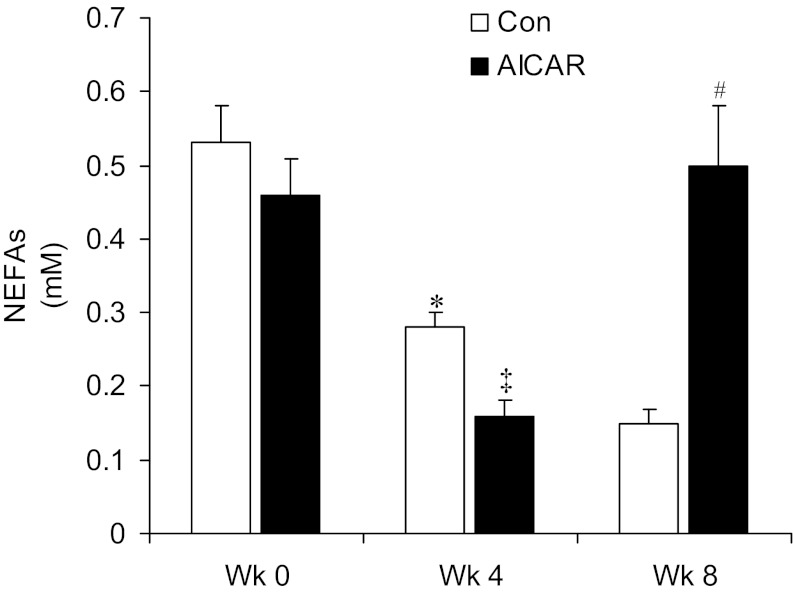
At the beginning of the experiment (week 0), plasma concentrations of NEFAs (Fig. 3) did not differ between both groups of animals (0.53 ± 0.05 and 0.46 ± 0.05 mM). After 4 wk of treatment, both groups elicited a significant reduction in plasma NEFAs compared with week 0, but the reduction was significantly larger in AICAR-treated rats (0.28 ± 0.02 and 0.18 ± 0.02 mM in the control and AICAR groups, respectively; Fig. 3). After 8 wk of treatment, plasma NEFAs were further reduced in control rats reaching average values of 0.15 ± 0.02 mM, while this variable significantly increased to 0.5 ± 0.08 mM in AICAR-treated rats (Fig. 3).
Fig. 3.
Chronic AICAR treatment initially reduces and then increases serum concentrations of non-esterified fatty acids (NEFAs). Serum NEFAs were measured before week 0 (Wk 0) and after 4 wk (Wk 4) and 8 wk (Wk 8) of treatment; n = 6–8 per group. *P < 0.05 vs. all other conditions; ‡P < 0.05 vs. control and AICAR Wk 0, control Wk 4, and AICAR Wk 8; #P < 0.05 vs. control and AICAR Wk 4 and control Wk 8. Two-way ANOVA with Bonferroni post hoc tests.
Effects of AICAR treatment on the content and phosphorylation of HSL in VC and SC fat pads.
