Abstract
Alcohol affects total body sodium balance, but the molecular mechanism of its effect remains unclear. We used single-channel methods to examine how ethanol affects epithelial sodium channels (ENaC) in A6 distal nephron cells. The data showed that ethanol significantly increased both ENaC open probability (Po) and the number of active ENaC in patches (N). 1-Propanol and 1-butanol also increased ENaC activity, but iso-alcohols did not. The effects of ethanol were mimicked by acetaldehyde, the first metabolic product of ethanol, but not by acetone, the metabolic product of 2-propanol. Besides increasing open probability and apparent density of active channels, confocal microscopy and surface biotinylation showed that ethanol significantly increased α-ENaC protein in the apical membrane. The effects of ethanol on ENaC Po and N were abolished by a superoxide scavenger, 4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy (TEMPOL) and blocked by the phosphatidylinositol 3-kinase inhibitor LY294002. Consistent with an effect of ethanol-induced reactive oxygen species (ROS) on ENaC, primary alcohols and acetaldehyde elevated intracellular ROS, but secondary alcohols did not. Taken together with our previous finding that ROS stimulate ENaC, the current results suggest that ethanol stimulates ENaC by elevating intracellular ROS probably via its metabolic product acetaldehyde.
Keywords: ENaC, ethanol, acetaldehyde, reactive oxygen species, single channels, PI-3-kinase
the world health organization estimates that 55% of all adults worldwide consume alcohol and that there are 2.5 million alcohol-related deaths annually (http://www.who.int/substance_abuse/en/). Over and above the potential for abuse, alcohol can produce physiologic alterations in a number of organ systems. Chronic ethanol ingestion not only affects the central nervous system, but also adversely affects other organs including the liver, lung, and kidney. In the lung, for example, chronic ethanol ingestion results in fluid leak across the alveolar epithelial membrane. In a rat model of chronic ethanol ingestion, Guidot and coworkers (14) showed that ethanol prevented alveolar epithelial cells from forming the tight barrier which is imperative for normal alveolar function. They also demonstrated that glutathione depletion contributed to this disruption, suggesting that the disruption was due, at least in part, to oxidative stress (15). We have recently shown that reactive oxygen species (ROS) stimulate epithelial sodium channels (ENaC) in lung and renal epithelial cells (17, 24, 44). Since the epithelium depends on active transport of sodium and water out of the alveolar space and into the interstitium to keep proper lung fluid balance, the ROS increase in ENaC activity counteracts the ethanol-induced increase permeability. It has been known for some time that acute exposure to ethanol significantly reduced sodium and potassium excretion in rats. These effects are independent of glomerular filtration rate, plasma aldosterone concentration, or plasma renin activity (1). It is not yet clear how ethanol regulates electrolyte transport across the renal epithelial membrane. Since ethanol consumption produces ROS (2, 16, 42), we hypothesized that ethanol may affect sodium transport in sodium-transporting epithelial cells by ROS-mediated stimulation of ENaC.1
We used cell-attached patch-clamp methods and the Xenopus A6 epithelial cells as a model to investigate how ethanol acutely regulates ENaC. Specifically, we investigated the effect of ethanol on both ENaC open probability (Po) and the number of active ENaC in a patch (N). We also examined the effects of acetaldehyde, the first metabolic product of ethanol, as well as 1-propanol and 1-butanol, primary alcohols which can be oxidized to form aldehydes and contrasted the effects of these alcohols with the effect of 2-propanol and acetone. We used confocal microscopy combined with surface biotinylation to examine the effects of ethanol on ROS production and on the amount of α-ENaC protein in the apical membrane and determined whether the effect of ethanol on ENaC Po and N could be reduced by a superoxide scavenger, 4-hydroxy-2,2,6,6-tetramethyl-piperidinyloxy (TEMPOL).
MATERIALS AND METHODS
Cell culture.
A highly transporting clone, 2F3, of the Xenopus laevis distal nephron epithelial cell line, A6, was a gift from Dr. Thomas Kleyman and was maintained by standard tissue culture techniques as previously described (44–46). Briefly, a culture medium consisting of a 50% (vol/vol) mix of DMEM and Ham's F12 medium adjusted to amphibian tonicity plus 0.6% penicillin–1.0% streptomycin, 5% (vol/vol) fetal bovine serum, 1.5 μM aldosterone, 1 mM glutamine, and 25 mM NaHCO3 at 26°C and 4% CO2. For patch-clamp experiments, A6 cells were plated on permeable, glutaraldehyde-fixed, collagen-coated Millipore-CM filters (Millipore, Billerica, MA) attached to the bottoms of small Lucite rings at a density to allow them to be confluent and fully polarized after culturing for 10–14 days. For confocal and biotinylation experiments, the cells were plated on the polyester membrane of Transwell inserts at a density similar to that described above. Prior to the experiments, monolayers were washed with standard saline containing 96 mM NaCl, 3.4 mM KCl, 0.8 mM CaCl2, 0.8 mM MgCl2, 10 mM HEPES, adjusted to pH 7.4 with HCl or NaOH.
Patch-clamp recordings.
Cell-attached recordings of ENaC single-channel current from A6 distal nephron cells were carried out using an Axopatch 1D amplifier (Molecular Devices, Sunnyvale, CA). A6 cells were thoroughly washed with standard saline. Glass micropipettes with a pipette resistance of 7–10 MΩ were filled with standard saline. Standard saline was used for both the luminal and the basolateral baths. Single-channel currents were obtained with no applied pipette potential, filtered at 1 kHz, and sampled every 50 μs with PClamp 10 software. Experiments were conducted at room temperature. The total number of functional channels in the patch were estimated by observing the number of peaks detected on the current-amplitude histogram during at least a 10-min recording period. The open probability (Po) of ENaC before (−3 to 0 min) and after (25–30 min) each experimental manipulation was estimated using PClamp 10.
Addition of all agents involved completely replacing the standard saline on the apical surface of the cell monolayers with standard saline containing the agent of interest (e.g., 2% ethanol). Premixing of the agents was necessary especially for acetaldehyde, butanol, and acetone because of the turbulence associated with adding these agents directly to water.
Confocal microscopy imaging.
