Abstract
For the treatment of rabbit endocarditis, dalbavancin given once daily (10 mg/kg of body weight for 4 days) or as a single 40-mg/kg dose was active against Staphylococcus aureus with or without reduced susceptibility to glycopeptides, as expected from its good in vitro activity, even in broth supplemented with 90% serum and given its prolonged elimination half-life.
Worldwide emergence of strains of Staphylococcus aureus with reduced susceptibility to glycopeptides (glycopeptide-intermediate S. aureus [GISA]) (2, 3, 8) emphasizes the need for new therapeutic options. Previous studies showed that dalbavancin (BI-397), a new semisynthetic glycopeptide antibiotic, is active in vitro and in animal models (1, 4) against gram-positive microorganisms, including methicillin-resistant S. aureus. In addition, dalbavancin has unique pharmacokinetics, with high levels in plasma which are sustained in humans for a long time due to its very long elimination half-life of approximately 1 week (A. Leighton, E. Mrosczcak, R. White, D. Jabes, A. B. Gottlieb, M. Baylor, M. Perry, and T. Henkel, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-951, 2001).
The in vivo activity of dalbavancin against GISA strains has not been previously studied. Therefore, we investigated the activity of dalbavancin in the rabbit endocarditis model against two isogenic strains isolated in a patient before and after treatment with vancomycin and teicoplanin. S. aureus Lim-1 was susceptible to vancomycin and teicoplanin (MICs, 2 and 4 μg/ml, respectively), whereas Lim-2 had a reduced susceptibility to both glycopeptides (MICs, 8 and 16 μg/ml, respectively) (8). Resistance to both vancomycin and teicoplanin was homogeneously expressed by Lim-2 (6). In addition, S. aureus 218, a teicoplanin-resistant derivative of Lim-2 (MICs of vancomycin and teicoplanin, 8 and 32 μg/ml, respectively) isolated from a rabbit with endocarditis treated with teicoplanin, was observed during in vitro studies (6). MICs were determined by the broth microdilution method (5), and minimal bactericidal concentrations (MBCs) were determined by the macrodilution method in Mueller-Hinton broth or in broth supplemented with 90% rabbit serum (7). The in vitro bactericidal killing rates were determined by the broth macrodilution method in brain heart infusion (BHI) broth and in broth supplemented with 90% rabbit serum at 107 CFU/ml for dalbavancin (4, 20, or 50 μg/ml), vancomycin (50 μg/ml), or teicoplanin (50 μg/ml). Experimental staphylococcal endocarditis was induced in New Zealand female rabbits as previously described (6). Forty-eight hours after the inoculation of 106 CFU of S. aureus via the ear vein, rabbits were treated with 10 mg of dalbavancin per kg of body weight, which was administered intravenously once daily for 4 days or as a single 40-mg/kg dose. One group of untreated animals served as controls. Animals were sacrificed by intravenous injection of phenobarbital at 48 h after bacterial inoculation for the control animals, at 24 h after the last injection for the once-daily regimen, and at 96 h after drug administration for the single-dose regimen. Colony counts were determined after 24 h of incubation as previously described (6). In addition, 0.1 ml of vegetation homogenates was plated on BHI agar containing four times the MIC of dalbavancin against the strain Lim-2 and incubated for 48 h at 37°C to allow detection of the emergence of resistant subpopulations. Infected rabbits were sampled at 30 min (peak) and 24 h (trough) after the last injection for the once-daily regimen (n = 8) and at 30 min (peak) and 96 h (trough) after the injection for the single-dose regimen (n = 6). Uninfected rabbits were administered single injections of 10 or 40 mg of dalbavancin per kg (three rabbits per group) and were sampled at 0.05, 0.5, 1, 3, 6, 9, 12, 24, 48, 72, 96, and 120 h. Dalbavancin levels in serum were determined by liquid chromatography-mass spectrometry (Leighton et al., 41st ICAAC). Pharmacokinetic constants were calculated using a noncompartmental model. WinNonlin software (Scientific Consulting, Inc., Apex, N.C.) was used to fit the data. The terminal half-life was calculated using the last sampling times. The Scheffe test was used to compare the bacterial counts in vegetations from groups of animals infected by the same strain and that had been treated with various regimens.