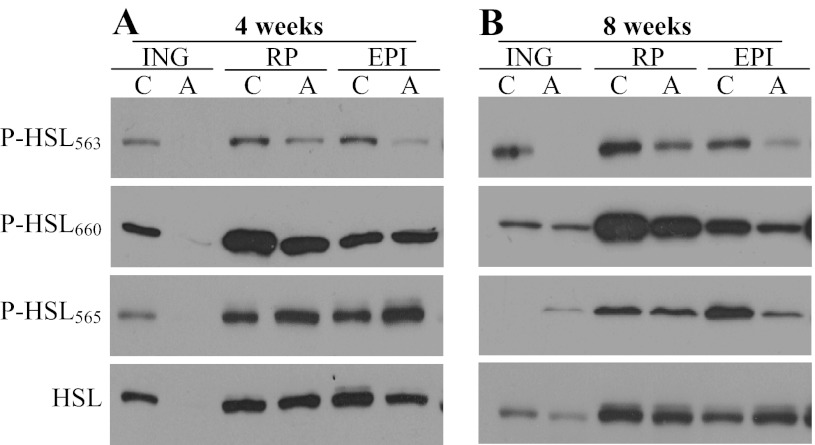
Western blot analyses revealed that HSL protein content was drastically reduced to undetectable values in the ING fat pad, although it remained unchanged in RP and EPI fat depots after 4 wk of AICAR treatment (Fig. 4A). At week 8 of treatment, HSL content was similar between control and AICAR-treated rats in all fat depots (Fig. 4B). Phosphorylation of HSL at the Ser563 residue was potently reduced in ING, RP, and EPI fat depots from AICAR-treated animals at 4 and 8 wk (Fig. 4, A and B). HSL phosphorylation at the Ser660 residue was undetectable in ING fat and did not seem to have been affected in RP and EPI fat depots after 4 wk of AICAR treatment (Fig. 4A). While HSL Ser660 phosphorylation in ING fat did not differ between control and AICAR-treated rats, this variable was reduced in RP and EPI fat pads after 8 wk of treatment with the AMPK activator (Fig. 4A). Phosphorylation of HSL at Ser565 was undetectable in ING fat and increased in RP and EPI depots after 4 wk of AICAR treatment (Fig. 4A). At the end of 8 wk of AICAR treatment, we found that phosphorylation of HSL at Ser565 was increased, unchanged, and reduced in ING, RP, and EPI fat depots, respectively (Fig. 4B).
Fig. 4.
HSL content and phosphorylation at Ser563, Ser660, and Ser565 residues are regulated in a tissue-specific and time-dependent manner in ING, RP, and EPI fat pads after 4 (A) and 8 wk (B) of treatment with either saline (control) or AICAR.
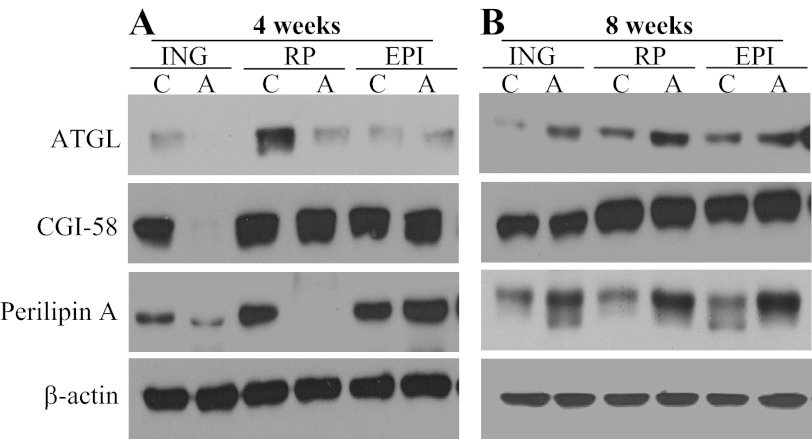
Effects of AICAR treatment on the content of ATGL, CGI-58, and perilipin A in VC and SC fat pads.
After 4 wk of treatment, ATGL was undetected and potently reduced in ING and RP fat depots of AICAR-treated rats, respectively, while in the EPI fat depot ATGL did not differ between control and AICAR-treated rats (Fig. 5A). However, at week 8, ATGL in ING, RP, and EPI fat depots was consistently upregulated in AICAR-treated rats (Fig. 5B). CGI-58 was only reduced to undetectable values in the ING fat depot with AICAR treatment at week 4 of treatment, and then remained unchanged in all fat depots at week 8 (Fig. 5, A and B). Perilipin A content was potently reduced in ING and RP fat depots but was slightly elevated in EPI fat after 4 wk of AICAR treatment (Fig. 5A). At week 8, perilipin content was consistently increased in ING, RP, and EPI of AICAR-treated rats (Fig. 5B).
Fig. 5.
The contents of adipose triglyceride lipase (ATGL), CGI-58, and perilipin A are regulated in a tissue-specific and time-dependent manner in ING, RP, and EPI fat pads after 4 (A) and 8 wk (B) of treatment with either saline (control) or AICAR. β-actin was used as loading control.