For detecting ENaC expression, A6 cells cultured on the polyester filter membrane of Transwell inserts were washed with standard saline. Untreated or treated cells were fixed with 2% paraformaldehyde for 10 min and permeabilized with 0.1% Triton X-100 in standard saline for 15 min. The cells were incubated with rabbit polyclonal antibody to α-ENaC (1:500) for 45 min and were then incubated with a secondary antibody (Alexa Fluor 488 goat anti-rabbit IgG, 2 μg/ml) for 30 min. Incubation of the cells with the secondary antibody alone served as a control and did not result in any fluorescence (data not shown). To monitor the apical level, the tight junction protein ZO-1 was labeled with mouse antibody to ZO-1 (Santa Cruz Biologics) according to the distributor's directions and stained with another antibody (Alexa Fluor 594 goat anti-mouse IgG, 2 μg/ml). Cells were washed twice following each step described above. Next, the polyester filter membrane was excised and mounted on a glass slide. For detecting intracellular ROS, live A6 cells were used. After experimental manipulations, the cells were stained with dihydroethidium at 2 μM for 15 min. Optical sections of A6 cell monolayer were performed with an Olympus FV-1000 confocal microscopy. In each set of experiments, images were obtained using the same parameter settings. All experiments were conducted at room temperature.
Biotinylation.
Four days after cells reached confluency on permeable supports, the apical side of A6 cells was labeled with 0.5 mg/ml sulfo-NHS-biotin (Pierce Chemical) in borate buffer (85 mM NaCl, 4 mM KCl, 15 mM Na2B4O7, pH 8.0) for 2 × 20 min on ice. Afterwards, cells were quenched with 100 mM glycine in PBS for 10 min and lysed in RIPA buffer (PBS with 0.1% SDS, 1% Nonidet P-40, 0.5% sodium deoxycholate) containing protease inhibitor cocktail (100 μM leupeptin, 1 mM phenylmethylsulfonyl fluoride, 100 μM antipain, 100 μM 1-chloro-3-tosylamido-7-amino-2-heptanone, and 100 μM l-1-tosylamido-2-phenylethyl chloromethyl ketone). Cellular debris was removed by centrifugation (1,200 g, 5 min).
The biotin-labeled proteins were precipitated by incubating with prewashed streptavidin-coated agarose beads for 18 h with gentle agitation at 4°C. Beads were then washed five times with RIPA (PBS with 0.1% SDS, 1% NP-40, 0.5% sodium deoxycholate), and the biotin-streptavidin complex was lysed by boiling in sample buffer containing 100 mM DDT and 5% SDS for 10 min. The apical biotin-labeled proteins were subsequently analyzed by Western blotting using a rabbit polyclonal antibody to α-ENaC. Briefly, the blot was transferred to nitrocellulose membrane, blocked in TBS buffer containing 5% milk and 0.1% Tween 20 and probed with the rabbit polyclonal antibody to α-ENaC at 1:500 dilution overnight. The membrane was then exposed to a goat anti-rabbit IgG secondary antibody coupled to alkaline phosphatase at 1:10,000 dilution. The antigen antibody complex was detected using a chemiluminescence detection system CDP-star (Tropix, Pearce Chemical) and Kodak 2000M camera system (Eastman Kodak, Rochester, NY).
Chemicals.
Unless explicitly stated in results, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Statistical analysis.
Data are reported as means ± SE. Statistical analysis was performed with SigmaPlot and SigmaStat software (Jandel Scientific). A Student's t-test was used for intergroup comparisons. Analysis of variance (ANOVA), followed by a Holm-Sidak post hoc test was used for multiple comparisons. A P < 0.05 was considered the minimum level for statistical significance.
RESULTS
Ethanol elevates ENaC Po and N in A6 epithelial cells.
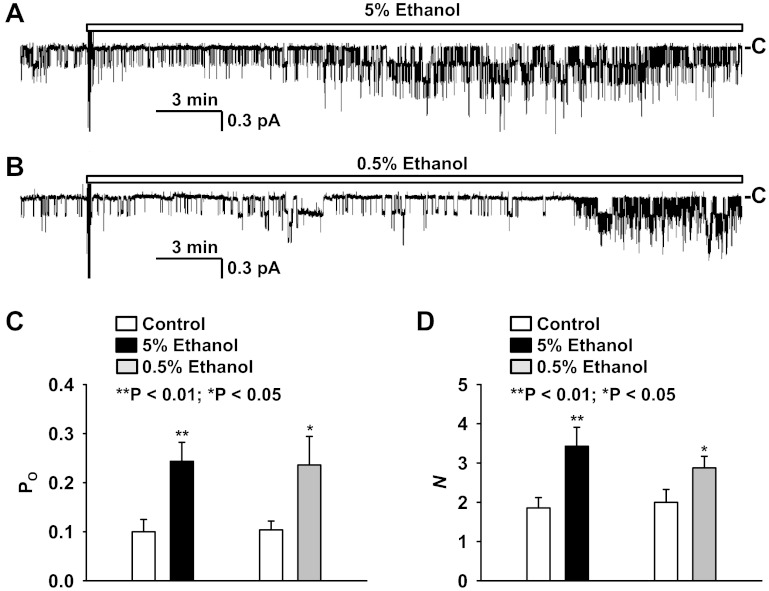
To determine whether ethanol affects ENaC activity, we performed cell-attached patch-clamp experiments using A6 cells as a model. To optimize our ability to detect any changes in activity, we initially used a relatively high concentration of ethanol. We found that addition of ethanol to the luminal bath at a final concentration of 5% (vol/vol) significantly stimulated ENaC in A6 cells (Fig. 1A). The effect occurred ∼10 min after the addition. A lower concentration (vol/vol = 0.5%) of ethanol also significantly elevated ENaC activity, but with an obviously longer latency (Fig. 1B). The effect occurred more than 20 min after the addition. Figure 1C shows the mean open probability (Po) before and after addition of either 5% or 0.5% ethanol, which was increased from 0.10 ± 0.03 (before addition) to 0.24 ± 0.04 (25–30 min after addition of 5% ethanol; P < 0.01; n = 7) and from 0.10 ± 0.02 (before addition) to 0.24 ± 0.06 (25–30 min after addition of 0.5% ethanol; P < 0.05; n = 8). Figure 1D shows that the number of active ENaC (N) in the patch was increased, from 1.9 ± 0.3 (before addition) to 3.4 ± 0.5 (25–30 min after addition of 5% ethanol; P < 0.01; n = 7) and from 2.0 ± 0.3 (before addition) to 2.9 ± 0.3 (25–30 min after addition of 0.5% ethanol; P < 0.05; n = 8).
Fig. 1.