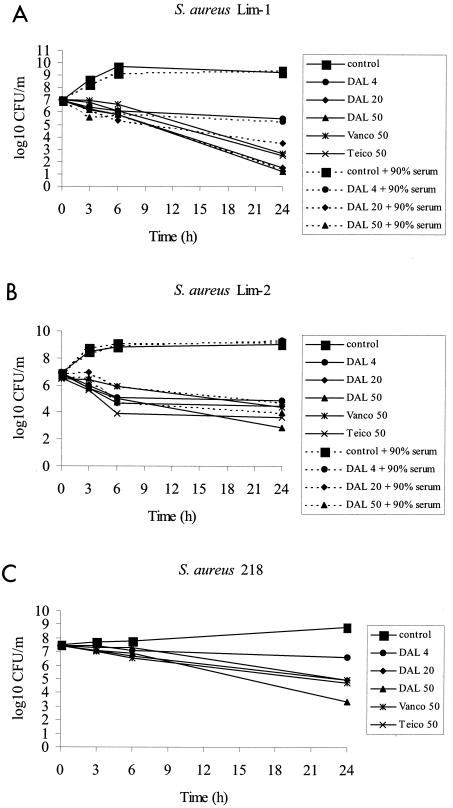
MICs of dalbavancin against the three strains of S. aureus were 0.5 μg/ml against Lim-1 and 4 μg/ml against Lim-2 and S. aureus 218. Dalbavancin was two- to fourfold more potent than vancomycin, the “gold standard” glycopeptide antibiotic, and was four- to eightfold more potent than teicoplanin against these strains—a result consistent with previous reports (4). MBCs of dalbavancin against Lim-1 and Lim-2 were identical to the MICs. The time-kill kinetics of the three antibiotics against the Lim-1, Lim-2, and S. aureus 218 strains are shown in Fig. 1. In unsupplemented BHI broth, 20 μg of dalbavancin per ml reduced the titer of Lim-1 by 5.5 log and by 2 log for Lim-2 within 24 h. At 50 μg/ml, the titer of Lim-2 was reduced by 4.0 log, suggesting some degree of concentration dependence under these conditions. In order to test the effect of the addition of serum, we calculated MICs and MBCs and plotted time-kill curves for broth supplemented with 90% rabbit serum. MICs in supplemented medium were 8 μg/ml against Lim-1 and 32 μg/ml against Lim-2. MBCs in supplemented medium were 32 μg/ml against Lim-1 and Lim-2 (i.e., 64- and 8-fold more than the MBC in broth against Lim-1 and Lim-2, respectively). Time-kill curves showed that addition of 90% rabbit serum to the medium had no effect on the bactericidal activity of dalbavancin against Lim-1 and significantly reduced the bactericidal activity against the Lim-2 strain only at the lowest concentration tested (4 μg/ml). This result contrasts with previous observations from our laboratory with oritavancin, another semisynthetic glycopeptide, which showed a 100-fold decrease in bactericidal activity in the presence of rabbit serum, a level that correlated with reduced in vivo activity (9). Against the teicoplanin-resistant mutant S. aureus 218, dalbavancin at a dosage of 50 μg/ml was bactericidal (reduction of 4.2 log) and was more active than vancomycin and teicoplanin at the same concentrations (Fig. 1C). At a dosage of 20 μg/ml, dalbavancin was as active as vancomycin and teicoplanin were at dosages of 50 μg/ml.
FIG. 1.
Time-kill study of dalbavancin (DAL), vancomycin (Vanco), and teicoplanin (Teico) at various concentrations against S. aureus Lim-1 (A) and Lim-2 (B) (with or without rabbit serum) and against S. aureus 218, a teicoplanin-resistant derivative of Lim-2 (C), all in BHI broth.
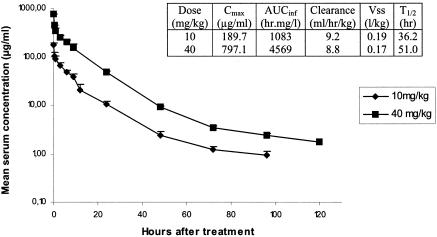
Mean concentrations of dalbavancin in serum following a single intravenous injection of 10 or 40 mg/kg in uninfected rabbits, and the corresponding pharmacokinetic parameters, are shown in Fig. 2. The serum elimination half-life was extremely long and ranged from 36 to 51 h. As shown in Table 1, the activities of dalbavancin in experimental endocarditis were similar for rabbits infected with Lim-1 or Lim-2, regardless of the dosing regimen used, and was thus not influenced by the acquisition of reduced susceptibility to glycopeptides. The 10-mg/kg once-daily regimen was bactericidal and tended to be more potent than the single-dose regimen, producing a reduction of 3.5 to 3.9 log10 CFU per gram of vegetation, while the 40-mg/kg single dose produced a reduction of 2.1 to 2.5 log10 CFU per gram of vegetation (P < 0.01). Note that the once-daily regimen of dalbavancin was at least as effective in this model as a daily regimen of vancomycin administered three times daily (mean peak and trough levels in serum, 36 and 15 μg/ml, respectively) against the same two strains (reduction of 2.9 to 3.6 log10 CFU per gram of vegetation) (6). One possible explanation for the lower in vivo activity of the single-dose regimen was the prolonged exposure to concentrations in serum that were insufficient to maintain a bactericidal activity during the entire course of therapy. Indeed, concentrations in serum obtained with the once-daily regimen were higher than the MBC in supplemented serum for approximately 42% of the time, whereas for the single-dose regimen, concentrations in serum exceeded the MBC in supplemented serum for approximatively only 35% of the time. This result does not exclude the possible use of dalbavancin with single-dose regimens or dosing intervals of several days for the treatment of infections in humans, since levels achieved in sera of healthy volunteers are much higher than those obtained in rabbits. Indeed, after a single 1,000-mg intravenous infusion to healthy humans, the maximum concentration of drug in serum was shown to be 301 μg/ml, and the mean concentration in plasma 1 week after the infusion was approximatively 40 μg/ml (J. A. Dowell, A. B. Gottlieb, C. Van Saders, M. B. Dorr, A. Leighton, M. Cavaleri, M. Guanci, and L. Colombo, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1386, 2002), a concentration that was still higher than the MBC in the presence of serum against the GISA strain. In addition, in the same study, the half-life was shown to be 257 h, which is also much higher than that obtained in small animals like rabbits.