DISCUSSION
Here, we report novel findings that chronic AICAR-induced AMPK activation promotes time-dependent and depot-specific effects on WAT lipolysis. These effects were characterized by an initial (first 4 wk of treatment) reduction in basal and catecholamine-stimulated lipolysis, which then reversed to an increase in SC and VC adipocytes at week 8 of AICAR treatment. Using the same in vivo approach, we have recently demonstrated (12) that the ability of SC and VC isolated adipocytes to oxidize palmitate increased and reduced after 4 and 8 wk of AICAR treatment, respectively. These time-dependent effects on FA oxidation in SC and VC fat depots were also accompanied by increased energy expenditure and reduced adiposity in rats chronically treated with AICAR (12). Since WAT lipolysis is crucial for providing substrate for energy production in peripheral tissues, the adaptive responses in SC and VC WAT must have occurred to meet the increase in whole body energy expenditure as treatment with AICAR progressed. Interestingly, plasma levels of NEFAs were also decreased and subsequently increased at 4 and 8 wk, respectively, indicating that our in vitro lipolysis data reflected the in vivo alterations in systemic energy demand with AICAR treatment (12). The rates of lipolysis in VC and SC fat depots have been repeatedly reported to differ (9, 30, 42) and our findings are in agreement with these observations. However, we found that after 8 wk of AICAR treatment the lipolytic responses of VC and SC adipocytes practically disappeared. In fact, even though the molecular mechanisms underlying the regulation of lipolysis differed between VC and SC adipocytes, similar lipolytic responses were found in adipocytes from both tissues under AICAR treatment. Thus it appears that VC and SC fat depots contribute similarly to FA metabolism at a whole body level under conditions of chronic pharmacological AMPK activation.
As previously mentioned, the regulatory effect of AMPK on adipocyte lipolysis has been reported to occur through suppression of PKA-mediated phosphorylation of key HSL serine residues. In its activated state, AMPK phosphorylates HSL at the Ser565 residue, an effect that has been proposed to prevent phosphorylation of the PKA-targeted serine residues and impair HSL activation and lipolysis (13). As we have recently reported (12), regardless of alterations in the total AMPK content, phosphorylation of this kinase was increased in all fat depots at both 4 and 8 wk of AICAR treatment. Our data also show that while adipocytes from animals treated for 4 wk with AICAR had a blunted lipolytic response, phosphorylation of Ser565 of HSL in RP and EPI fat pads was increased. Additionally, a consistent reduction in HSL Ser563 phosphorylation was observed in all three fat depots after 4 and 8 wk of AICAR treatment. These findings seem to fit well with AMPK impairing PKA-mediated HSL phosphorylation/activation as originally proposed by Garton et al. (13). However, our data also show that in the ING fat pad of chronically AICAR-treated rats HSL Ser565, Ser563, and Ser660 phosphorylations were all undetected. These observations suggested that, at least in the ING fat pad, AMPK-induced HSL Ser565 phosphorylation was not a potential mechanism by which lipolysis was inhibited under conditions of chronic AICAR treatment. In fact, previous studies have reported that phosphorylation of Ser563 was neither required for HSL activation in vitro (2) nor for the translocation of HSL to the surface of the lipid droplet (21), although a site-directed serine to alanine mutation of the Ser565 residue of HSL prevented the translocation of this lipase to the lipid droplet in 3T3-L1 adipocytes (21). This led to the hypothesis that instead of inhibiting lipolysis by interfering with PKA-mediated phosphorylation of HSL Ser563, phosphorylation of HSL Ser565 by AMPK would cause a structural alteration that prevents the conformational change required for the translocation and activation of HSL upon PKA-mediated phosphorylation of the crucial Ser659 and Ser660 residues (21). Despite the debate regarding the potential mechanism(s) by which AMPK could suppress lipolysis, our data show that in the ING fat pad of chronically AICAR-treated rats the total HSL content and the phosphorylation of all HSL serine residues investigated were undetectable. Thus regardless of the phosphorylation state of HSL Ser565, the overall marked reduction in the content and phosphorylation of all HSL serine residues assessed could account for the reduction in lipolysis in ING fat pads of AICAR-treated rats. Importantly, the contents of other major regulators of lipolysis such as ATGL and perilipin A also markedly dropped in ING and RP fat depots at week 4 of AICAR treatment. Together, all these alterations must have led to the suppression of basal and catecholamine-stimulated lipolysis in the first 4 wk of AICAR treatment.