Effects of 5% or 0.5% ethanol on epithelial sodium channel (ENaC) open probability (Po) and the number of active ENaC in patches (N). A: a representative cell-attached recording from an A6 cell before and after addition of 5% ethanol to the luminal bath. B: a representative cell-attached recording from another A6 cell before and after addition of 0.5% ethanol to the luminal bath. Downward transitions show the channel openings. “−C” shows the baseline when the channel is closed. C and D: summary plots of ENaC Po (C) and N (D) before (open bars) and after addition of either 5% (black bars) or 0.5% ethanol (gray bars) to the luminal bath.
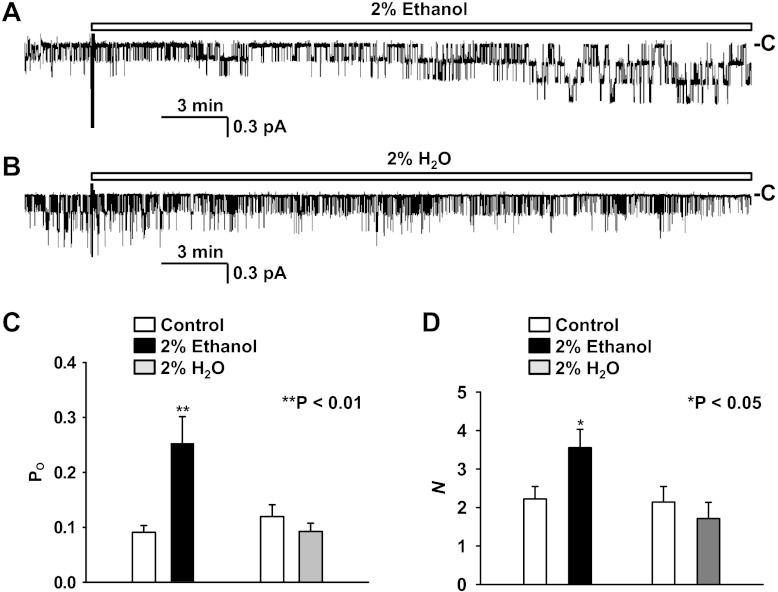
There were no significant differences in single-channel effects between the several discrete ethanol concentrations we studied. One exception was that it required a relatively long latency for 0.5% ethanol (or lower concentrations) to stimulate ENaC. Since ENaC activity is gradually decreased even under control conditions (often referred to as channel activity rundown), investigating an effect with a long latency is problematic. Therefore, we used the pharmacologic concentration of ethanol (vol/vol = 2%) to investigate the acute effect of ethanol on ENaC activity in the following experiments. The data (Fig. 2, A and C) showed that addition of 2% ethanol to the luminal bath increased Po from 0.09 ± 0.01 (before addition) to 0.22 ± 0.06 (25–30 min after addition of 2% ethanol; P < 0.01; n = 8) as well as N from 2.2 ± 0.3 (before addition) to 3.6 ± 0.5 (25–30 min after addition of 2% ethanol; P < 0.05; n = 9, Fig. 2D). As a control, addition of 2% H2O to the luminal bath (Fig. 2, B–D) affected neither Po, 0.12 ± 0.02 (before addition) versus 0.09 ± 0.02 (25–30 min after addition of 2% H2O; P = 0.1; n = 7), nor N, 2.1 ± 0.4 (before addition) versus 1.7 ± 0.4 (25–30 min after addition of 2% H2O; P = 0.1; n = 7).
Fig. 2.
Effects of 2% ethanol on ENaC Po and N. A: a representative cell-attached recording from an A6 cell before and after addition of 2% ethanol to the luminal bath. B: a representative cell-attached recording from another A6 cell before and after addition of 2% H2O to the luminal bath. C and D: summary plots of ENaC Po (C) and N (D) before (open bars) and after addition of either 2% ethanol (black bars) or 2% H2O (gray bars) to the luminal bath.
Acetaldehyde mimics the effects of ethanol on ENaC Po and N.
It has been reported that acetaldehyde, the first product arising from ethanol metabolism, accounts for most of effects of ethanol (9). On the basis of this information, and the temporal relationship between ethanol exposure determined in the present study, we hypothesized that acetaldehyde might actually be responsible for the effects of ethanol on ENaC Po and N. Data from the current investigation demonstrated that addition of 1% acetaldehyde to the luminal bath mimicked the effect of ethanol and significantly elevated ENaC activity (Fig. 3A). As shown in Fig. 3B, Po was increased, from 0.13 ± 0.03 (before addition) to 0.23 ± 0.04 (shortly minutes after addition of 1% acetaldehyde; P < 0.01; n = 10); N was also increased, from 2.0 ± 0.3 (before addition) to 3.2 ± 0.4 (10–15 min after addition of 1% acetaldehyde; P < 0.01; n = 10, Fig. 3C).
Fig. 3.
Effects of acetaldehyde on ENaC Po and N. A: a representative cell-attached recording from an A6 cell before and after addition of 1% acetaldehyde to the luminal bath. B and C: summary plots of ENaC Po (B) and N (C) in A6 cells before (open bars) and after addition of 1% acetaldehyde (black bars) to the luminal bath.
n-Propanol, but not iso-propanol, mimics the effects of ethanol.
n-Propanol and iso-propanol were used to investigate whether other alcohols also stimulate ENaC. The data demonstrated that addition of 2% n-propanol, which can be metabolized to an aldehyde, to the luminal bath increased ENaC activity (Fig. 4A). As shown in Fig. 4, C and D, Po was elevated, from 0.11 ± 0.04 (before addition) to 0.20 ± 0.04 (25–30 min after addition of 2% n-propanol; P < 0.01; n = 9); N was also elevated, from 1.6 ± 0.3 (before addition) to 2.7 ± 0.4 (25–30 min after addition of 2% n-propanol; P < 0.01; n = 9). In contrast, addition of iso-propanol, which is metabolized to acetone rather than an aldehyde, to the luminal bath had no effect on ENaC activity (Fig. 4B). As shown in Fig. 4, C and D, Po was 0.13 ± 0.04 (before addition) versus 0.10 ± 0.03 (25–30 min after addition of 2% iso-propanol; P = 0.1; n = 10); N was 2.0 ± 0.3 (before addition) to 1.8 ± 0.3 (25–30 min after addition of 2% iso-propanol; P = 0.3; n = 10). In data not shown, 1% n-butanol also increased ENaC Po and N, but iso-butanol did not. Addition of the first metabolic product of n-propanol, acetone, has no effect on ENaC Po or N. Taken together with the effects of acetaldehyde, these data suggest that normal alcohols stimulate ENaC through their metabolic products, namely aldehydes, while iso-alcohols and their metabolic products, ketones, have no effect.