FIG. 2.
Mean concentrations of dalbavancin in serum following a single intravenous injection of 10 or 40 mg/kg in uninfected rabbits. Corresponding pharmacokinetic parameters of dalbavancin following administration of 10 or 40 mg/kg are shown in the embedded table.
TABLE 1.
Results of treatment with dalbavancin in rabbits with experimental S. aureus endocarditisa
| Regimen | Antibiotic levels in serum, μg/ml (mean ± SD)
|
Log10 CFU/g of vegetation (mean ± SD) (no. of sterile/treated animals)
|
||
|---|---|---|---|---|
| Peak (h) | Trough (h) | Lim-1 | Lim-2 | |
| Controls | 9.1 ± 1.3 (0/17) | 9.4 ± 1.1 (0/15) | ||
| DAL 10 mg/kg q.d.i.v., 4 days | 114 ± 17 (0.5) | 19 ± 9 (24) | 5.6 ± 1.6b (0/7) | 5.5 ± 2.1b (1/10) |
| DAL 40 mg/kg i.v., single dose | 255 ± 53 (0.5) | 3 ± 2 (96) | 7.0 ± 1.4b (0/8) | 6.9 ± 2.1b (0/10) |
Abbreviations: DAL, dalbavancin; q.d., once a day; i.v., intravenously.
P < 0.01 versus controls.
No resistant subpopulations for any strain were detected following therapy with dalbavancin with either dosing regimen tested. The lack of selection for subpopulations resistant to dalbavancin can probably be explained by the potency of dalbavancin against the GISA strain (Lim-2), the high serum drug concentration/MIC ratio, and the persistent activity against the teicoplanin-resistant derivative, as shown by the MIC and time-kill studies (Fig. 1).
In conclusion, our study provides strong evidence that dalbavancin is at least as potent as vancomycin against methicillin-resistant S. aureus with or without reduced susceptibility to vancomycin. In addition, the prolonged serum drug elimination half-life may allow the use of infrequent dosing intervals in humans.
Acknowledgments
This work was supported by a grant from Biosearch Italia S.P.A.
REFERENCES
- 1.Candiani, G., M. Abbondi, M. Borgonovi, M. Romano, and F. Parenti. 1999. In-vitro and in-vivo antibacterial activity of BI-397, a new semi-synthetic glycopeptide antibiotic. J. Antimicrob. Chemother. 44:179-192. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1997. Staphylococcus aureus with reduced susceptibility to vancomycin—United States 1997. Morb. Mortal. Wkly. Rep. 46:765-766. [PubMed] [Google Scholar]
- 3.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 4.Jones, R. N., D. J. Biedenbach, D. M. Johnson, and M. A. Pfaller. 2001. In vitro evaluation of BI-397, a novel glycopeptide antimicrobial agent. J. Chemother. 13:244-254. [DOI] [PubMed] [Google Scholar]
- 5.National Committee for Clinical Laboratory Standards. 2001. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 6.Pavie J., A. Lefort, M. C. Ploy, L. Garry, F. Denis, and B. Fantin. 2003. Influence of reduced susceptibility to glycopeptides on the activity of vancomycin and teicoplanin in experimental endocarditis due to Staphylococcus aureus. Antimicrob. Agents Chemother. 47:2018-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson, R., R. T. Steigbigel, H. T. Davis, and S. W. Chapman. 1980. Method for reliable determination of minimal lethal antibiotic concentrations. Antimicrob. Agents Chemother. 18:699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ploy, M. C., C. Grelaud, C. Martin, L. de Lumley, and F. Denis. 1998. First clinical isolate of vancomycin-intermediate Staphylococcus aureus in a French hospital. Lancet 351:1212. [DOI] [PubMed] [Google Scholar]
- 9.Saleh-Mghir, A., A. Lefort, Y. Petegnef, S. Dautrey, J. M. Vallois, D. Le Guludec, C. Carbon, and B. Fantin. 1999. Activity and diffusion of LY333328 in experimental endocarditis due to vancomycin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 43:115-120. [DOI] [PMC free article] [PubMed] [Google Scholar]