Clearly, the lipolytic cascade of the ING fat depot was the most drastically affected by the treatment, since, as mentioned above, phosphorylation and content of most proteins involved in lipolysis were consistently undetected or very low in this tissue after 4 wk of AICAR administration. Conversely, the enhancement of catecholamine-induced lipolysis in isolated VC and SC adipocytes after 8 wk of AICAR treatment correlated well with increased ATGL and perilipin contents in all fat depots. Even though HSL content was similar in RP and EPI fat depots of both groups at week 8, phosphorylation of this lipase at Ser563 and Ser660 was still lower in AICAR-treated than controls. These findings again undermine the role of AMPK regulating HSL phosphorylation/activity in mediating the response to AICAR and indicate that at week 8 a more relevant role seemed to have been played by increased ATGL and perilipin contents in enhancing lipolysis in ING, RP, and EPI fat depots of AICAR-treated rats. In fact, this is compatible with previous observations that adipocytes from ATGL knockout mice exhibit a more profound reduction in FA release compared with mice lacking HSL (19). Also, a recent study by Ahmadian et al. (1) has identified the Ser406 residue of ATGL as a target for AMPK. It was demonstrated that phosphorylation of ATGL Ser406 by AMPK increased lipolysis in HEK293 cells and 3T3-L1 adipocytes after exposure to AICAR. Furthermore, increased in vivo lipolytic response to chronic AICAR treatment was observed in wild-type but not in adipose-specific ATGL knock out mice (1). This is also consistent with previous reports that prolonged AICAR-induced AMPK activation leads to an increase in the release of FAs by primary rat adipocytes with an increase in ATGL content and TAG lipase activity in these cells (10). These data support our findings of increased basal and catecholamine-induced lipolysis in a context of elevated ATGL content in SC and VC WAT after 8 wk of AICAR treatment. Importantly, increased perilipin A content, a protein associated with the lipid droplet, must also have contributed to the increased lipolytic response after 8 wk of AICAR treatment. In fact, upon phosphorylation by PKA, perilipin A triggers a massive remodeling of lipid droplets, whereby large perinuclear lipid droplets fragment into myriad lipid microdroplets that can then be hydrolased by lipases such as ATGL and HSL (7, 17). Perilipin A has also been shown to be essential for the translocation of HSL to the lipid droplet during lipolytic activation (20). Thus, from our data, it appears that after 8 wk of AICAR treatment upregulation of ATGL and perilipin A played major roles in enhancing catecholamine-stimulated lipolysis in all fat depots. In summary, our novel findings provide evidence that chronic in vivo AICAR-induced AMPK activation caused alterations in WAT lipolysis that were compatible with increases in whole body energy demand. Even though major alterations in HSL phosphorylation occurred in SC and VC WAT in the course of chronic AICAR-induced AMPK activation, the most relevant mechanisms underlying the time-dependent and depot-specific effects on lipolysis seem to have depended on the regulation of ATGL and perilipin. Furthermore, this study provides evidence that adipocyte lipolysis can be effectively modified through chronic pharmacologic AMPK activation. This can be of great therapeutic relevance for the treatment of obesity and type 2 diabetes, conditions in which the storage and release of FAs by VC and SC adipocytes are defective (4, 11). Additional studies investigating the chronic effects of other pharmacological activators of AMPK in vivo will help assess the potential therapeutic effects of these agents on WAT metabolism.