Fig. 4.
Effects of n-propanol or iso-propanol on ENaC Po and N. A: a representative cell-attached recording from an A6 cell before and after addition of 2% n-propanol to the luminal bath. B: a representative cell-attached recording from another A6 cell before and after addition of 2% iso-propanol to the luminal bath. C and D: summary plots of ENaC Po (C) and N (D) before (open bars) and after addition of either 2% n-propanol (black bars) or 2% iso-propanol (gray bars) to the luminal bath.
Ethanol, acetaldehyde, and n-propanol, but not iso-propanol, elevate ROS in A6 cells.
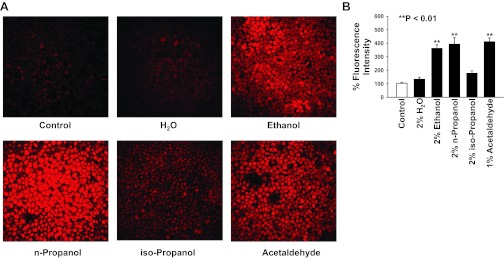
To test how ethanol, acetaldehyde, n-propanol, and iso-propanol affect the levels of intracellular ROS, confluent A6 cells were stained with dihydroethidium, a ROS-sensitive dye. Confocal microscopy optical sections were performed across A6 monolayers. As shown in Fig. 5, A and B, compared with ROS in control A6 cells (104% ± 8%; n = 5), intracellular ROS was significantly elevated in A6 cells treated for 15 min with 2% ethanol (361% ± 27%; P < 0.001; n = 5), 2% n-propanol (393% ± 48%; P < 0.001; n = 5), or 1% acetaldehyde (412 ± 27%; P < 0.001; n = 5), but not in A6 cells treated for 15 min with 2% H2O (134% ± 15%; P > 0.05; n = 5) or 2% iso-propanol (177% ± 19%; P > 0.05; n = 5). These data taken together with the results from patch-clamp experiments suggest that ethanol stimulates ENaC by elevating ROS in A6 cells probably via its metabolic product, acetaldehyde.
Fig. 5.
Confocal images of reactive oxygen species (ROS) in A6 cells either under control conditions or treated with 2% H2O, 2% ethanol, 2% n-propanol, 2% iso-propanol, or 1% acetaldehyde. Confluent A6 cells were stained with dihydroethidium, a membrane-permeable ROS-sensitive dye. A: confocal microscopy XY scanning was performed across A6 cell monolayers. B: summary plots of % fluorescence intensity from five experiments for each condition.
TEMPOL, a ROS scavenger, abolishes the effects of ethanol on ENaC Po and N.
To determine whether ethanol stimulates ENaC by elevating intracellular ROS, we pretreated A6 epithelial cells with 250 μM TEMPOL, a ROS scavenger, for 15 min. As shown in Fig. 6, A and B, after the pretreatment, addition of 2% ethanol to the luminal bath affected neither Po [0.08 ± 0.02 (before) versus 0.07 ± 0.02 (25–30 min after ethanol, Fig. 6C); P = 0.1; n = 8] nor N [1.9 ± 0.3 (before) versus 2.1 ± 0.3 (25–30 min after ethanol, Fig. 6D); P = 0.3; n = 8]. These data suggest that the effects of ethanol on ENaC Po and N are mediated by intracellular ROS. As a control, the effects of TEMPOL on ENaC activity were also studied (Fig. 6A). Addition of 250 μM TEMPOL to the luminal bath significantly reduced the basal ENaC Po from 0.23 ± 0.04 (before) to 0.06 ± 0.02 (25–30 min after TEMPOL; P < 0.01; n = 6), but did not significantly affect the basal N [2.7 ± 0.5 (before) versus 1.8 ± 0.3 (25–30 min after TEMPOL); P = 0.093; n = 6], indicating that basal ROS in A6 cells is important for maintaining Po but not N. However, ethanol-induced ROS is critical for the maintenance of both Po and N.
Fig. 6.
TEMPOL, a superoxide scavenger, abolishes the effects of ethanol on ENaC Po and N. A: a representative cell-attached recording from a control 2F3 cell before and after addition of 250 μM TEMPOL to the luminal bath. B: a representative cell-attached recording from another A6 cell pretreated with 250 μM TEMPOL before and after addition of 2% ethanol to the luminal bath. C and D: summary plots of ENaC Po (C) and N (D) either in control A6 cells before (open bars) and after addition of TEMPOL (gray bars) or in A6 cells pretreated with TEMPOL before (gray bars) and after addition of 2% ethanol (black bars) to the luminal bath.
Ethanol elevates ENaC density in the apical membrane of A6 epithelial cells.
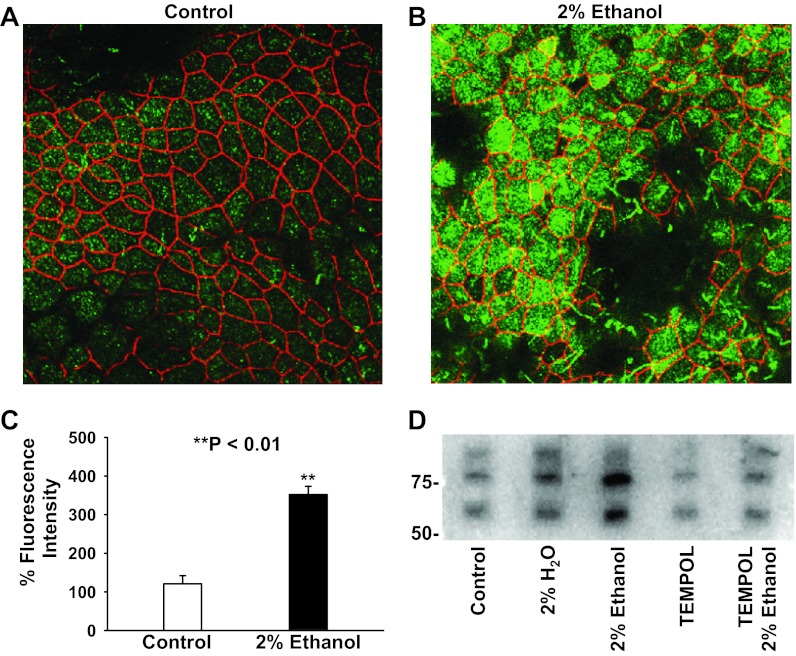
Results from patch-clamp experiments showed that ethanol elevated ENaC Po and N probably via its metabolic product acetaldehyde. To determine how ethanol elevates ENaC N, first, we performed confocal microscopy experiments to detect the density of α-ENaC in or near the apical membrane by labeling α-ENaC with its antibody (shown in green in Fig. 7, A and B). To make XY optical sections in or near the apical membrane, the tight junction protein ZO-1 was also labeled with antibody (shown in red in Fig. 7, A and B). We found that the density of ENaC in or near the apical membrane was significantly elevated after treatment of A6 cells with 2% ethanol for 30 min (Fig. 7, A–C). To further determine whether ethanol elevates ENaC density in the apical membrane, we biotinylated the proteins in the apical membrane, separated the biotinylated surface proteins with streptavidin, and performed Western blot experiments on the precipitated material. The data showed that treatment of the cells with 2% ethanol significantly increased the density of α-ENaC in the apical membrane (Fig. 7D). The effect was abolished when the cells were pretreated with TEMPOL. In contrast, treatment of the cells with 2% H2O or TEMPOL only slightly increased or decreased ENaC apical membrane expression, respectively. These data suggest that ethanol elevates ENaC apical density probably via ROS.