GRANTS
This research was funded by a Discovery Grant from the Natural Science and Engineering Research Council of Canada and by infrastructure grants from the Canada Foundation for Innovation and the Ontario Research Fund (awarded to R. B. Ceddia). R. B. Ceddia is also a recipient of the Canadian Institutes of Health Research (CIHR) New Investigator Award and the Early Research Award from the Ontario Ministry of Research and Innovation. M. P. Gaidhu was supported by the CIHR Michael Smith Foreign Study Supplement and by the Canadian Diabetes Association Doctoral Student Research Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.P.G. and R.B.C. conception and design of research; M.P.G. performed experiments; M.P.G., G.B., and R.B.C. analyzed data; M.P.G., G.B., and R.B.C. interpreted results of experiments; M.P.G. and G.B. prepared figures; G.B. and R.B.C. edited and revised manuscript; R.B.C. drafted manuscript; R.B.C. approved final version of manuscript.
REFERENCES
- 1. Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, Wang Y, Duncan RE, Kang C, Sul HS. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab 13: 739–748, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem 273: 215–221, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Anthony NM, Gaidhu MP, Ceddia RB. Regulation of visceral and subcutaneous adipocyte lipolysis by acute AICAR-induced AMPK activation. Obesity (Silver Spring) 17: 1312–1317, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Arner P. Human fat cell lipolysis: biochemistry regulation and clinical role. Best Pract Res Clin Endocrinol Metab 19: 471–482, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Bergeron R, Previs SF, Cline G, Perret P, Russell RR, III, Young LH, Shulman GI. Effect of 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes 50: 1076–1082, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Boon H, Bosselaar M, Praet SF, Blaak EE, Saris WH, Wagenmakers AJ, McGee SL, Tack CJ, Smits P, Hargreaves M, van Loon LJ. Intravenous AICAR administration reduces hepatic glucose output and inhibits whole body lipolysis in type 2 diabetic patients. Diabetologia 51: 1893–1900, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Brasaemle DL, Subramanian V, Garcia A, Marcinkiewicz A, Rothenberg A. Perilipin A and the control of triacylglycerol metabolism. Mol Cell Biochem 326: 15–21, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Daval M, Diot-Dupuy F, Bazin R, Hainault I, Viollet B, Vaulont S, Hajduch E, Ferre P, Foufelle F. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem 280: 25250–25257, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Di Girolamo M, Fine JB. Cellularity measurements. Methods Mol Biol 155: 65–75, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Gaidhu MP, Fediuc S, Anthony NM, So M, Mirpourian M, Perry RL, Ceddia RB. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J Lipid Res 50: 704–715, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaidhu MP, Anthony NM, Patel P, Hawke TJ, Ceddia RB. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol 298: C961–C971, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Gaidhu MP, Frontini A, Hung S, Pistor K, Cinti S, Ceddia RB. Chronic AMP-kinase activation with AICAR reduces adiposity by remodeling adipocyte metabolism and increasing leptin sensitivity. J Lipid Res 52: 1702–1711, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garton AJ, Campbell DG, Carling D, Hardie DG, Colbran RJ, Yeaman SJ. Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase. A possible antilipolytic mechanism. Eur J Biochem 179: 249–254, 1989 [DOI] [PubMed] [Google Scholar]
- 14. Granneman JG, Moore HP, Granneman RL, Greenberg AS, Obin MS, Zhu Z. Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem 282: 5726–5735, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res 48: 275–297, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab 3: 309–319, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Marcinkiewicz A, Gauthier D, Garcia A, Brasaemle DL. The phosphorylation of serine 492 of perilipin a directs lipid droplet fragmentation and dispersion. J Biol Chem 281: 11901–11909, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPARdelta agonists are exercise mimetics. Cell 134: 405–415, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 281: 40236–40241, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, Londos C. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol 161: 1093–1103, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Su CL, Sztalryd C, Contreras JA, Holm C, Kimmel AR, Londos C. Mutational analysis of the hormone-sensitive lipase translocation reaction in adipocytes. J Biol Chem 278: 43615–43619, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Sullivan JE, Brocklehurst KJ, Marley AE, Carey F, Carling D, Beri RK. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett 353: 33–36, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol 88: 2219–2226, 2000 [DOI] [PubMed] [Google Scholar]