Fig. 7.
Ethanol elevates apical expression of α-ENaC. A and B: confocal microscopy images of A6 cells either in control conditions (A) or treated with 2% ethanol for 30 min (B). Confluent A6 cells were labeled with antibodies to α-ENaC (shown in green) or ZO-1 (shown in red). C: summary plots of % green fluorescence intensity (α-ENaC) from five experiments in control A6 cells (open bar) and A6 cells treated with 2% ethanol (black bar). D: Western blot of α-ENaC in the apical membrane of either control A6 cells or A6 cells treated with 2% H2O, 2% ethanol, 250 μM TEMPOL alone, or 250 μM TEMPOL plus 2% ethanol. The data represent three experiments showing similar results.
A phosphatidylinositol 3-kinase inhibitor blocks ethanol-induced ENaC activation.
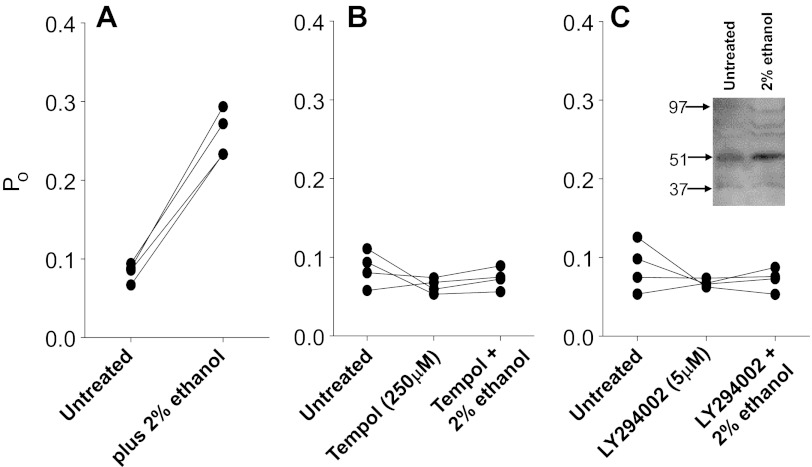
Ethanol and other normal alcohols increase ENaC activity and increase the density of ENaC channels in the apical membrane. The ethanol-induced increase in ENaC appears to depend upon the ability of ethanol to generate ROS. There are several mechanisms that increase both the activity of single ENaC channels and apical channel density. The most important one is an increase in phosphatidylinositol phosphate especially the tris-phosphate (PIP3) that directly increases ENaC open probability and reduces ENaC internalization, thereby increasing surface density. PIP3 is produced by the action of phosphatidylinositol 3-kinase (PI-3-K) which can be activated by ROS (19, 40). To investigate the role of PI-3-K in ethanol-induced ENaC activation, we pretreated 2F3 cells with the PI-3-K inhibitor LY294002 before examining the effect of ethanol. Figure 8 shows that LY294002 prevents ethanol activation of ENaC as well as TEMPOL, implying that ethanol-induced activation of ENaC requires PI-3-K.
Fig. 8.
A phosphatidylinositol 3-kinase (PI-3-kinase) inhibitor blocks the effect of ethanol as strongly as a ROS scavenger. ENaC Po from individual patches was measured, always starting with untreated patches before application of treatments to the same patch. This protocol used individual patches as their own controls and therefore reduced the effect of patch-to-patch variability in Po. A: untreated followed by 2% ethanol. B: untreated followed by TEMPOL followed by 2% ethanol. TEMPOL blocked the effect of ethanol. C: untreated followed by the PI-3-kinase inhibitor LY294002 followed by ethanol. LY294002 blocked the action of ethanol as effectively as TEMPOL. There was no significant effect of ethanol after ROS removal or after PI-3-kinase inhibition to prevent production of PIP3. Inset: Western blot of SGK before and 20 min after the addition of 2% ethanol. In this and three other experiments, SGK was increased by a comparable amount.
DISCUSSION
The major findings from the present work are as follow: 1) ethanol elevates ENaC Po and N in A6 epithelial cells; 2) acetaldehyde mimics the effects of ethanol on ENaC Po and N; 3) 1- (i.e., normal) alcohols, but not secondary alcohols, mimic the effects of ethanol; 4) ethanol, acetaldehyde, and n-propanol, but not iso-propanol, elevate ROS in A6 epithelial cells; 5) TEMPOL, a ROS scavenger, abolishes the effects of ethanol on ENaC Po and N; 6) ethanol elevates ENaC density in the apical membrane of A6 epithelial cells; and 7) the effect of ethanol depends upon PI-3-K.
Physiologically relevant ethanol concentrations.
Some of the concentrations of ethanol we used were pharmacological rather than physiological. Five percent(vol/vol) ethanol in saline is the seemingly high concentration of 857 mM. Even the lowest concentration we used, 0.1% (vol/vol) ethanol (data not shown), is 17 mM, but it can still stimulate ENaC. The primary difference between high concentrations and low concentrations is the rapidity of the action; the various concentrations all finally produce approximately the same effect on ENaC open probability and apical channel density, but the low concentrations require longer to produce the effect. Because cell-attached patches are not necessarily stable for long periods of time, we generally used higher concentrations of alcohol to produce the ENaC-activating effect more quickly. However, even moderately high concentrations of ethanol are not physiologically unprecedented. There are numerous reports in the popular press of very high blood alcohol concentrations (in extraordinary cases >1%) (13, 33, 34, 36). After ingestion of alcohol, the urine alcohol concentration compared with the blood alcohol concentration varies but is always higher by 1.2- to 1.6-fold (28). In the present study, we found that even the lowest concentration we applied on the apical surface of cells (0.1% ethanol) can stimulate ENaC. The 0.1% concentration is consistent with that found in the urine of chronic alcoholics, suggesting that our findings are clinically relevant (35).
The physiological distinction between the effects of chronic and acute ethanol ingestion in humans is often difficult to make because of confounding factors such as a prior history of alcohol consumption or preexisting hypertension. Previous reports have shown that chronic ethanol ingestion resulted in increased sodium transport in both lung and renal epithelium (1, 14). One study that did examine the acute effects of ethanol administration to rats and controlled for ethanol effects that could confound the results (changes in renin or aldosterone production, in particular) found that acute exposure to ethanol that would increase blood alcohol to ∼0.1% did decrease sodium excretion and increase blood sodium levels (1).
Acetaldehyde mimics the effects of ethanol on ENaC Po and N.
The addition of 1% acetaldehyde to the luminal bath mimicked the effect of ethanol and resulted in significantly elevated ENaC activity (Fig. 3). As we showed for ethanol, the acetaldehyde-mediated increase in ENaC activity was a result of significant increases in both the open probability (Po) and the number of active channels in the patches (N). In contrast to channel responses to ethanol, the onset of the increased activity in the experiments with acetaldehyde occurred almost immediately and was relatively stable for the entire recording period of 25–35 min. This observation would appear to imply that the production of acetaldehyde or acetaldehyde, itself, is responsible for all the effects of ethanol. This expectation is consistent with our observation that only alcohols that can be metabolized to aldehydes increase ENaC activity while alcohols metabolized to ketones do not. However, the production and effect of acetaldehyde alone are not consistent with the observation that a superoxide scavenger completely eliminates the effects of ethanol. Our fluorescent imaging of epithelial monolayers shows that ethanol or n-propanol metabolism to aldehydes increases ROS (although metabolism of iso-alcohols does not), but acetaldehyde by itself also increases ROS. These observations imply that the presence of aldehydes is intimately related to the production of ROS. In fact, aldehydes are known to inhibit both superoxide dismutase (11) and catalase (40), the inhibition of which would lead to increased cellular levels of superoxide and hydrogen peroxide.
These results are consistent with those of Helms and coworkers, who demonstrated the importance of ROS in the regulation of ENaC in lung and kidney cells (17, 24, 44). The results are also consistent with the observation that antioxidants like glutathione and procysteine prevent the ethanol-induced to increase the ENaC-mediated sodium transport in alveolar epithelial cells (14).
Ethanol elevates ENaC density in the apical membrane of A6 epithelial cells.
Patch-clamp experiments showed that increases in ENaC induced by ethanol and/or acetaldehyde were due to increases in both open probability (Po) and the number of active channels (N). Confocal microscopy experiments showed that the density of α-ENaC in or near the apical membrane was significantly elevated after treatment of A6 epithelial cells with 2% ethanol for 30 min (see Fig. 7). To further verify that ethanol elevates ENaC density in the apical membrane, we biotinylated the proteins in the apical membrane and performed Western blot experiments. The data showed that treatment of the cells with 2% ethanol significantly increased the density of α-ENaC in the apical membrane. The effect was abolished when the cells were pretreated with TEMPOL. This increase in N could be due to either an accelerated transport of ENaC to the apical membrane or a reduction in the removal of ENaC from the apical membrane.
Mechanism of ethanol activation of ENaC.
We were initially surprised that ethanol stimulated ENaC. Conventional wisdom suggests that ethanol is a central nervous system depressant consistent with the anesthetic effects of longer-chain alcohols. In fact, long-chain alcohols like n-octanol do reduce ENaC activity. However, our realization that ethanol metabolism produced an increase in cellular ROS that mediated the ethanol-induced increase in ENaC activity altered our expectation. We had previously shown that ROS could activate ENaC (17, 24, 38, 44). Others have shown that ROS activates p21 Ras (37, 43) and activated Ras activates phosphatidylinositol 3-kinase (PI-3-K) to produce phosphatidylinositol-tris-phosphate (PIP3) (4–6). In addition, ROS is known to inhibit the inositol lipid phosphatase, PTEN (10, 20, 21). Inhibition of PTEN will lead to increasing levels of PIP3 in the apical membrane. We have established in prior work that increasing PIP3 increases ENaC open probability (18, 25–27, 31, 47). Such an increase is what we observe and the increase is blocked by an inhibitor of PI-3-K.
However, besides an increase in open probability, we also observe an increase in the density of ENaC channels in the apical membrane. But this increase is also consistent with an increase in PIP3. PIP3 activates phosphatidylinositol-dependent kinase 1/2 (PDK1/2) and PDK1/2 activates serum and glucocorticoid-dependent kinase (SGK1) (7, 30, 41). SGK1, in turn, phosphorylates and inactivates the ubiquitin ligase Nedd4–2 (22, 29). Nedd4–2 ubiquinates ENaC, and the ubiquitination promotes ENaC internalization (12, 32). Inhibition of Nedd4–2 (by SGK1) reduces internalization and increases ENaC in the surface membrane of epithelial cells (8, 22). Thus, an increase in PIP3 will increase both ENaC open probability and the density of ENaC in the apical membrane.
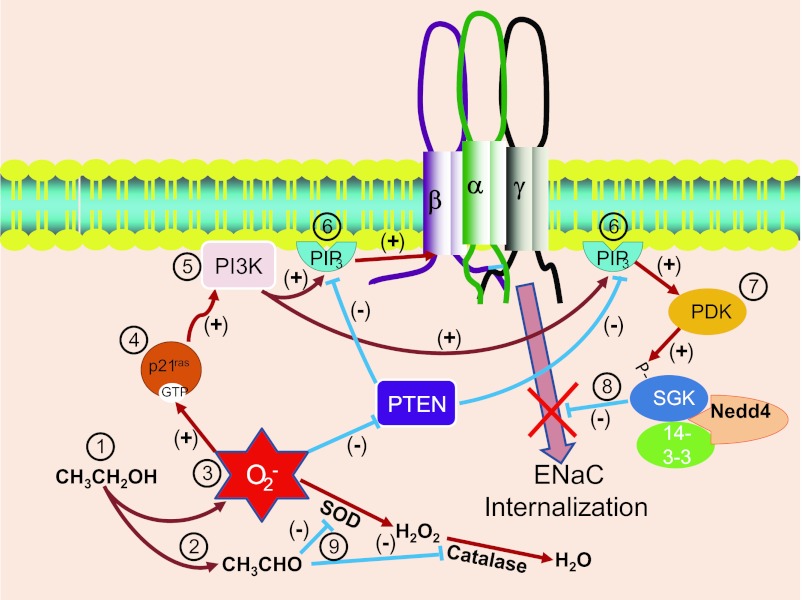
Primary alcohols generate ROS, and an increase in ROS should activate ENaC, but, typically, in epithelial cells there are enzymatic mechanisms to reduce the concentration of ROS. Superoxide dismutase (SOD1) converts superoxide into hydrogen peroxide and catalase converts hydrogen peroxide into water and free oxygen. We have previously shown that 2F3 cells have very high levels of catalase (24) which can metabolize hydrogen peroxide. Our observations suggest that one important component of the action of ethanol and other alcohols is the requirement that the alcohols be metabolized to aldehydes. Interestingly, aldehydes (but not ketones) are capable of inhibiting both SOD1 (11) and catalase (40). Thus, at the same time that ethanol metabolism is generating ROS, the aldehydic products of ethanol metabolism are preventing the breakdown of the ROS and further increasing the effects of ethanol. This effect of aldehydes on ROS breakdown is consistent with our observation that the effect of ethanol is somewhat larger than the effect of actetaldehyde since acetaldehyde causes an increase in ROS only by preventing a breakdown in ROS, while ethanol prevents the breakdown but also generates new ROS. These mechanisms are all schematically represented in Fig. 9.
Fig. 9.
Schematic diagram of the mechanism for ENaC stimulation by ethanol. At 1, ethanol is metabolized to produce acetaldehyde (at 2) and ROS (at 3). ROS activates p21 K-Ras (at 4) and K-Ras stimulates PI-3-kinase (PI3K) (at 5). PI-3-kinase produces phosphatidylinositol-tris-phosphate (PIP3) (at 6). PIP3 can directly interact with ENaC to increase open probability (as we have shown in this paper). In addition, PIP3 can activate other molecules with pleckstrin homology domains including phosphatidylinositol-dependent kinase (PDK) (at 7). PDK phosphorylates serum- and glucocorticoid-dependent kinase (SGK1) that phosphorylates Nedd4–2 to prevent Nedd4-mediated ENaC internalization (at 8) and thereby allow an accumulation of ENaC in the surface membrane (also as we observed in this paper). All of these ROS-mediated events are facilitated by the original production of acetaldehyde since acetaldehyde inhibits both superoxide dismutase (SOD) and catalase (at 9), preventing the usual breakdown of the ethanol-generated ROS.
The variation in the time to the peak effect of ethanol is consistent with a simple model in which the production of reactive oxygen species is proportional to the production of acetaldehyde.
Overall, the results from the current experiments add further support to the hypothesis that ROS is important in the regulation of ENaC in epithelial cells.
GRANTS
This work was supported by Department of Health and Human Services (National Institutes of Health Grants 2R01-DK067110 to H.-P. Ma and R37-DK037963 to D. C. Eaton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.-F.B., J.Z.S., B.J.D., and H.-P.M. performed the experiments; H.-F.B., H.-P.M., and D.C.E. analyzed the data; H.-F.B., H.-P.M., and D.C.E. interpreted the results of the experiments; H.-F.B., H.-P.M., D.D.D., and D.C.E. approved the final version of the manuscript; H.-P.M. and D.C.E. conception and design of the research; H.-P.M. and D.C.E. prepared the figures; H.-P.M., D.D.D., and D.C.E. edited and revised the manuscript; D.D.D. and D.C.E. drafted the manuscript.
Footnotes
This article is the topic of an Editorial Focus by Peter M. Snyder (35a).
REFERENCES
- 1. Assadi FK. Acute effect of ethanol on renal electrolyte excretion in rats. Alcohol 6: 257–260, 1989 [DOI] [PubMed] [Google Scholar]
- 2. Bailey SM, Cunningham CC. Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology 28: 1318–1326, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Castellano E, Downward J. Role of RAS in the regulation of PI 3-kinase. Curr Top Microbiol Immunol 346: 143–169, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Castellano E, Downward J. RAS interaction with PI3K: more than just another effector pathway. Genes Cancer 2: 261–274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castellano E, Santos E. Functional specificity of ras isoforms: so similar but so different. Genes Cancer 2: 216–231, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci USA 96: 2514–2519, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4–2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J 20: 7052–7059, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deng XS, Deitrich RA. Putative role of brain acetaldehyde in ethanol addiction. Curr Drug Abuse Rev 1: 3–8, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Downes CP, Ross S, Maccario H, Perera N, Davidson L, Leslie NR. Stimulation of PI 3-kinase signaling via inhibition of the tumor suppressor phosphatase, PTEN. Adv Enzyme Regul 47: 184–194, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Eom SY, Zhang YW, Ogawa M, Oyama T, Isse T, Kang JW, Lee CJ, Kim YD, Kawamoto T, Kim H. Activities of antioxidant enzymes induced by ethanol exposure in aldehyde dehydrogenase 2 knockout mice. J Health Sci 53: 378–381, 2007 [Google Scholar]
- 12. Flores SY, Debonneville C, Staub O. The role of Nedd4/Nedd4-like dependant ubiquitylation in epithelial transport processes. Pflügers Arch 446: 334–338, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Goldman R. Extreme drunk driving [Online]. ABC News; 7-24-2008. http://abcnews.go.com/Health/story?id=5436334#.UHnVCnndHIM [Google Scholar]
- 14. Guidot DM, Modelska K, Lois M, Jain L, Moss IM, Pittet JF, Brown LA. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. Am J Physiol Lung Cell Mol Physiol 279: L127–L135, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Guidot DM, Moss M, Holguin F, Lois M, Brown LA. Ethanol ingestion impairs alveolar epithelial glutathione homeostasis and function, and predisposes to endotoxin-mediated acute lung injury. Chest 116: 82S, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Heaton MB, Paiva M, Mayer J, Miller R. Ethanol-mediated generation of reactive oxygen species in developing rat cerebellum. Neurosci Lett 334: 83–86, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Helms MN, Jain L, Self JL, Eaton DC. Redox regulation of epithelial sodium channels examined in alveolar type 1 and 2 cells patch-clamped in lung slice tissue. J Biol Chem 283: 22875–22883, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Helms MN, Liu L, Liang YY, Al-Khalili O, Vandewalle A, Saxena S, Eaton DC, Ma HP. Phosphatidylinositol 3,4,5-trisphosphate mediates aldosterone stimulation of epithelial sodium channel (ENaC) and interacts with gamma-ENaC. J Biol Chem 280: 40885–40891, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Kohli R, Pan X, Malladi P, Wainwright MS, Whitington PF. Mitochondrial reactive oxygen species signal hepatocyte steatosis by regulating the phosphatidylinositol 3-kinase cell survival pathway. J Biol Chem 282: 21327–21336, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J 22: 5501–5510, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leslie NR, Lindsay Y, Ross SH, Downes CP. Redox regulation of phosphatase function. Biochem Soc Trans 32: 1018–1020, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Loffing J, Zecevic M, Feraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Renal Physiol 280: F675–F682, 2001. [DOI] [PubMed] [Google Scholar]
- 24. Ma HP. Hydrogen peroxide stimulates the epithelial sodium channel through a phosphatidylinositide 3-kinase-dependent pathway. J Biol Chem 286: 32444–32453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma HP, Chou CF, Wei SP, Eaton DC. Regulation of the epithelial sodium channel by phosphatidylinositides: experiments, implications, and speculations. Pflügers Arch 455: 169–180, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Ma HP, Eaton DC. Acute regulation of epithelial sodium channel by anionic phospholipids. J Am Soc Nephrol 16: 3182–3187, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Ma HP, Saxena S, Warnock DG. Anionic phospholipids regulate native and expressed ENaC. J Biol Chem 277: 7641–7644, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Miles WR. The comparative concentrations of alcohol in human blood and urine at intervals after ingestion. J Pharmacol Exp Ther 20: 265–319, 1922 [Google Scholar]
- 29. Pearce D. The role of SGK1 in hormone-regulated sodium transport. Trends Endocrinol Metab 12: 341–347, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Pearce D, Verrey F, Chen SY, Mastroberardino L, Meijer OC, Wang J, Bhargava A. Role of SGK in mineralocorticoid-regulated sodium transport. Kidney Int 57: 1283–1289, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Pochynyuk O, Tong Q, Staruschenko A, Ma HP, Stockand JD. Regulation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. Am J Physiol Renal Physiol 290: F949–F957, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Rotin D, Staub O, Haguenauer-Tsapis R. Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J Membr Biol 176: 1–17, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Saksensa V. R.I. police arrest man with record .491 blood alcohol level. Pawtuckett Times, Rhode Island, July 28, 2008 [Google Scholar]
- 34. Schoetz D. DUI suspect's ‘lethal dose’ earns $50K bail. ABC News, December 28, 2007. http://abcnews.go.com/US/story?id=4061172#.UHnXPXndHIM [Google Scholar]
- 35. Silverstein SJ, Nathan PE, Taylor HA. Blood alcohol level estimation and controlled drinking by chronic alcoholics. Behavior Therapy 5: 1–15, 1974 [Google Scholar]
- 35a. Snyder PM. Intoxicated Na+ channels. Focus on “Ethanol stimulates epithelial sodium channels by elevating reactive oxygen species.” Am J Physiol Cell Physiol (September 19, 2012). doi:10.1152/ajpcell.00301.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stanglin D. Woman's .708 blood alcohol level may set state record. USA Today January 10, 2008. http://content.usatoday.com/communities/ondeadline/post/2009/12/womans-blood-alcohol-content-may-set-state-record/1#.UHnXi3ndHIM [Google Scholar]
- 37. Tai P, Ascoli M. Reactive oxygen species (ROS) play a critical role in the cAMP-induced activation of Ras and the phosphorylation of ERK1/2 in Leydig cells. Mol Endocrinol 25: 885–893, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takemura Y, Goodson P, Bao HF, Jain L, Helms MN. Rac1-mediated NADPH oxidase release of O2− regulates epithelial sodium channel activity in the alveolar epithelium. Am J Physiol Lung Cell Mol Physiol 298: L509–L520, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trac D, Brewer E, Helms MN. Chronic ethanol ingestion increases epithelial sodium channel activity in C57BL/6 lung (Abstract). FASEB J 26: 884.–6., 2012 [Google Scholar]
- 40. Venkatesan B, Mahimainathan L, Das F, Ghosh-Choudhury N, Ghosh CG. Downregulation of catalase by reactive oxygen species via PI 3 kinase/Akt signaling in mesangial cells. J Cell Physiol 211: 457–467, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Wang J, Barbry P, Maiyar AC, Rozansky DJ, Bhargava A, Leong M, Firestone GL, Pearce D. SGK integrates insulin and mineralocorticoid regulation of epithelial sodium transport. Am J Physiol Renal Physiol 280: F303–F313, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health 27: 277–284, 2003 [PMC free article] [PubMed] [Google Scholar]
- 43. Xiao L, Pimentel DR, Wang J, Singh K, Colucci WS, Sawyer DB. Role of reactive oxygen species and NAD(P)H oxidase in alpha(1)-adrenoceptor signaling in adult rat cardiac myocytes. Am J Physiol Cell Physiol 282: C926–C934, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Yu L, Bao HF, Self JL, Eaton DC, Helms MN. Aldosterone-induced increases in superoxide production counters nitric oxide inhibition of epithelial Na channel activity in A6 distal nephron cells. Am J Physiol Renal Physiol 293: F1666–F1677, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Yu L, Eaton DC, Helms MN. The effect of divalent heavy metals on epithelial Na channels in A6 cells. Am J Physiol Renal Physiol 293: F236–F244, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Yu L, Helms MN, Yue Q, Eaton DC. Single-channel analysis of functional epithelial sodium channel (ENaC) stability at the apical membrane of A6 distal kidney cells. Am J Physiol Renal Physiol 295: F1519–F1527, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yue G, Malik B, Yue G, Eaton DC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem 277: 11965–11969, 2002 [DOI] [PubMed] [Google Scholar]